Autosomal dominantly inherited myopathy likely caused by the TNNT1 variant p.(Asp65Ala)
Abstract
Nemaline myopathies (NEMs) are genetically and clinically heterogenous. Biallelic or monoallelic variants in TNNT1, encoding slow skeletal troponin T1 (TnT1), cause NEM. We report a 2-year-old patient and his mother carrying the heterozygous TNNT1 variant c.194A>C/p.(Asp65Ala) that occurred de novo in the mother. Both had muscle hypotrophy and muscle weakness. Muscle pathology in the proband's mother revealed slow twitch type 1 fiber hypotrophy and fast twitch type 2 fiber hypertrophy that was confirmed by a reduced ratio of slow skeletal myosin to fast skeletal myosin type 2a. Reverse transcription polymerase chain reaction and immunoblotting data demonstrated increased levels of high-molecular-weight TnT1 isoforms in skeletal muscle of the proband's mother that were also observed in some controls. In an overexpression system, complex formation of TnT1-D65A with tropomyosin 3 (TPM3) was enhanced. The previously reported TnT1-E104V and TnT1-L96P mutants showed reduced or no co-immunoprecipitation with TPM3. Our studies support pathogenicity of the TNNT1 p.(Asp65Ala) variant.
Congenital myopathies are a group of rare hereditary muscle diseases that are subdivided into five subgroups, including nemaline myopathies (NEMs) (Claeys, 2020). NEMs are genetically and clinically heterogenous and caused by de novo, autosomal dominantly or recessively inherited variants in at least 13 genes. The clinical spectrum is broad, ranges from severe neonatal presentations to milder disorders with onset in childhood, and includes variable and progressive muscle weakness, hypoventilation, dysphagia, and speech impairment. The name NEM originated from the presence of pathological structures that are rod-like or ovoid in shape in the sarcoplasm. These nemaline bodies or rods can be detected by electron microscopy or stained red with the modified Gömöri trichrome technique visualized by light microscopy (Sewry et al., 2019). Nemaline myopathy 5, Amish type (NEM5; MIM# 605355), is a lethal myopathy with infantile onset, characterized by tremors, muscle weakness, contractures, and progressive pectus carinatum. Median survival is 18 months (Fox et al., 2018; Johnston et al., 2000; Mondal & Jin, 2016). NEM5 is caused by biallelic TNNT1 variants, including nonsense and splice variants, small deletion–insertion or insertion variants, and deletions of single or multiple exons (Abdulhaq et al., 2016; D'Amico et al., 2019; Fattahi et al., 2017; Fox et al., 2018; Geraud et al., 2021; Johnston et al., 2000; Marra et al., 2015; Pergande et al., 2020; Sharifi et al., 2021; Streff et al., 2019; van der Pol et al., 2014). These pathogenic TNNT1 variants likely represent null alleles as they cause complete loss of the encoded slow skeletal muscle troponin T1 (TnT1) (Amarasinghe et al., 2016; Fox et al., 2018; Geraud et al., 2021; Jin et al., 2003; Johnston et al., 2000). A homozygous TNNT1 missense variant, c.287T>C/p.(Leu96Pro), has been reported in four French Canadians with a slowly progressive core-rod myopathy (Pellerin et al., 2020). Recently, an adult female with an autosomal recessive mild form of nemaline myopathy and the homozygous 1-bp deletion c.786delG in TNNT1 has been described. This variant is located in the penultimate exon of TNNT1, likely escapes nonsense-mediated messenger RNA (mRNA) decay, and possibly leads to the production of a partially functioning TnT1 protein truncated by 16 C-terminally amino acid residues and extended by 35 TnT1-unrelated residues at the C-terminus [p.(Lys263Serfs*36)] (Petrucci et al., 2021). Another mild form of nemaline myopathy that is inherited in an autosomal dominant manner has been reported in a large family with 10 affected relatives (Gonatas et al., 1966; Spiro & Kennedy, 1965). Clinical severity in the affected individuals ranges from mild difficulty with weight-training exercises to a Gowers' maneuver in early childhood (Supporting Information: Table S1). In eight affected and genetically tested family members the heterozygous TNNT1 missense variant c.311A>T/p.(Glu104Val) was identified (Konersman et al., 2017).
