The TALE never ends: A comprehensive overview of the role of PBX1, a TALE transcription factor, in human developmental defects
Abstract
PBX1 is a highly conserved atypical homeodomain transcription factor (TF) belonging to the TALE (three amino acid loop extension) family. Dimerized with other TALE proteins, it can interact with numerous partners and reach dozens of regulating sequences, suggesting its role as a pioneer factor. PBX1 is expressed throughout the embryonic stages (as early as the blastula stage) in vertebrates. In human, PBX1 germline variations are linked to syndromic renal anomalies (CAKUTHED). In this review, we summarized available data on PBX1 functions, PBX1-deficient animal models, and PBX1 germline variations in humans. Two types of genetic alterations were identified in PBX1 gene. PBX1 missense variations generate a severe phenotype including lung hypoplasia, cardiac malformations, and sexual development defects (DSDs). Conversely, truncating variants generate milder phenotypes (mainly cryptorchidism and deafness). We suggest that defects in PBX1 interactions with various partners, including proteins from the HOX (HOXA7, HOXA10, etc.), WNT (WNT9B, WNT3), and Polycomb (BMI1, EED) families are responsible for abnormal proliferation and differentiation of the embryonic mesenchyme. These alterations could explain most of the defects observed in humans. However, some phenotype variability (especially DSDs) remains poorly understood. Further studies are needed to explore the TALE family in greater depth.
1 INTRODUCTION
1.1 The PBC family of transcription factors (TFs)
Numerous classes of homeodomain (HD) TFs have been described in the animal kingdom. One of these highly conserved super-classes of TFs is characterized by the insertion of a three aminoacid loop in the HD which has been named the TALE TF (for three amino acid loop extension [Bertolino et al., 1995]). Two TALE families, the MEINOX and Pbx, ceh-20, extra-denticle (PBC) TFs, are able to interact with HOX and non-HOX proteins (reviews by Jozwik & Carroll, 2012; Laurent et al., 2008; Merabet & Mann, 2016; Penkov et al., 2013; Pi et al., 2020). They also have the ability to recruit regulators of chromatin accessibility and transcriptional corepressors (Asahara et al., 1999; Laurent et al., 2008; Longobardi et al., 2014). These highly versatile functions make MEINOX and PBC proteins key factors in development, cell maintenance, and cancer (see reviews by Blasi et al. [2017] for cancer and Licia Selleri et al. [2019] development).
The PBC family is composed of four proteins, namely PBX 1–4, with highly conserved sequences among vertebrates. As TALE TFs, their structure is composed of an HD and two protein–protein interacting domains named PBC-A and B. The HD enables PBX proteins to interact with other TFs and DNA, but the PBC domains are critical for interactions between PBX and MEINOX (PKNOX [also named PREP] and MEIS) proteins (Longobardi et al., 2014). Hetero-dimerization of these proteins is essential to allow PBX DNA-binding capacities, inducing: (1) the import of PBX proteins into the nucleus by hiding their nuclear export signals (NES) (Abu-Shaar et al., 1999; Jaw et al., 2000), (2) conformational changes of both partners stabilizing their interaction with DNA (Mathiasen et al., 2016), (3) the selection of targeted DNA with PBX protein binding properties relying on the DNA consensus sequence of their MEINOX partner (Blasi et al., 2017). Since the phenomena of PKNOX or MEIS binding to PBX proteins are mutually exclusive (Blasi et al., 2017), the properties of PBX are linked to its partner. Briefly, PKNOX and MEIS proteins have more or less opposite effects on cell fate. PKNOX proteins favor transcription initiation and preferentially bind to the promoter regions of housekeeping genes. PKNOX1 has been extensively studied in the setting of both development and cancer (Laurent et al., 2008; Penkov et al., 2013); it is ubiquitously expressed during development and is also considered as a tumor-suppressing gene. PKNOX2 is less well known, and shares structure and functions that are close to PKNOX1. PKNOX2 is mainly expressed in the heart, the brain, muscles, limb buds, and the ovaries, and regulates gene expression, with less effective binding properties to PBX proteins than PKNOX1 (Fognani et al., 2002; Haller et al., 2002; Zhou et al., 2013). MEIS proteins target promoter-remote regions of developmental genes and favor cellular survival and proliferation. MEIS1, expressed early and ubiquitously, is involved in cancer, hematopoiesis, and embryonic development, especially for eye, limb, heart, and vasculature formation (review by Paul et al. [2020]). MEIS2 shares common functions with MEIS1 and is expressed during development mainly in neural structures, neural crest-derived cells, the head, and the gut, with overlapping patterns with MEIS1 (Berenguer & Duester, 2021; Fujita et al., 2016). Finally, MEIS3 has been far less studied in mammals. Its role in cancer varies with the tumor type (Meng et al., 2021). Meis3 has been shown to regulate neural crest cell migration, neural plate organization, and pancreatic formation during mouse development (J. Liu et al., 2010).
