First splicing variant in HECW2 with an autosomal recessive pattern of inheritance and associated with NDHSAL
María Elena Rodríguez-García and Francisco Javier Cotrina-Vinagre contributed equally to this study.
Abstract
We report the clinical and genetic features of a Caucasian girl who presented a severe neurodevelopmental disorder with drug-resistant epilepsy, hypotonia, severe gastro-esophageal reflux and brain magnetic resonance imaging anomalies. WES uncovered a novel variant in homozygosis (g.197092814_197092824delinsC) in HECW2 gene that encodes the E3 ubiquitin-protein ligase HECW2. This protein induces ubiquitination and is implicated in the regulation of several important pathways involved in neurodevelopment and neurogenesis. Furthermore, de novo heterozygous missense variants in this gene have been associated with neurodevelopmental disorder with hypotonia, seizures, and absent language (NDHSAL). The homozygous variant of our patient disrupts the splice donor site of intron 22 and causes the elimination of exon 22 (r.3766_3917+1del) leading to an in-frame deletion of the protein (p.Leu1256_Trp1306del). Functional studies showed a twofold increase of its RNA expression, while the protein expression level was reduced by 60%, suggesting a partial loss-of-function mechanism of pathogenesis. Thus, this is the first patient with NDHSAL caused by an autosomal recessive splicing variant in HECW2.
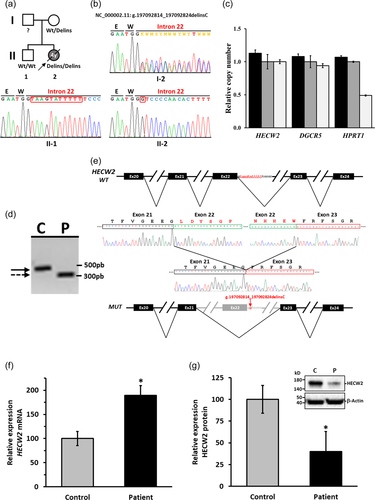
Herein, we describe a Spanish patient with a severe developmental delay, drug-resistant epilepsy and hypotonia (see Supporting Information Case report). She was a girl of nonconsanguineous healthy parents with a negative family history for congenital anomalies, epilepsy and intellectual disability (Figure 1a). An older brother, diagnosed with attention deficit hyperactivity disorder, was otherwise healthy. CNVs (Copy Number Variations), which are common in neurodevelopmental disorders were ruled out by aCGH (microarray-based Comparative Genomic Hybridization) study performed with the patient's sample (Supporting Information Chromosomal Array Study). Then, by using WES data and an analytic pipeline that prioritizes variants in genes that were both rare and predicted to be deleterious (Supporting Information Whole-exome sequencing), we were able to identify 24 variants (Table S1). However, only the novel homozygous variant in HECW2 gene (HECT, C2 and WW domain containing E3 ubiquitin protein ligase 2; MIM# 617245) was compatible with the patient's phenotype. The homozygous state of the variant (NC_000002.11 (GRCh37.p13):g.197092814_197092824delinsC; NM_020760.4:c.3917+2_3917+12delinsG in intron 22 as numbered in NG_053156.1) was assessed by Sanger sequencing. The patient carried the variation in HECW2 in homozygosity, whereas her healthy mother was heterozygous for the variant and her healthy brother was homozygous for wild-type form (Figure 1b). Because the father's DNA was not available, a quantitative PCR was performed to determine the homozygosity or hemizygosity (deletion of/in one allele) of the variant in the patient. The homozygous state of the variant was confirmed (Figure 1c), since the primers used in the determination of the gene dose were inside of the area surrounding the variant site that was sequenced by Sanger. These data confirmed the recessive pattern of inheritance, although the heterozygosity of the variant in the father is unknown. The chromosome 2 has a ROH (region of homozygosity) of 11.9 Mb that includes HECW2 gene (Figure S1), so a segmental uniparental disomy from the mother could be a possibility. However, the distribution of the ROHs in the other chromosomes (Table S2) indicates at least a parental consanguinity of fifth degree (see Supporting Information Homozygosity Study).