The troponin complex plays a central role in calcium-mediated skeletal muscle contraction. It is composed of the calcium-binding subunit troponin C, the inhibitory subunit troponin I, and the tropomyosin binding subunit TnT. Binding of TnT to tropomyosin is necessary to link the troponin complex to actin. The two highly conserved tropomyosin binding sites correspond to residues 64–102 (tropomyosin-binding site 1) and residues 164–203 (tropomyosin-binding site 2) in the slow skeletal muscle TnT1 (Jin & Chong, 2010; Mondal & Jin, 2016). TnT1 is specifically expressed in slow-contracting type 1 muscle fibers (Mondal & Jin, 2016; Wei & Jin, 2016). Skeletal muscle biopsies from individuals with biallelic TNNT1 variants show type 1 fiber hypotrophy, type 2 fiber hypertrophy, and the presence of nemaline rods (Abdulhaq et al., 2016; D'Amico et al., 2019; Fattahi et al., 2017; Fox et al., 2018; Johnston et al., 2000; Pellerin et al., 2020; Petrucci et al., 2021; van der Pol et al., 2014; Wei et al., 2014). Similarly, in muscle biopsies of subjects with the heterozygous TNNT1 variant p.(Glu104Val) hypotrophy and nemaline rods restricted to the type 1 fiber population and compensatory type 2 fiber hypertrophy have been observed (Konersman et al., 2017).
Here, we describe a 2-year-old boy and his 33-year-old mother with an autosomal dominantly inherited myopathy (Supporting Information: Table S1). The male index patient was born at 36 weeks of gestation to nonconsanguineous parents (Supporting Information: Figure S1). Pregnancy was achieved by intracytoplasmic sperm injection and was complicated by maternal arterial hypertension and gestational diabetes. Birth measurements were in the normal range with length of 48 cm (−0.48z), weight of 2350 g (−1.15z), and occipitofrontal head circumference (OFC) of 33 cm (−0.5z). Postnatal cardiorespiratory adaptation was normal, however, he was a floppy infant with reduced muscle tone and spontaneous movements. A small ventricular septal defect was diagnosed postnatally. During the first weeks of life, the boy had feeding difficulties that improved and eventually disappeared over time. At the age of 3 months, ophthalmologic evaluation, electroencephalogram, motor nerve conduction velocity of the upper limbs, and cerebral ultrasound revealed normal results. Creatine kinase (CK) levels and basic metabolic work-up in blood and urine were normal. His motor development was delayed: at the age of 13 months, he was able to crawl, to get on his knees, and to sit unaided for few minutes after being seated, but he had a poor head control. He has been undergoing physiotherapy since birth. At the age of 21 months, he was able to stand up and walk without help and to climb up stairs with support. At last examination at the age of 2 years and 11 months, his length was 94 cm (−0.39z), weight was 10.8 kg (−2.16z), and OFC was 50 cm (−0.46z). He showed muscle hypotrophy, generalized muscle weakness, most prominent in the proximal muscles and in the neck and legs, and absent deep tendon reflexes. He could stand up with one hand support on leg and could walk independently with compensatory hyperlordosis and positive Trendelenburg sign. He could pick up speed but could not run. He climbed four stairs one foot at a time using both arms on handrails. Head control was still poor, but facial weakness or ophthalmoplegia was absent as were contractures and scoliosis. Swallowing was reported to be normal. He had expressive language delay and used 35 words and 3-word-sentences with slurred speech. Speech therapy was ongoing. According to his parents, cognitive abilities were appropriate for his age. There were no clinical signs of respiratory insufficiency and/or pulmonary infections.
The family history was remarkable as the 33-year-old proband's mother had a similar phenotype as her son (Supporting Information: Figure S1). She was born to healthy parents who did not report additional family members with clinical signs of myopathy. At last appointment, her healthy father was 60 years old and her mother was 55 years old. Her mother was healthy until the age of 53 years when she developed symptoms of Parkinson's disease. She received medical treatment for Parkinson's disease and her symptoms improved slightly. The proband's mother developed muscular hypotonia after birth and underwent physiotherapy in infancy and childhood. She started walking at the age of 16 months. Her motor development was reported to be normal, but she described a nonprogressive generalized muscle weakness. During childhood clinical examination revealed muscle hypotrophy with proximal muscle weakness, absent deep tendon reflexes, thoracolumbar scoliosis, high-arched palate, and a long face. Diagnostic work-up demonstrated normal CK levels and normal motor and sensory nerve conduction velocities. Muscle ultrasound showed neurogenic rather than myogenic tissue changes. At the age of 11 years, she underwent a first muscle biopsy. Histological examination demonstrated fiber type disproportion with predominance of type I fibers. Her cognitive development was normal. Since the age of 13 years, she received noninvasive ventilation at night because of chronic respiratory insufficiency. At the age of 18 years, she underwent spondylodesis due to severe thoracolumbar scoliosis. Last cardiac examination at the age of 30 years revealed normal results. She underwent a second muscle biopsy of the right vastus lateralis at the age of 32 years. At last examination at the age of 33 years, she presented with a slender build, proximal muscle weakness, most prominent in legs, and mild myopathic facies with bilateral ptosis. She was able to stand up without help, to walk with positive Trendelenburg sign, to walk a few steps on heels and tiptoes, to pick up speed, and to ascend stairs without help. Her walking distance was about 5 km. Routine chromosome analysis and a muscular dystrophies next-generation sequencing panel (179 genes) revealed normal results.