1.2 PBX1 expression and role
Most of the PBX proteins are expressed throughout the embryonic stages (as early as the blastula stage in vertebrates [Choe et al., 2009]) and maintained in mature tissues. During development, PBX1 is able to bind to promoters and promote histone modifications and chromatin accessibility, promoting gene transcription (Choe et al., 2014; Grebbin et al., 2016). This binding can precede efficient gene transcription by hours or days (Choe et al., 2014). In addition, PBX1 (and TALE TFs, in general) can use distinct DNA-binding motifs and protein partners depending on the embryonic stage. This suggests key roles for PBC proteins, from early development and throughout life (for an extensive review on TALE TFs and development, see Licia Selleri et al. [2019]).
In the PBC family, PBX1 and 2 are the most widely expressed both during development and in mature organs (Roberts et al., 1995; L. Selleri et al., 2001), with overlapping patterns (Capellini et al., 2006). Intriguingly, only PBX1 seems to be mandatory for embryonic development, since Pbx2-knock-down (KO) mice are viable and healthy (Licia Selleri et al., 2004). Multiple hypotheses may explain such a discrepancy, but a quantitative model, relying on global cell concentration of Pbx proteins, seemed the most accurate (see Licia Selleri et al. [2004] and paragraph 3.3). Numerous studies have investigated ubiquitous patterns of PBX1 expression during development, mainly in mouse embryos (Capellini et al., 2010, p. 20; 2008; DiMartino et al., 2001; Golonzhka et al., 2015; Grebbin et al., 2016; Lampe et al., 2008; Lescroart & Zaffran, 2018; Manley et al., 2004; Schnabel, Godin, et al., 2003; L. Selleri et al., 2001; Sonnet et al., 2012; Vitobello et al., 2011). In human adults, PBX1 is ubiquitously expressed, predominantly in glandular tissues, female tissues, and the bladder (Fagerberg et al., 2014). It remains difficult to define the role of PBX1 proteins because alternative splicing can produce up to seven isoforms for PBX1 in humans, only three with known functions (PBX1a, PBX1b, and PBX1d [Asahara et al., 1999; Sengupta et al., 2012]). All proteins exhibit a degree of specificity in their patterns of expression (Laurent et al., 2008; C. A. Schnabel et al., 2001; Sengupta et al., 2012). PBX1a is mainly expressed in the brain and during adulthood. Conversely, PBX1b is expressed ubiquitously and preferentially during the embryonic period (Schnabel, Godin, et al., 2003). The PBX1d isoform seems specific to CD4+ T cells and is considered as a susceptibility factor for lupus (Cuda et al., 2012; Niu et al., 2017). PBX1 has numerous partners leading to hetero-dimerization or hetero-trimerization (PKNOX1 and 2 proteins, MEIS1 and 2, FOXC1, HOX proteins, and so forth [Asahara et al., 1999; Berry et al., 2005; Dard et al., 2019; Laurent et al., 2008]). In these larger complexes, PBX1 is able to recruit chromatin-remodeling factors with either repressing or enhancing activity (Asahara et al., 1999; Laurent et al., 2008; Penkov et al., 2013). PBX1 target genes include ubiquitous developmental genes (PAX1/PAX9, WNT9B/WNT3, PITX3, and so forth (Capellini et al., 2008; Ma et al., 2015; Villaescusa et al., 2016) and organ-specific genes (HIF1A, DCX, TH, GNRH genes, etc. [Grebbin & Schulte, 2017; Kocabas et al., 2015; Rave-Harel et al., 2004]), driving cell proliferation, pluripotency, and differentiation (Fukuda et al., 2015; Gemel et al., 1999; Kocabas et al., 2015; Wei et al., 2018). Table 1 sums up the most relevant PBX1 cofactors and target genes with implications in development demonstrated in functional studies. For extensive gene modifications induced by PBX1 alone or by the chimeric E2A-PBX1 protein, see (Diakos et al., 2014; Thiaville et al., 2012).