HECW2 encodes the E3 ubiquitin-protein ligase HECW2 (UniProt: Q9P2P5), which is a member of the E3 ubiquitin ligases of the HECT (homologous to E6AP C terminus) family, and one of the nine members of the NEDD4 (neural precursor cell expressed developmentally downregulated protein) subfamily (Rotin & Kumar, 2009; Scheffner & Kumar, 2014; Sluimer & Distel, 2018). The proteins in this subfamily share a similar domain composition, but despite being similar in structure, each of the nine NEDD4 subfamily members shows different functions (Rotin & Kumar, 2009; Scheffner & Kumar, 2014; Sluimer & Distel, 2018). Thus, HECW2 contains an N-terminal HECW domain (its function is not clearly understood), a calcium/lipid-binding (C2) domain involved in membrane targeting, two WW domains responsible for cellular localization and substrate recognition, and a catalytic HECT (Homologous to E6-associated protein Carboxyl Terminus) domain in the C-terminus (Figure S2a) (Rotin & Kumar, 2009; Scheffner & Kumar, 2014; Sluimer & Distel, 2018). The protein induces the covalent attachment of ubiquitin to the target, and this process can be regulated by protein–protein interactions, posttranslational modification, binding of calcium ions, and other means (Sluimer & Distel, 2018). As a consequence of the ubiquitination, the target can be stabilized or tagged for degradation (Sluimer & Distel, 2018). Thus, HECW2 protein plays an important role in several pathways involved in neurodevelopment and neurogenesis by stabilizing TP73 (tumor protein p73; O15350) (Berko et al., 2017; Lu et al., 2013; Miyazaki et al., 2003); angiogenesis through stabilization of endothelial cell-to-cell junctions by regulating AMOTL1 (angiomotin-like protein 1; Q8IY63) (Choi et al., 2016); nuclear organization by interacting with both lamin A-binding proteins (PCNA [proliferating cell nuclear antigen; P12004], and lamin-B1 [P20700]) (Krishnamoorthy et al., 2018b) and with both HP1α (chromobox protein homolog 5; P45973) and HP1β (chromobox protein homolog 1; P83916) (Krishnamoorthy et al., 2018a); enteric nervous system and kidney development as positive a regulator of GDNF/Ret/Akt signaling during enteric neurogenesis (Qiu et al., 2016; Wei et al., 2015); and fertilization by playing a role in sperm chromatin remodeling and acrosome formation (Mao et al., 2021). In animal models, the Hecw2 null mouse did not display a brain-related phenotype but a gastrointestinal phenotype due to a reduction of the enteric nervous system (Wei et al., 2015). However, the transcriptional knockdown of zebrafish hecw2a led to early morphological abnormalities in the brain tissues, suggesting a functional link between HECW2 dysfunction and brain development (Lu et al., 2021).
Recently, de novo variants in HECW2 have been recognized as a cause of a neurodevelopmental disorder with hypotonia, seizures, and absent language (NDHSAL; MIM# 617268) (Acharya et al., 2021; Berko et al., 2017; Halvardson et al., 2016; Heide et al., 2021; Kritioti et al., 2021; Lu et al., 2021; Nakamura et al., 2018; Peikes et al., 2021; Ullman et al., 2018; Yanagishita et al., 2021). They have also been associated to autism spectrum disorder (Iossifov et al., 2014; Krumm et al., 2015), developmental disorder (Deciphering Developmental Disorders Study 2015; Deciphering Developmental Disorders Study 2017), intellectual disability (Taşkıran et al., 2021), and epileptic encephalopathy (Euro et al., 2014; Hamdan et al., 2017). Currently, 54 patients from 53 families with 32 missense variants in HECW2 have been published (Table 1). These variants showed an autosomal dominant pattern of inheritance, and most of them were de novo (Acharya et al., 2021; Berko et al., 2017; Deciphering Developmental Disorders Study 2015; Deciphering Developmental Disorders Study 2017; Euro et al., 2014; Halvardson et al., 2016; Hamdan et al., 2017; Heide et al., 2021; Iossifov et al., 2014; Kritioti et al., 2021; Krumm et al., 2015; Lu et al., 2021; Nakamura et al., 2018; Peikes et al., 2021; Taşkıran et al., 2021; Ullman et al., 2018; Yanagishita et al., 2021). Of these 32 variants, 18 are described as pathogenic and 7 as likely pathogenic, the rest being of uncertain significance. However, during the review process of this study, the first case of a homozygous nonsense variant in HECW2 has been published (Table 1) (Krami et al., 2022).