Skeletal muscle biopsy of the vastus lateralis in the proband's mother showed type 1 fiber predominance accompanied by marked differences in fiber size due to numerous hypotrophic fibers restricted to type 1 fibers and scattered hypertrophic type 2 fibers (Supporting Information: Figure S2a,e,f). Red/purple-staining rod-like structures (i.e., nemaline rods) on Gömöri trichome were absent (Supporting Information: Figure S2b). Mild irregular disruption of the myofibrillar network was observed on NADH and SDH oxidative stains (Supporting Information: Figure S2c,d).
Research analysis of clinical trio WES data identified a heterozygous missense variant in TNNT1 in the proband and his mother that is absent in the father: NM_003283.6:c.194A>C/p.(Asp65Ala) at position GRCh38/hg38: chr19:55,141,301 (Supporting Information: Figure S1). TNNT1 is intolerant of functional genetic variation, as only 79% of the expected missense variants (observed/expected score: 0.79) are found in gnomAD (v.2.1.1) (Karczewski et al., 2020). The variant c.194A>C/p.(Asp65Ala) is absent in gnomAD (versions 2.1.1 and 3.1.1) and predicted to be damaging by multiple variant annotation tools (CADD score: 33; REVEL score: 0.823; M-CAP score: 0.895). Aspartate 65 is intolerant of variation (Wiel et al., 2019) and represents the first amino acid residue in the tropomyosin-binding site 1 (Jin & Chong, 2010). c.194A>C affects the second nucleotide of exon 8 and may alter splicing of the TNNT1 pre-mRNA. However, the splicing predictors varSEAK (https://varseak.bio/), MaxEntScan, NNSPLICE (BDGP/Fruitfly), and GeneSplicer (Pertea et al., 2001; Reese et al., 1997; Yeo & Burge, 2004) did not predict an altered recognition of the natural splice acceptor site in intron 7. Sanger sequencing confirmed the TNNT1 variant in the heterozygous state in the proband and his mother. The variant was absent in the healthy father of the proband and his healthy maternal grandparents indicating that it occurred de novo in the affected proband's mother (Supporting Information: Figure S1).
TNNT1 consists of 14 exons and is alternatively spliced (Figure 1a). Alternative splicing of exon 5 encoding 11 amino acid (aa) residues (Ala-Pro-Glu-Glu-Pro-Glu-Pro-Val-Ala-Glu-Pro) generates two slow TnT1 isoforms which differ in their N-terminal region and functionalities (NM_001126132.3 including exon 5, calculated molecular mass of 31 kDa; NM_001126133.3 excluding exon 5, calculated molecular mass of 30 kDa). In addition to the two aforementioned TNNT1 transcripts, a third TNNT1 transcript variant with exon 5 and a longer version of exon 12 (+48 bp in the 5′ region; NM_003283.6, calculated molecular mass of 33 kDa) exists in the skeletal muscle (Gahlmann et al., 1987; Zhang et al., 2014). Normal adult slow muscles express mainly the high-molecular-weight (HMW) isoform(s) (including the 11 N-terminal aa) (Gahlmann et al., 1987; Jin et al., 1998; Samson et al., 1994). The low-molecular-weight (LMW) slow TnT1 isoform (without the 11 N-terminal aa) is less acidic and generates more specific Ca2+-activated contractile force than the HMW isoform (Larsson et al., 2008).