| Protein | Cofactor or target | Interaction with PBX1 | Interaction consequence | Cell or tissue type studied | Source |
|---|---|---|---|---|---|
| PBX1 cofactor/enhancer | |||||
| N CoR/SMRT | Cofactor | Recruited by PBX1 | Repression of target. Sensitive to PBX1 isoform (PBX1a) | (HEK) 293T cells | Asahara et al. (1999) |
| PDX1 | Cofactor | Forms heterodimers with PBX1 | Activation/repression of target | ||
| CREB binding protein | Cofactor | Recruited by PBX1 | Activation of target. Sensitive to PBX1 isoform (PBX1b) | ||
| FLNA | Cofactor | Involved in proper subnuclear localization of PBX1 through phosphorylation | Inhibition of PBX1 export to the nucleus | A7 melanoma cells | Berry et al. (2005) |
| FoxC1 | Cofactor | Recruited by PBX1 | Cooperative binding of DNA | ||
| TLX1 | Cofactor | Forms heterodimers with PBX1 in spleen | Splenic formation and progenitor proliferation | Splenic progenitors | Brendolan et al. (2005) |
| Emx2 | Cofactor | Recruited by PBX1 | Scapula blade patterning | Dermomyotome | Capellini et al. (2010) |
| HoxA7 | Cofactor | Binds to PBX1-Meis complex | Trimer formation under control of DNA topology and surrounding proteins | HEK, HeLa, and MCF7 cells | Dard et al. (2019) |
| HoxB3 | Cofactor | Binds to PBX1-Meis complex | |||
| HoxC8 | Cofactor | Binds to PBX1-Meis complex | |||
| HoxA9 | Cofactor | Binds to PBX1-Meis complex | Trimer formation insensitive to DNA topology and surrounding proteins | ||
| HoxA10 | Cofactor | Forms heterodimers with PBX1 | Repress HOXA10 target genes activation | Osteoblasts and C3H10T1/2 and MC3T3-E1 cells | Gordon et al. (2010) |
| NMHCB | Cofactor | Downregulation of PBX1 | Compete with Meis1 for PBX1 dimerization, retains PBX1 in the cytoplasm | HEK293T, Cos-7 cells, and Drosophila S2 cells | Huang et al. (2003) |
| AP2γ | Cofactor | Putative PBX1 cofactor in estrogen receptor (ER)-dependant cancers | Allows the accessibility to chromatin of ER | ER-dependant cancer cells | Jozwik & Carroll (2012) |
| FOXA1 | Cofactor | Putative PBX1 cofactor in ER-dependant cancers | MCF-7 cells | ||
| ZFPIP | Cofactor | Binds to PBX1-HOXA9 complex | Inhibit PBX1-HoxA9 DNA binding | Embryonic female genital tract, head, and limb bud | Laurent et al. (2008) |
| HDAC, HAT | Cofactor | Recruited by PBX1 | Repression/activation of target | Laurent et al. (2008); Penkov et al. (2013) | |
| MEIS1 | Cofactor | Needed for PBX1 function (binding mutually exclusive with PKNOX) | Targets promoter-remote regions and developmental genes, favors transcriptional regulation and tumorigenesis | MEF cells, mouse embryos | Blasi et al. (2017); Laurent et al. (2008); Penkov et al. (2013) |
| MEIS2 | Cofactor | Needed for PBX1 function (binding mutually exclusive with PKNOX) | Tumorigenesis | HepG2 cells | Bjerke et al. (2011) |
| KLF4 | Cofactor | Binds to PBX1-Meis2 complex | |||
| SIN3B | Cofactor | Recruited by PBX1 | Part of a repressor complex including HDAC1, HDAC3, mSIN3B, and N-CoR/SMRT | Laurent et al. (2008) | |
| PKNOX1 | Cofactor | Needed for PBX1 function (binding mutually exclusive with Meis) | Promoters and house-keeping genes, transcription initiation, tumor suppression | MEF cells, mouse embryos | Blasi et al. (2017); Laurent et al. (2008); Penkov et al. (2013) |
| PKNOX2 | Cofactor | Needed for PBX1 function (binding mutually exclusive with Meis) | Binds TALE and HoxA1 promoters in early development and removed after retinoic acid-induced differentiation | Mouse embryos | De Kumar et al. (2017) |
| CDX2 | Cofactor | Forms heterodimers with PBX1 | Dimer enhances the activation of proglucagon | Pancreatic islets | Liu et al. (2006) |
| HoxA5 | Cofactor | Forms heterodimers with PBX1 | Hox target gene activation | Bl21 transfected cells | Lu & Kamps (1996) |
| HoxB5 | Cofactor | Forms heterodimers with PBX1 | |||
| HoxB8 | Cofactor | Forms heterodimers with PBX1 | |||
| RUNX1 | Cofactor | Regulated by chimeric E2A-PBX1 | Promote cell proliferation and tumorigenesis | pre-B ALL cells | Pi et al. (2020) |
| Sp2 | Cofactor | Recruited by PBX1-Prep1 + Nf-y | Enhances PBX1-Prep DNA binding on promoters | Mouse embryonic fibroblasts | Völkel et al. (2018) |
| PBXIP1 | Cofactor | Repress PBX1-Hox DNA binding | Erythroid differentiation, various implications in cancer | Hematopoietic Stem cells (HSC) | Khumukcham & Manavathi (2021) |
| MyoD | Cofactor | Recruited by PBX1 | Myogenin expression during somitogenesis | Mouse embryo | Cho et al. (2015) |
| Evi-1 | Enhancer | Enhancer of PBX1 | Evi-1 promotes PBX1 expression | HSC | Fukuda et al. (2015) |
| TET | Enhancer | Favors TALE binding on DNA | Histone 5mC oxidation | P19 mouse embryonal carcinoma cells | Mahé et al. (2017) |
| PBX1 target | |||||