| HECW2 (NM_020760.4) | AA change | ACMG Clasificationa | Inheritance | Phenotype | Reference |
|---|---|---|---|---|---|
| c.412A>G (de novo) | p.(Ile138Val) | Uncertain significance | Dominant | NDHSAL | (15) |
| c.794A>G (de novo) | p.(Asp265Gly) | Uncertain significance | Dominant | DD | (3), (7) |
| c.1105A>G (de novo) | p.(Asn369Asp) | Uncertain significance | Dominant | ID | (13) |
| c.2587T>C (de novo) | p.(Tyr863His) | Pathogenic | Dominant | NDHSAL | (15) |
| c.2818G>A (unknown) | p.(Ala940Thr) | Uncertain significance | Dominant | NDHSAL | (15) |
| c.3175C>T (unknown) | p.(Pro1059Ser) | Uncertain significance | Dominant | NDHSAL | (15) |
| c.3542C>G (de novo) | p.(Ala1181Gly) | Pathogenic | Dominant | NDHSAL | (16)b |
| c.3571C>T (de novo, inher) | p.(Arg1191Trp) | Likely pathogenic | Dominant | ASD, NDHSAL | (2), (17) |
| c.3572G>A (de novo) | p.(Arg1191Gln) | Likely pathogenic | Dominant | NDHSAL (±microcephaly) | (6), (10), (15) |
| c.3577T>G (de novo) | p.(Phe1193Val) | Pathogenic | Dominant | NDHSAL | (6) |
| c.3583G>C (de novo) | p.(Ala1195Pro) | Pathogenic | Dominant | NDHSAL | (15) |
| c.3587A>G (de novo) | p.(Lys1196Arg) | Pathogenic | Dominant | NDHSAL | (16)b |
| c.3597C>A (de novo) | p.(Asn1199Lys) | Likely pathogenic | Dominant | EE-IS, NDHSAL | (1), (15) |
| c.3668A>T (de novo) | p.(Asp1223Val) | Uncertain significance | Dominant | DD | (7) |
| c.3940C>T (de novo) | p.(Leu1314Phe) | Likely pathogenic | Dominant | NDHSAL | (14) |
| c.3980T>C (unknown) | p.(Phe1327Ser) | Pathogenic | Dominant | NDHSAL | (15) |
| c.3988C>T (de novo) | p.(Arg1330Trp) | Pathogenic | Dominant | NDHSAL, DD, NDHSAL | (5-7), (9), (12), (15) |
| c.4323T>G (de novo) | p.(Phe1441Leu) | Pathogenic | Dominant | NDHSAL | (15) |
| c.4325A>G (de novo) | p.(Asp1442Gly) | Pathogenic | Dominant | ASD | (4) |
| c.4331G>C (de novo) | p.(Arg1444Thr) | Pathogenic | Dominant | NDHSAL | (14) |
| c.4333G>C (de novo) | p.(Glu1445Gln) | Pathogenic | Dominant | NDHSAL | (15) |
| c.4334A>G (de novo) | p.(Glu1445Gly) | Pathogenic | Dominant | NDHSAL | (6) |
| c.4335A>C (de novo) | p.(Glu1445Asp) | Likely pathogenic | Dominant | DD | (3), (7) |
| c.4355G>T (de novo) | p.(Gly1452Val) | Likely pathogenic | Dominant | NDHSAL | (15) |
| c.4484G>A (de novo) | p.(Arg1495Lys) | Pathogenic | Dominant | DD, DEE | (7), (8) |
| c.4485G>T (de novo) | p.(Arg1495Ser) | Likely pathogenic | Dominant | NDHSAL (+microcephaly) | (11) |
| c.4507A>G (de novo) | p.(Thr1503Ala) | Pathogenic | Dominant | NDHSAL | (15) |
| c.4511C>A (de novo) | p.(Ser1504Tyr) | Pathogenic | Dominant | NDHSAL | (15) |
| c.4511C>G (de novo) | p.(Ser1504Cys) | Pathogenic | Dominant | NDHSAL | (14)b |
| c.4514G>C (de novo) | p.(Ser1505Thr) | Pathogenic | Dominant | NDHSAL | (15) |
| c.4590A>C (de novo) | p.(Lys1530Asn) | Uncertain significance | Dominant | NDHSAL | (14)b |
| c.4690G>A (de novo) | p.(Glu1564Lys) | Pathogenic | Dominant | NDHSAL | (14) |
| c.736C>T (Hom) | p.(Arg246Ter) | Pathogenic | Recessive | NDHSAL | (18) |
| c.3917+2_3917+12delinsG (Hom) | p.(Leu1256_Trp1306del) | Pathogenic | Recessive | NDHSAL | This study |