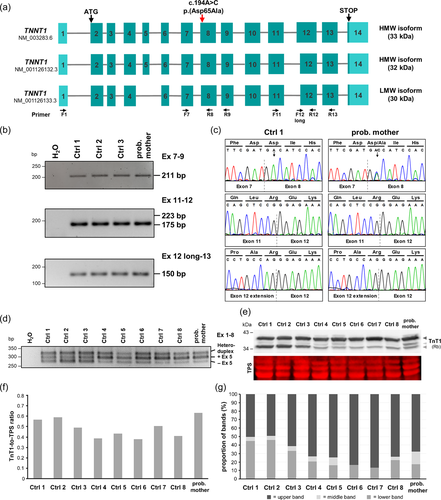
We first analyzed whether the c.194A>C variant causes aberrant splicing of exon 8 in RNA isolated from a vastus lateralis muscle biopsy of the proband's mother. By qualitative reverse transcription polymerase chain reaction (RT-PCR) using primers in exons 7 and 9 followed by Sanger sequencing, we identified TNNT1 mRNAs that include exon 8 in the proband's mother and controls and observed similar ratios of wild-type and mutant transcripts (Figure 1b,c). We next studied alternative splicing of TNNT1 pre-mRNAs and possible inclusion of the larger version of exon 12 (+48 bp in the 5′ region). RT-PCR with primers in exons 11 and 12 followed by direct sequencing of the single 175-bp band in the proband's mother and controls (no products of 223 bp) revealed only TNNT1 transcripts with the shorter version of exon 12 (Figure 1b,c). By using a forward primer in the 5′ extended region (48 bp) of exon 12 and a reverse primer in exon 13, we could amplify an RT-PCR product in the proband's mother and controls (Figure 1b). Direct sequencing of the 150-bp amplicon confirmed the presence of the larger exon 12 in the TNNT1 transcripts (Figure 1c). Alternative splicing of exon 5 was studied by using primers in exons 1 and 8. We observed three amplicons (~250, ~280, and ~330 bp) in the proband's mother and controls (Figure 1d), however, the intensity of the three bands was different: the two upper bands in the proband's mother and in controls 4–8 had a greater intensity than those in controls 1–3 (Figure 1d). To determine the identity of the three bands, we cloned the amplicons from the proband's mother and control 1, performed PCRs from 53 single Escherichia coli colonies each, and directly sequenced the amplicons. For control 1, 32 cloned amplicons represent TNNT1 transcripts including exon 5 (60%) (corresponding to the 280-bp band) and 21 cloned amplicons represent transcripts without exon 5 (40%) (corresponding to the 250-bp band) (Supporting Information: Figure S3a). For the proband's mother, we identified 40 clones with TNNT1 transcripts including exon 5 (75%) and 13 clones representing transcripts without exon 5 (25%) (Supporting Information: Figure S3a). As we could not detect a third TNNT1 transcript in any of the cloned amplicons that corresponds to the 330-bp amplicon (Figure 1d and Supporting Information: Figure S3b, upper panel), we assumed the formation of a heteroduplex by the two amplicons representing TNNT1 transcripts with and without exon 5. This assumption was corroborated by running the exon 1–8 amplicons of the proband's mother and three controls on an 8% PAGE gel that revealed only two bands with sizes of 280 bp (+exon 5) and 250 bp (−exon 5) (Supporting Information: Figure S3b, lower panel). Although we identified a relatively high abundance of TNNT1 transcripts with exon 5 in the vastus lateralis muscle specimen of the proband's mother, this finding does not seem to be related to the TNNT1 variant as a similar TNNT1 transcript pattern was identified in muscle specimen from several control individuals.