| MEOX1 | Target | Regulated by PBX1 | Mediates the cellular growth signal of PBX1 in ovarian cancers | Ovarian cancer cells (OVCAR3) | Thiaville et al. (2012) |
| Jun | Target | Regulated by PBX1/PKNOX1 or Pbx1/MEIS1 + Oct-1 | Nonsmall cell lung cancer | Blasi et al. (2017) | |
| HoxA4 | Target | Indirectly regulated by PBX1 through regulation of BMI1 and EED | Partial control of mesoderm patterning | Mouse embryo limb mesoderm | Capellini et al. (2008) |
| HoxB5 | Target | ||||
| HoxB8 | Target | ||||
| HoxC6 | Target | ||||
| HoxD3 | Target | ||||
| Pax1 | Target | Regulated by PBX1 | Partial control of Polycomb gene mesoderm patterning | ||
| BMI1 | Target | ||||
| EED | Target | ||||
| Polycomb | Target | ||||
| Smad | Target | Recruited by PBX1-PKNOX1 | Mediators of activin signaling | LβT2 cells | Bailey, Rave-Harel, McGillivray, Coss, & Mellon (2004); Bailey et al. (2004) |
| FSHB( | Target | Regulated by PBX1 | Regulated by PBX1 | ||
| Alx1 | Target | Regulated by PBX1-Emx2 | Scapula blade patterning | Mouse embryo dermomyotome | Capellini et al. (2010) |
| DBX1 | Target | Repressed by PBX1 | Control of dorsoventral patterning of the spinal cord | Pbx mutant cortical progenitors | Golonzhka et al. (2015) |
| DMRTA1 | Target | Repressed by PBX1 | Control of dorsoventral patterning of ventral zone cortical progenitors | ||
| Dcx | Target | Regulated by PBX1 | Promoter early bookmarked by pioneer factor PBX1 | Progenitor cells and newborn neuroblasts | Grebbin & Schulte (2017); Grebbin et al. (2016) |
| Th | Target | Regulated by PBX1 | |||
| STAT3 | Target | Activated by PBX1 | Promote STAT3/JAK2 pathway and chemoresistance | Ovarian cancer | Jung et al. (2016) |
| Hif1a | Target | Activated by PBX1-Meis-HoxA9 | Favors hypoxic metabolism in hematopoietic stem cells (HSC) | HSC | Kocabas et al. (2015) |
| HoxA2 | Target | Regulated by PBX1-Hox | Hox-Pbx responsive cis-regulatory sequence = Hoxa2 regulation in rhombomere 4 | P19 and COS7 cells | Lampe et al. (2008) |
| Fgf10 | Target | Activated by PBX1-Meis1-HoxB4 | Promote lung development and surfactant secretion | Mouse embryonic lungs | Li et al. (2014) |
| Wnt9b/Wnt3 | Target | Regulated by PBX1 | Mouse face morphogenesis | Mouse embryos | Ferretti et al. (2011); Maili et al. (2020) |
| CD44 | Target | Regulated by PBX1 | Activation of T cells, risk factor of lupus disease (isoform PBX1-d) | Jurkat T cells | Niu et al. (2017) |
| BMP4 | Target | Regulated by PBX1 in presence of retinoic acid | Promote differentiation of P19 cells under retinoic acid stimulation | P19 mouse embryonal carcinoma cells | Qin et al. (2004) |
| DCN | Target | Regulated by PBX1 in presence of retinoic acid | Promote differentiation of P19 cells under retinoic acid stimulation | ||
| GNRH1 | Target | Regulated by PBX1/Prep1 or Pbx1/Meis1 + Oct-1 | Promote restricted expression of GnRH in hypothalamic neurons | GT1-7 cells | Rave-Harel et al. (2004) |
| NFE2L1 | Target | Activated by PBX1 | Ventral midbrain specification of dopaminergic neurons | Ventral midbrain E12.5 tissues | Villaescusa et al. (2016) |
| Pitx3 | Target | Activated by PBX1 | |||
| GBF1 | Target | Activated by PBX1 | |||
| TPCN1 | Target | Activated by PBX1 | |||
| TMEM218 | Target | Activated by PBX1 | |||
| B3GNTL1 | Target | Activated by PBX1 | |||
| CANX | Target | Activated by PBX1 | |||
| IQCD | Target | Activated by PBX1 | |||
| LRRC48 | Target | Activated by PBX1 | |||
| ABHD2 | Target | Activated by PBX1 | |||
| CDK5R2 | Target | Repressed by PBX1 | |||
| ANKRD54 | Target | Repressed by PBX1 | |||
| GPR108 | Target | Repressed by PBX1 | |||
| PRR13 | Target | Repressed by PBX1 | |||
| RAB40C | Target | Repressed by PBX1 | |||
| ACYP1 | Target | Repressed by PBX1 | |||
| COQ7 | Target | Repressed by PBX1 | |||
| NFATC2IP | Target | Repressed by PBX1 | |||
| GTPBP1 | Target | Repressed by PBX1 | |||
| ZFP810 | Target | Repressed by PBX1 | |||
| RNF5 | Target | Repressed by PBX1 | |||
| RWDD4A | Target | Repressed by PBX1 | |||
| MED30 | Target | Repressed by PBX1 | |||
| IER5L | Target | Repressed by PBX1 | |||
| SIX4IP | Target | Repressed by PBX1 | |||
| ZSWIM4 | Target | Repressed by PBX1 | |||
| ONECUT2 | Target | Repressed by PBX1 | |||
| ALDH1A2 | Target | Targeted by PBX1-HoxA1 | Retinoic acid synthesis in the hindbrain | Mouse embryos | Vitobello et al. (2011) |
| CCND1 | Target | Targeted by STAT3, STAT3 phosphorylation enhanced by PBX1 | Promote STAT3/JAK2 pathway and chemoresistance | ccRCC cells | Wei et al. (2018) |
| Pax6 | Target | Regulated by PBX1 | Pancreatic development | Mouse model | Zhang et al. (2006) |
- Note: "Interaction consequence" means either the direct molecular effects induced by PBX1 interaction with a cofactor or the cellular (or developmental) consequence of PBX1 binding on a target gene's promoter.