- Note: Krumm et al. (2015); (3) Deciphering Developmental Disorders Study (2015); (4) Krumm et al. (2015); (5) Halvardson et al. (2016); (6) Berko et al. (2017); (7) Deciphering Developmental Disorders Study (2017); (8) Hamdan et al. (2017); (9) Nakamura et al. (2018); (10) Ullman et al. (2018); (11) Peikes et al. (2021); (12) Lu et al. (2021); (13) Taşkıran et al. (2021); (14) Yanagishita et al. (2021); (15) Acharya et al. (2021); (16) Kritioti et al. (2021); (17) Heide et al. (2021); (18) Krami et al. (2022).
- Abbreviations: ASD, autism spectrum disorder; DD, developmental disorder; EE-IS, epileptic encephalopathy and infantile spasm; ID, intellectual disability; NDHSAL, neurodevelopmental disorder with hypotonia, seizures, and absent language; inher, inherited.
- a Data from Varsome (https://varsome.com/).
- b Same allele.
The effect of the intronic variant in HECW2 RNA was evaluated in skin fibroblasts from the patient and a genetically unrelated control of similar age through RT-PCR. Since the variant is located in intron 22, a PCR product was amplified by using primers situated in exon 19/20 (HecF19/20) and exon 23/24 (HecR23/24). The analyses from agarose gel of this product showed a smaller transcript in the patient respect to the control (Figure 1d). In the patient's transcript, an in-frame elimination of exon 22 due to differential splicing processing of the mRNA (NM_020760.4:r.3766_3917+1del) was identified by Sanger sequencing (Figure 1e). The variant disrupts the splice donor site of intron 22 and causes the deletion of 51 aa (p.Leu1256_Trp1306del) in the HECT domain of HECW2 (Figure S2a). The computer modeling by Swiss Model program (Biasini et al., 2014) allows to determine the change in the 3D structure of HECW2 carrying p.Leu1256_Trp1306del compared to the wild-type. The final modeled structures were visualized by Chimera (Pettersen et al., 2004) and showed that the deletion of 51 aa in the HECT domain of HECW2 (1216–1572 aa) was close to the catalytic cysteine residue (p.Cys1540) (Figure S2b).
The effect of the HECW2 variant in the steady-state level of mRNA and protein was also evaluated in skin fibroblasts from the patient and control. The steady-state level of HECW2 transcripts was evaluated by qRT-PCR, and the analysis showed an increase of approximately 100% in the patient respect to the control (Figure 1f). On the other hand, the expression of HECW2 protein in the patient, evaluated by western blot with an antibody against an internal region non affected by the deletion, showed a dramatic decrease (~60%) compared to the control (Figure 1g). Thereby, the variant in the HECW2 gene specifically affected the production of the protein. The lower levels of HECW2, with a deletion of 51 aa in the catalytic domain (HECT domain), are probably due to the instability of the protein, because most variants that alter amino acids destabilize the proteins and result in gradual decline in their performance (Tokuriki et al., 2007). Then, these findings suggest that a compensatory adaptation occurs at transcriptional level in an attempt to maintain normal levels of protein in response to the protein instability. In addition to the lower level of deleted protein, the internal deletion in the HECT domain could modify the catalytic activity due to the proximity of the catalytic cysteine 1540 (Choi et al., 2016) (Figure S2b). Furthermore, none of the aforementioned pathogenic variants is located in exon 22 (between the aa 1256–1306) (Table 1). In view of these results, the pathogenic role of this homozygous variant in HECW2 gene is strongly supported.