We next performed immunoblotting using total protein extracts from muscle biopsy of the proband's mother and controls. We applied a rabbit polyclonal anti-TnT1 antibody to detect the LMW and HMW slow TnT1 isoforms. In all controls and the proband's mother, two slow TnT1 isoforms of ~35 and ~37 kDa were observed, while an additional third isoform of about 36 kDa (“middle band”) was detected in the proband's mother and controls 1–5 and 8 and not in controls 6 and 7 (Figure 1e and Supporting Information: Figure S4). The ~37 and ~36-kDa slow TnT1 isoforms likely represent the two HMW isoforms expressed by TNNT1 transcripts with exon 5 together with the long or short version of exon 12, while the ~35-kDa slow TnT1 isoform corresponds to the LMW isoform encoded by TNNT1 mRNAs without exon 5 (Figure 1a,e). The total amount of slow TnT1 was in a similar range in the proband's mother and controls (Figure 1f). The HMW-to-LMW slow TnT1 isoform ratio was 83:17 and 87:13 in controls 6 and 7 (showing one HMW and one LMW slow TnT1 isoform), respectively, and a ratio of 49:5(two HMW isoforms):46(LMW) to 75:9(two HMW):16(LMW) was found in controls 1–5 and 8 and of 68:15(two HMW):17(LMW) in the proband's mother (Figure 1g). We also performed immunoblotting with the mouse monoclonal antislow troponin T antibody (CT3). The CT3 antibody detected the ~37-kDa HMW and the ~35-kDa LMW slow TnT1 isoforms in a similar pattern in controls and the proband's mother as compared to the rabbit polyclonal anti-TnT1 antibody (Supporting Information: Figure S5). A weak “middle band” corresponding to the ~36-kDa slow TnT1 isoform was detected in the muscle protein extract of the proband's mother by this antibody in one of three immunoblots (technical replicates using the same samples) (Supporting Information: Figure S5b). Remarkably, total TnT1 levels detected by the CT3 antibody were reduced in the proband's mother compared to the eight controls (Supporting Information: Figure S5a). The amino acid substitution p.(Asp65Ala) in slow TnT1 is located in the epitope region of the CT3 antibody, which was mapped to the exon 7-encoded region and flanking areas of slow TnT1 (Jin & Chong, 2010). Reduced total levels of slow TnT1 detected by the monoclonal CT3 antibody (Supporting Information: Figure S5a), but not by the polyclonal anti-TnT1 antibody in the proband's mother (Figure 1e,f) suggest that slow TnT1 isoforms expressed from the TNNT1 allele with the p.(Asp65Ala) variant escape detection by the CT3 antibody in the muscle of the proband's mother. Thus, total slow TnT1 levels determined by the CT3 antibody likely represent only wild-type slow TnT1 expressed from the normal TNNT1 allele in the proband's mother.
The histology findings in the muscle biopsy of the proband's mother are typical of TNNT1 myopathy (Fox et al., 2018) and included type 1 fiber hypotrophy and type 2 fiber hypertrophy (Supporting Information: Figure S2). In muscle specimen of patients with biallelic TNNT1 variants, a reduced amount of slow skeletal fiber myosin (MYH7) accompanied by a modest increase in fast skeletal fiber myosin type 2a (MYH2) has been described (Fox et al., 2018). Therefore, we next detected slow skeletal fiber myosin and fast skeletal fiber myosin type 2a in the muscle protein extracts of the proband's mother and controls. The amount of slow skeletal fiber myosin (MYH7) was similar in the proband's mother and controls (Supporting Information: Figure S6a), while that of fast skeletal fiber myosin type 2a (MYH2) was increased three- to sevenfold in the muscle of the proband's mother compared to controls (Supporting Information: Figure S6b). We then determined the ratio of slow skeletal fiber myosin (type 1 fibers) to fast skeletal fiber myosin type 2a (type 2 fibers) that was reduced fourfold in the proband's mother muscle specimen compared to the mean of controls (Supporting Information: Figure S6c).
Troponin and tropomyosin are bound to the actin thin filament to control the contraction of striated muscle (Mondal & Jin, 2016). To detect possible changes in levels of actin (all forms) in the muscle specimen of the proband's mother, we used a pan actin antibody in immunoblot analysis. The amount of actin was increased 1.7- to 3-fold in the muscle of the proband's mother compared to controls (Supporting Information: Figure S5a, lower part).
The two amino acid substitutions p.(Leu96Pro) and p.(Asp65Ala) reported in individuals with a mild TNNT1 myopathy (Pellerin et al., 2020) and in mother and son here, respectively, are both located within the tropomyosin-binding site 1 of slow TnT1 (residues 64–102; Supporting Information: Figure S7). The third TNNT1 myopathy-associated amino acid substitution p.(Glu104Val) affects a residue just outside the tropomyosin-binding site 1 (two C-terminal residues apart) (Supporting Information: Figure S7) (Jin & Chong, 2010). All three amino acid substitutions are predicted to affect slow TnT1's affinity for tropomyosin because (i) a negatively charged amino acid residue is substituted with a nonpolar one and (ii) the leucine-to-proline exchange disrupts the alpha-helical secondary structure of the tropomyosin-binding site 1 (Konersman et al., 2017; Pellerin et al., 2020). To study complex formation between wild-type or mutant slow TnT1 and tropomyosin, we coexpressed both proteins in HEK293T cells and performed coimmunoprecipitation (co-IP) experiments. We used the coding region of tropomyosin 3 (TPM3) and of the longest TNNT1 transcript variant (NM_003283.6), including exon 5 and the long version of exon 12, and introduced the c.194A>C, the c.311A>T, and the c.287T>C changes in the coding region.