PBX1 structure is similar to what is observed in other PBC family members, with three functional domains: an HD and two protein–protein interacting domains, PBC-A and B (Figure 1). The heptad 77-84 in PBC-A and two regions (around Lys153 and heptad 197-211) in PBC-B are required for PBX1-MEINOX interactions (Bruckmann et al., 2020). Between the PBC-A and PBC-B domains, a poly-alanine stretch (108-150) confers a flexibility on PBX1 and thus enhances its DNA-binding ability by favoring a conformational change upon binding (Blasi et al., 2017). DNA binding of PBX1 HD relies strongly on an alpha-helix (located in position 271-288) (Farber & Mittermaier, 2011). PBX1 HD is able to recognize a generic octameric motif (TGATNNAT), where N represents nucleotides that are specified by the HOX partner of PBX1 alone or PBX1-PKNOX/MEIS dimers (Longobardi et al., 2014). However, DNA motif recognition of PBX1 varies through development and depends on its partners (Ladam et al., 2018). Initially, PBX1 was thought to interact only with HOX proteins carrying a specific hexapeptide motif in their HD called a W motif (Neuteboom et al., 1995; L. Selleri et al., 2001). However, recent studies have demonstrated that PBX1 is also able to interact with other amino acid motifs of HOX HD in place of, or in addition to, the W motif (Dard et al., 2019; Merabet & Mann, 2016). The use of these alternative motifs allows HOX proteins to adopt different conformations, increasing the range of binding sites that are recognized. This increase in DNA recognition and binding gives the HOX-PBX1 complex the ability to recognize and bind low-affinity, nonconsensus binding sites (Merabet & Mann, 2016) and thus increase the number of genes regulated by PBX1-HOX partners (Dard et al., 2019).
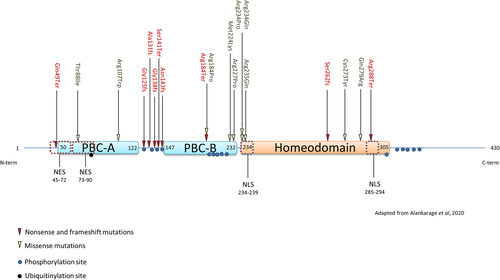
Localization of PBX1 is under the control of two nuclear localization signals (NLS) located in the HD (Berthelsen et al., 1999), and two NES located close to and in the PBC-A domain (Saleh et al., 2000). As a monomer, PBX1 is folded in such a way that the NES (N-terminus) comes into close contact with the HD, hiding the NLS and thus retaining PBX1 in the cytosol (Saleh et al., 2000). PBX1 hetero-dimerization changes the conformation of PBX1, exposing the NLSs (Longobardi et al., 2014). The nuclear import of PBX1 can also be based on serine phosphorylation, depending on the cell type (Kilstrup-Nielsen et al., 2003).
Even if the target genes of PBXs are not fully discovered yet, these proteins, and especially PBX1, have long been linked to cell fate, embryonic development, and cancer in humans. The first human phenotype linked to PBX1 dysfunction was acute leukemia by way of the formation of an E2A-PBX1 fusion transcript (Kamps et al., 1991; C.-H. Lin et al., 2019). Further studies have also highlighted the role of PBX1 in promoting the proliferation of various solid tumors (Blasi et al., 2017; He et al., 2017; Jung et al., 2016; Magnani et al., 2015; Wei et al., 2018). More recently, PBX1 constitutional heterozygous deletions or point variations have been linked to developmental defects, especially to congenital anomalies of the kidney and urinary tract (CAKUTHED; MIM# 617641) (Le Tanno et al., 2017). Despite growing knowledge of the role of PBX1, human developmental phenotypes induced by PBX1 variations are not yet fully understood. The increasing number of cases and the pleiotropy of the symptoms are presented in this review focused on the human developmental phenotype induced by PBX1 variations.
2 PBX1 VARIATIONS IN HUMANS
The PBX1a transcript (NM_002585.4) will be used as a reference in the present manuscript. Point variations and structural variants with an allele count of 1 were omitted since we could not exclude the presence of very mildly affected individuals in the databases used (gnomADv2, DGV database and Alsmadi et al., 2014; Audano et al., 2019; Thareja et al., 2015; Uddin et al., 2015; Wong et al., 2013). Two hundred and fifty-eight point variations or small indels and 24 structural variations (15 deletions, 6 duplications, and 3 insertions) have been reported to date in PBX1. Only two of these late deletions have an impact on the PBX1 coding region (exon 1 [Alsmadi et al., 2014; Thareja et al., 2015]). The exact count for each variation type is reported in Supporting Information: Table S1.