All the variants in HECW2 associated with an autosomal dominant disease are heterozygous missense variants, and most of them are clustering at the last third of the protein (27/32 are located between aa 1000 and 1572), and more specifically in C-terminal HECT domain (19/32 are located between aa 1216 and 1572). Furthermore, HECW2 is intolerant to haploinsufficiency and loss-of-function (LOF), and hypomorphic variants (nonsense, frameshift, or variant in the catalytic cysteine 1540) with LOF have not been described to date (Acharya et al., 2021). This suggests that these missense variants could act through a gain-of-function (GOF) or dominant negative (DN) mechanism (Acharya et al., 2021; Backwell & Marsh, 2022). The DN variants act by interfering with the activity of wild-type protein through competition, by competing with the wild-type subunit in homomeric complexes or reducing the availability of free ligand for the wild-type protein. On the other hand, the GOF variants act by increasing protein activity (hypermorphic) or introducing a new function (neomorphic), but the specific molecular mechanisms can be complex (Backwell & Marsh, 2022). It has been demonstrated previously that the four recurrent variants (p.(Arg1191Gln), p.(Asn1199Lys), p.(Phe1327Ser), and p.(Arg1330Trp)), showed a difference in protein surface property and conformation compared with wild-type (Acharya et al., 2021). This suggests that, at less these variants, could act by DN mechanism. Other variants could act by GOF by increasing ubiquitination activity. Thus, missense variants in the HECT domain of NEDD4L (E3 ubiquitin-protein ligase NEDD4-like; Q96PU5) lead to conformation changes and constitutive activation of the catalytic function that mimic the increased activity resulting from the overexpression of wild-type NEDD4L (Broix et al., 2016). In contrast, LOF variants involve a loss of the normal biological function of the protein, and this can be complete (amorphic), or only partial (hypomorphic) (Backwell & Marsh, 2022). Furthermore, most of the recessive variants result in LOF conditions, and usually are associated with protein loss (Turner et al., 2015). Thus, the mechanism of the recently published homozygous nonsense HECW2 variant p.(Arg246Ter) (Krami et al., 2022) could be a complete LOF (amorphic variant), while a partial LOF (hypomorphic variant) is likely to be the mechanism of our patient's homozygous splice variant.
In conclusion, this is the first report of a patient with NDHSAL due to an autosomal recessive splicing variant in HECW2, expanding the genotype associated to this syndrome.
WEB RESOURCES
ANNOVAR: https://wannovar.wglab.org/
gnomAD: https://gnomad.broadinstitute.org/
OMIM: https://omim.org/
UniProt: https://www.uniprot.org/
ACKNOWLEDGMENTS
The authors thank the patient and his family for their contribution, and Alicia Torrado and Lucía del Pozo Filíu for valuable comments on the manuscript. This work was supported by the Spanish Instituto de Salud Carlos III (ISCIII) and European Regional Development Fund (ERDF) (grant PI17/00487 and PI20/00150 to F.M-A.). F.J.C-V. was supported by fellowship from the Instituto de Investigación Hospital 12 de Octubre (i+12) and fellowship from ISCIII and ERDF (PI20/00150). M.E.R-G. was supported by fellowship from ISCIII and ERDF (PI17/00487).
CONFLICTS OF INTEREST
The authors declare no conflicts of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data of the study (variant and phenotype) have been submitted to LOVD database (https://databases.lovd.nl/shared/variants/0000825318; patient ID 00393155). The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.