We cotransfected HEK293T cells with Flag-tagged TPM3 and HA-tagged slow TnT1 wildtype or either of the three TnT1 mutants and detected the slow TnT1 wildtype and mutants as well as Flag-tagged TPM3 in whole-cell lysates. Flag-tagged TPM3 was uniformly expressed in HEK293T cells (Figure 2a,c are representative examples [Experiment 1] drawn from Supporting Information: Figure S8a,b; Input). While the amount of HA-tagged TnT1 wildtype, TnT1-D65A, and TnT1-E104V in whole-cell lysates was similar, the level of TnT1-L96P was markedly increased (Figure 2a,c and Supporting Information: S8a,b; Input). We next immunoprecipitated TPM3-Flag and detected co-immunoprecipitated (co-IPed) HA-tagged TnT1. Wild-type TnT1, TnT1-D65A, and TnT1-E104V efficiently co-IPed with TPM3, while we did only observe a very small amount of co-IPed TnT1-L96P (Figure 2a and Supporting Information: S8a). Quantification of immunoblot data revealed a similar amount of co-IPed TnT1 wildtype and TnT1-E104V with TPM3-Flag, significantly enhanced co-IPed TnT1-D65A by ~37% and significantly reduced co-IPed TnT1-L96P by ~88% (Figure 2b).
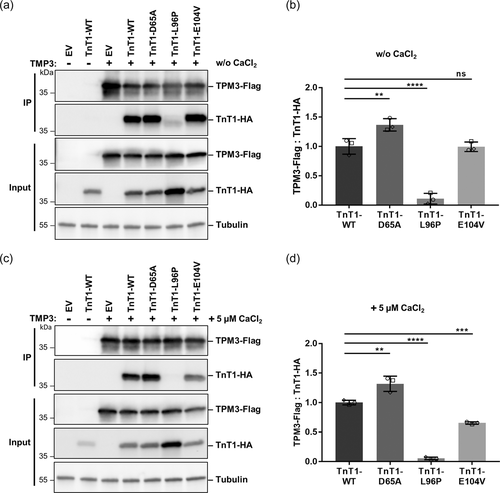
The troponin complex is critical in regulation of the Ca2+-mediated cooperative interactions among the three troponin subunits, tropomyosin, and the actin thin filament (Mondal & Jin, 2016). Therefore, we repeated the co-IPs in Ca2+-containing buffer. We could confirm effective co-IP of wild-type TnT1, TnT1-D65A, and TnT1-E104V with TPM3, while TnT1-L96P did not co-immunoprecipitate with TPM3 (Figure 2c and Supporting Information: Figure S8b). Quantitative analysis revealed statistically significant enrichment of the TnT1-D65A mutant compared with TnT1 wildtype in the precipitates (by ~32%; Figure 2d). In contrast to the co-IPs performed without Ca2+, the TnT1-E104V mutant showed a significantly reduced co-IP with TPM3 by ~35% (Figure 2d). Almost no co-IP of the TnT1-L96P mutant with Flag-tagged TPM3 was observed (Figure 2d). Together, these data show that the p.(Leu96Pro) variant abrogates complex formation between TnT1 and TPM3, while the two amino acid substitutions p.(Asp65Ala) and p.(Glu104Val) differentially affect co-IP of TnT1 with TPM3.
Here, we report another family, mother and son, with an autosomal dominantly inherited myopathy likely caused by the heterozygous TNNT1 variant c.194A>C/p.(Asp65Ala). Both individuals shared muscle hypotrophy, muscle weakness, hyperlordosis or thoracolumbar scoliosis, and absent deep tendon reflexes. In addition, the proband's mother had mild myopathic facies with ptosis and respiratory insufficiency. The clinical picture is comparable to that reported in 10 family members with autosomal dominant TNNT1-associated nemaline myopathy and lies at the mild end of the spectrum. The heterozygous TNNT1 variant c.311A>T/p.(Glu104Val) segregated with the disease in this family. Slowly progressive proximal weakness, slender habitus, myopathic face, high-arched palate, pectus carinatum, and mild kyphoscoliosis were the most common clinical hallmarks in the subjects. All five affected individuals older than 60 years were still independently ambulatory at that age (Supporting Information: Table S1) (Konersman et al., 2017). The severe end of the TNNT1 nemaline myopathy is characterized by progressive muscle atrophy and proximal stiffness, with a median survival of 18 months due to respiratory failure. Biallelic TNNT1 loss-of-function variants cause complete loss of protein and are associated with this infantile-onset and lethal disease (Fox et al., 2018; Geraud et al., 2021). Two specific homozygous variants in TNNT1, the missense variant c.287T>C/p.(Leu96Pro) and the frameshift variant c.786del/p.(Lys263Serfs*35), have been associated with a milder and slowly progressive autosomal recessive nemaline myopathy compared with the severe infantile-onset form (Pellerin et al., 2020; Petrucci et al., 2021). Possibly, both variants lead to production of a partially functioning slow TnT1. The 49-year-old female with the TNNT1 truncating variant had slowly progressive exercise intolerance, muscle hypotrophy, respiratory involvement, rigidity of the chest wall, contractures, short stature, dyspnea, mild dysphagia, and myalgia since infancy (Petrucci et al., 2021). The phenotype of the individuals with the homozygous p.(Leu96Pro) variant was characterized by limb-girdle weakness and contractures with onset in childhood, rigid spine, and kyphoscoliosis. Variable restrictive lung disease and short stature were also reported (Pellerin et al., 2020). Together, these data demonstrate a broad clinical spectrum in patients with TNNT1-associated myopathy. The inheritance pattern and the type of the TNNT1 variant likely determine the clinical outcome in the affected individuals.