Thirty patients with heterozygous pathogenic mutations and partial or complete heterozygous deletions of PBX1 have been reported in the literature (Alankarage et al., 2020; Bertoli-Avella et al., 2021; Eozenou et al., 2019; Fitzgerald et al., 2021; Fromer et al., 2014; Heidet et al., 2017; Le Tanno et al., 2017; Riedhammer et al., 2017; Slavotinek et al., 2017), and 10 additional patients are reported in the ClinVar, DECIPHER, and HGMD databases. Their clinical data are summarized in Table 2. The PBX1 structure and its related variants are summarized in Figure 1 and Supporting Information: Figure S1. None of these variations was reported in gnomAD, except one (NM_001204961.1:c.862C>T, p.(Arg288Ter) in a heterozygous state and at a frequency of 0.3.10−4).
| Heterozygous variant (NM_002585.4) | Protein change (NP_002576.1) | Expected effect | Functional studies | Chromosomal sex | Face and head anlies | Renal and urinary anlies | External genitalia anlies | Gonadal/uterine anlies | Skeletal anlies | Cardiac malformations | Pulmonary hypoplasia | Deafness | NCS anlies | ID | Other | Article or accession number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.145C>T | p.(Gln49Ter) | Truncated protein or NMD (LoF) | No | XY | NC | Yes | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar VCV000666556 |
| c.263C>T | p.(Thr88Ile) | Impaired protein function | No | XY | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | Yes | Fromer et al. (2014) |
| c.319C>T | p.(Arg107Trp) | Impaired protein function | No | XX | Yes | Yes | No | Yes | No | No | Yes | NC | No | NC | Yes | Arts et al. (2020) |
| c.319C>T | p.(Arg107Trp) | Impaired protein function | No | XY | Yes | Yes | Yes | Yes | No | Yes | Yes | NC | No | NC | Yes | Arts et al. (2020) |
| c.370_371dup | p.(Gly125fs) | Truncated protein or NMD (LoF) | No | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar VCV000817312.1 |
| c.392del | p.(Ala131fs) | Truncated protein or NMD (LoF) | No | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar RCV001008581.1 |
| c.413_419del | p.(Gly138fs) | Truncated protein or NMD (LoF) | No | XY | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | No | Riedhammer et al. (2017) |
| c.422C>G | p.(Ser141Ter) | Truncated protein or NMD (LoF) | No | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | NC | Heidet et al. (2017) |
| c.428del | p.(Asn143fs) | Truncated protein or NMD (LoF) | No | XX | No | Yes | No | No | Yes | No | No | Yes | No | No | No | |
| NG_028246.2(PBX1):c.511-2A>G | Splice defect | Truncated protein or NMD (LoF) | No | XY | No | Yes | No | No | No | No | No | No | No | No | No | |
| c.550C>T | p.(Arg184Ter) | Truncated protein or NMD (LoF) | No | XX | Yes | Yes | No | No | No | No | No | No | No | Yes | No | |
| NC | p.(Arg184Pro) | Impaired protein function | No | XY | Yes | Yes | No | No | Yes | Yes | No | No | No | Yes | No | Alankarage et al. (2020) |
| c.671T>A | p.(Met224Lys) | Impaired protein function | No | XY | Yes | No | Yes | No | No | Yes | Yes | No | No | Yes | Yes | Slavotinek et al. (2017) |
| c.680G>C | p.(Arg227Pro) | Impaired protein function | Yes | XY | Yes | No | Yes | No | No | No | No | No | No | Yes | No | |
| c.701G>A | p.(Arg234Gln) | Impaired protein function | No | XX | NC | Yes | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar VCV000559855 |
| c.701G>C | p.(Arg234Pro) | Impaired protein function | Yes | NC | No | Yes | No | No | No | Yes | No | No | No | Yes | No | Slavotinek et al. (2017) |
| c.704G>A | p.(Arg235Gln) | Impaired protein function | Yes | XY | Yes | No | Yes | Yes | No | No | No | No | No | Yes | No | Kia et al. (2019); Slavotinek et al. (2017) |
| c.704G>A | p.(Arg235Gln) | Impaired protein function | Yes | XY | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | No | No | Slavotinek et al. (2017) |
| c.704G>T | p.(Arg235Gln) | Impaired protein function | Yes | XY | No | No | Yes | Yes | Yes | No | No | No | No | No | No | Eozenou et al. (2019) |
| c.783dup | p.(Ser262fs) | Truncated protein or NMD (LoF) | Yes | NC | Yes | No | No | No | No | No | No | Yes | No | Yes | No | Slavotinek et al. (2017) |
| c.818G>A | p.(Cys273Tyr) | Impaired protein function | No | NC | NC | Yes | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar VCV000598775 |
| c.836A>G | p.(Gln279Arg) | Impaired protein function | No | NC | NC | Yes | NC | NC | NC | NC | NC | NC | NC | NC | NC | ClinVar RCV001251145.1 |