Typical histological features of TNNT1-related myopathies are fiber type disproportion with type 1 fiber hypotrophy and type 2 fiber hypertrophy and nemaline rods (see table 1 in Petrucci [Fox et al., 2018; Petrucci et al., 2021]). Muscle pathology in the mother of our proband is consistent with these findings except for the absence of nemaline rods. Smallness of the type 1 myofiber population and hypertrophy of type 2 myofibers in the proband's mother was confirmed by immunoblotting data demonstrating a reduced ratio of slow skeletal fiber myosin (MYH7) to fast skeletal fiber myosin type 2a (MYH2). Hypertrophy of type 2 fibers has been presumed to represent a compensatory reaction to hypotrophic type 1 fibers and may mitigate muscular weakness (Konersman et al., 2017). Another adaptive response likely are increased actin levels in the muscle of the proband's mother. Similarly, in muscle of individuals with biallelic KLHL40 variants and a severe form of autosomal recessive nemaline myopathy, an increase in sarcomeric α-actin has been observed (Ravenscroft et al., 2013). Nemaline rods, primarily restricted to type 1 fibers, have been reported in most of the patients with an autosomal recessive or dominant form of TNNT1-related myopathy (Fox et al., 2018; Konersman et al., 2017; table 1 in Petrucci et al., 2021). However, these structures were absent in muscle biopsies of a few patients with TNNT1 myopathy (Abdulhaq et al., 2016; Pellerin et al., 2020; Pergande et al., 2020; Streff et al., 2019), including the mother of our proband. These data indicate that nemaline rods are a variable finding in muscle specimen of affected individuals, while fiber size variation is the most consistent feature in TNNT1-associated myopathies (table 1 in Pellerin et al., 2020; Petrucci et al., 2021; and this study). Muscle histology also identified mild irregular disruption of the myofibrillar network in the proband's mother. A similar myofibrillar disorganization on NADH staining was found in patients with a mild form of nemaline myopathy, such as individuals with the homozygous p.(Leu96Pro) and p.(Lys263Serfs*35) variants (Pellerin et al., 2020; Petrucci et al., 2021). Possibly, an irregular myofibrillar network may be a pathological feature in individuals with “mild” pathogenic TNNT1 variants and a more benign course.
Alternative splicing of TNNT1 exon 5 generates the HMW and LMW slow TnT1 isoforms with different functionalities. Regulation of TnT1 isoforms through alternative splicing plays a role in modulating muscle contractility under pathophysiological conditions, such as in Charcot–Marie–Tooth disease type 1 and overused prior polio muscle, and during adaptation to functional demands (Larsson et al., 2008). For example, resistance training increased the relative abundance of TNNT1 transcripts without exon 5 and decreased that of TNNT1 mRNAs with exon 5 (Zhang et al., 2014). As LMW slow TnT1 generates more Ca2+-activated contractile force than HMW slow TnT1 (Larsson et al., 2008), the LMW/HMW ratio is beneficial for human muscle function (Zhang et al., 2014). Thus, a possible physiological response to nemaline myopathy is preferential skipping of exon 5 in pre-TNNT1-mRNAs to upregulate LMW slow TnT1 that has been observed in skeletal muscle of individuals with the heterozygous p.(Glu104Val) variant (Konersman et al., 2017). Similarly, in muscle biopsies of patients with the homozygous p.(Leu96Pro) variant, the levels of HMW slow TnT1 isoforms were markedly reduced, while those of LMW slow TnT1 were retained (Pellerin et al., 2020). In the muscle of the proband's mother reported here, we determined levels of HMW and LMW slow TnT1 isoforms by using two different anti-TnT1 antibodies. With the rabbit anti-TnT1 antibody the ratio of the two HMW isoforms to the LMW isoform was 68:15(HMW):17(LMW) in the proband's mother. Similar ratios of HMW-to-LMW slow TnT1 isoforms were identified in several control muscle specimen. Immunoblot data produced with the mouse anti-TnT1 (CT3) antibody indicated a similar slow TnT1 isoform pattern in the proband's mother and some controls, however, the ~36-kDa isoform (“middle band”) was only visible in one of three immunoblots (technical replicates) (Supporting Information: Figure S5b). Higher levels of HMW versus LMW slow TnT1 isoforms in the proband's mother may suggest a possible adaptive response to increase production of HMW slow TnT1 isoforms with a more acidic N-terminal region. The N-terminal variable region of slow TnT1 differentially modulates the interaction between TnT1 and tropomyosin by reducing the binding affinity of slow TnT1 for tropomyosin (Amarasinghe & Jin, 2015). Accordingly, LMW slow TnT1 has a higher binding affinity for tropomyosin than the HMW isoform (Larsson et al., 2008). By co-IP studies, we demonstrated enhanced complex formation between the slow TnT1-D65A mutant and TPM3. We hypothesize that increased production of HMW slow TnT1s with a naturally lower binding affinity for tropomyosin from the wild-type TNNT1 allele could be a pathophysiological adaptation in the proband's mother to compensate for the increased binding capacity of the TnT1-D65A mutant for tropomyosin. co-IP studies with the slow TnT1 mutants L96P and E104V revealed no complex formation (independent of Ca2+) and reduced complex formation in the presence of Ca2+ with tropomyosin, respectively. Accordingly, upregulation or retention of the LMW slow TnT1 isoform in the muscle of patients with the TNNT1 variants p.(Leu96Pro) and p.(Glu104Val) may represent a pathophysiological response in the other direction as compared to the mother of the proband with the p.(Asp65Ala) variant.
Autosomal dominant pathogenic variants in TNNT2, encoding cardiac muscle TnT, cause hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) (Yotti et al., 2019). Amino acid residues in cardiac TnT analogous to the residues Asp65 and Leu96 mutated in slow skeletal TnT1 have not yet been reported to be changed by HCM- or DCM-causing variants (Supporting Information: Figure S7). However, the amino acid residue Glu104 in slow skeletal TnT that corresponds to Glu128 in cardiac TnT was affected by an HCM-causing sequence variant and resulted in substitution to lysine [p.(Glu128Lys)] (Supporting Information: Figure S7) (Liu et al., 2013). DCM- and HCM-associated cardiac TnT mutants with amino acid substitutions in the tropomyosin-binding site show a strong correlation between changes in the binding affinity of cardiac TnT for tropomyosin and changes in the calcium sensitivity of regulated actomyosin ATPase activities. These data suggest that alterations in the affinity of cardiac TnT for tropomyosin are the common pathomechanism underlying both DCM and HCM (Gangadharan et al., 2017). Similarly, we hypothesize that nemaline myopathy-associated TNNT1 missense variants located in the tropomyosin-binding site 1 primarily alter muscle contractility and/or relaxation by changing the affinity of slow TnT1 for tropomyosin.
In conclusion, we report an autosomal dominantly inherited myopathy likely associated with the TNNT1 missense variant c.194A>C/p.(Asp65Ala). Clinical features of our patient and his mother are at the milder end of the clinical spectrum. The absence of nemaline rods in the mother's muscle biopsy did not contradict with TNNT1 myopathy as several individuals with pathogenic variants in other NEM genes, such as NEB and ACTA1, also had no rods in biopsies (Kaindl et al., 2004; Wallgren-Pettersson et al., 2007; Zukosky et al., 2015). Our in vitro studies demonstrate enhanced complex formation of the TnT1-D65A mutant with tropomyosin, however, the data is limited to an overexpression system and needs to be confirmed in a more physiological setting.
ACKNOWLEDGMENTS
We are grateful to the family for participation in this study. We thank Inka Jantke, Barbara Schröder, Celina Soltwedel, and Dennis Zorndt for skillful technical assistance. This study was supported by a grant from the Deutsche Forschungsgemeinschaft (KO 4576/1–2 to K. K.). Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