| c.862C>T | p.(Arg288Ter) | Truncated protein or NMD (LoF) | Yes | NC | Yes | Yes | No | No | Yes | No | No | No | No | Yes | No | Eozenou et al. (2019) |
| arr[hg19] 1q23.3(164749027_164786500)x1 | p.? Deletion of exon 3-6 |
Truncated protein or NMD (LoF) | No | XY | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | Yes | Fitzgerald et al. (2021) |
| arr[hg19] 1q23.3(164749027_164786500)x1 | p.? Deletion of exon 3-6 |
Truncated protein or NMD (LoF) | No | XX | Yes | Yes | No | No | Yes | No | No | No | Yes | No | Yes | |
| arr[hg19] 1q23.3(164749027_164786500)x1 | p.? Deletion of exon 3-6 |
Truncated protein or NMD (LoF) | No | XX | Yes | Yes | No | No | Yes | No | No | No | No | No | Yes | |
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | Yes | No | No | No | No | No | No | Yes | Yes | Yes | Le Tanno et al. (2017) | |
| Whole gene deletion | Haploinsufficiency | No | XX | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | No | ||
| Whole gene deletion | Haploinsufficiency | No | XX | Yes | Yes | No | No | No | Yes | No | No | No | Yes | No | ||
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | Yes | Yes | No | Yes | No | No | No | No | No | No | ||
| Whole gene deletion | Haploinsufficiency | No | XX | Yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Yes | ||
| Whole gene deletion | Haploinsufficiency | No | XY | No | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | No | ||
| Whole gene deletion | Haploinsufficiency | No | XX | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | Yes | ||
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | Yes | Yes | No | No | No | No | Yes | No | Yes | No | ||
| Whole gene deletion | Haploinsufficiency | No | XX | No | Yes | No | No | No | No | No | Yes | No | No | No | Heidet et al. (2017) | |
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | Yes | No | No | No | No | No | No | No | Yes | No | ||
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | NC | NC | NC | Yes | Yes | No | No | No | Yes | Yes | DECIPHER 398083 | |
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | Yes | No | Yes | No | No | No | No | No | Yes | No | DECIPHER 387270 | |
| Whole gene deletion | Haploinsufficiency | No | XX | Yes | No | No | No | No | No | No | No | No | No | No | DECIPHER 276516 | |
| Whole gene deletion | Haploinsufficiency | No | XY | Yes | No | Yes | No | Yes | No | No | No | No | Yes | Yes | DECIPHER 262739 |
- Abbreviations: anlies, anomalies; CNS, central nervous system; ID, intellectual disability; LoF, loss of function; NC, not communicated; NMD, nonsense-mediated decay.
2.1 PBX1 loss-of-function (LOF)
Whole or partial gene deletions account for 43% of all the PBX1-mutated patients, and nonsense variations (stop gains) or frame-shift mutations (including indels) amount to 23% of the patients. Nonsense or frame-shift variations mainly affect the polyalanine stretch between PBC-A and PBC-B (56%). Only one nonsense variation was located outside a functional domain (Figure 1).
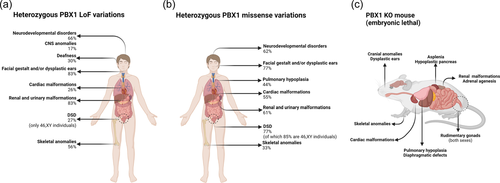
To date, 17 patients have been reported with whole or partial deletions of PBX1 (Fitzgerald et al., 2021; Heidet et al., 2017; Le Tanno et al., 2017) of whom 3 were siblings (Fitzgerald et al., 2021). The size of these deletions ranged from 37 kb (Fitzgerald et al., 2021) to 18 Mb (patient 398083 from DECIPHER). Nine patients were described as presenting LOF variations (nonsense, splice, or frame-shift variations, including indels) and six had documented phenotypes. The clinical data of these patients are summarized in Figure 2A and extensively reported in Supporting Information: Table S2. Phenotypes are highly similar between variants resulting in LOF and PBX1 gene deletions; we, therefore, considered them as the same entity. Most of these patients (83%) displayed various combinations of renal and/or urinary tract anomalies, the most common defect being renal hypoplasia. The only genital anomaly reported was cryptorchidism. Dysplastic ears were frequently reported (43%), sometimes associated with deafness. Cardiac malformations were mainly conotruncal defects and patent ductus arteriosus. Rare cases of arrhythmia were also documented. Interestingly, these cardiac anomalies were only reported in patients with PBX1 deletions. The hypothesis of the contiguous gene syndrome to explain certain extra-renal phenotypes has been proposed (Le Tanno et al., 2017) but the cardiac phenotype could not be linked to another gene in close proximity to PBX1. The implication of PBX1 in the cardiac phenotype is discussed below.
2.2 PBX1 missense variations
Missense variations were diagnosed for 13 (33%) of the PBX1-mutated patients. Most of these missense variations identified in the HD of PBX1 lie in the NLS (Figure 1), with two recurrent positions affected (Arg234 and Arg235), which were studied using functional analyses (Slavotinek et al., 2017; Eozenou et al., 2019) (see below).
Of these 13 patients, 9 had detailed clinical features (Arts et al., 2020; Eozenou et al., 2019; Kia et al., 2019; Slavotinek et al., 2017) (Figure 2B and Supporting Information: Table S2). Renal or urinary anomalies were less prevalent in this group than in the PBX1 LOF group and were milder (hypoplastic or horseshoe kidneys, ureteral dilatation, or hyperechogenic kidneys). Dysplastic ears were rarer. One-third of the patients had mild skeletal anomalies (brachydactyly, clinodactyly, radioulnar synostosis). Unlike PBX1 LOF patients, no central nervous system malformations were reported. Cardiac anomalies were frequent (55%) and similar to the cardiac defects found among patients carrying PBX1 deletions.
In contrast with PBX1 LOF patients, genital anomalies were frequent and severe. Only one 46,XX individual was described in this cohort as having a bicornuate uterus. Six of the 8 46,XY individuals had various severe phenotypes ranging from cryptorchidism and micropenis to complete sex reversal. These last phenotypes included a nearly normal female phenotype for both internal and external genitalia and gonads (one patient), various gonadal anomalies (ovotestis, streak gonads), and internal genitalia malformations. Lung hypoplasia was observed in four patients. For three patients, this lung hypoplasia was associated with diaphragmatic thinning or defect (different from diaphragmatic hernias).
2.2.1 Functional assessment of PBX1 HD variations
Slavotinek et al. (2017) studied the functional roles of PBX1 mutations, notably generating the p.(Arg234Pro) and the p.(Arg235Gln) mutants in HEK293T transfected cells with plasmids encoding these variants. They demonstrated that these variations affected neither levels of protein expression nor nuclear localization in their conditions. Both mutant proteins showed reduced transactivation properties of a luciferase gene preceded by a PBX1 promoter, but in a Pbx1-KO murine cell line, only p.(Arg234Pro) had reduced transactivation properties. The authors suggested that the interactions between endogenous PBX1 protein in HEK293T cells and mutated transfected PBX1 could adversely affect these results. Eozenou et al. (2019) studied the p.(Arg235Gln) mutant in HEK293T cells and demonstrated that, despite a few mutant proteins localized in the nucleus, the variation favored a cytoplasmic retention of mutated PBX1. Moreover, they demonstrated that the mutant protein was unable to form heterodimers with HOXB8. Although the binding of the mutant protein to PKNOX1 was maintained, the binding of mutant PBX1 to EMX2 was reduced and abolished with CBX2. The discrepancy in nuclear localization between these two publications that studied the same variation in the same cellular model raises interesting questions, such as the impact of in vitro approaches on PBX1 function. Eozenou et al. used myc-tagged PBX1 expression vectors, unlike Slavotinek et al., who transfected HEK293T cells with the cDNA of interest (plus PKNOX1 cDNA) and analyzed the protein content of the cell compartments by Western-blotting experiments. Variations might also result to the expression levels of PBX1 reached after plasmid transfection. However, mechanisms other than the use of NLS enable PBX1 to reach the nucleus: phosphorylation of serine residues in the PBC-B domain also has a role in PBX1 nuclear import (Figure 1 [Kilstrup-Nielsen et al., 2003]). In contrast, interaction with proteins of the cytoskeleton, namely NMHCB or FLNA, retains PBX1 in the cytosol (Berry et al., 2005; Huang et al., 2003). Both proteins compete with MEIS1 to bind PBX1, but it is the interaction between MEIS1 (or PKNOX1) and PBX1 that enables PBX1 conformational change and exposure of its NLS (Longobardi et al., 2014; Saleh et al., 2000). It is tempting to postulate that PKNOX1 overexpression could partially overcome mutant PBX1 cytoplasmic retention. On the contrary, a myc-tag may worsen an abnormal conformational change induced by a morbid variation in the PBX1 NLS domain.
Thus, these studies suggest that variations in the NLS lead to a partial loss of PBX1 nuclear localization, but that they also prevent, at least partly, PBX1 interaction with some of its cofactors, two defects that could explain the phenotype induced in humans which recalls, but does not fully phenocopy, the Pbx1-KO mouse phenotype.
Two other variations in the HD have been reported to date but not extensively studied: p.(Cys273Tyr) and p.(Gln279Arg) (ClinVar VCV000598775 and Bertoli-Avella et al. [2021]). Both native amino acids are highly conserved across species (Supporting Information: Figure S1). Both residues are positioned on a segment undergoing conformational changes (Ser271-Arg288 [Blasi et al., 2017]), which are important in increasing DNA binding ability. We can suggest that these variants alter DNA binding rather than PBX1 localization (as demonstrated with other variants by Slavotinek et al. [2017]) or they disrupt interaction with non-HOX partners. Functional studies are required to explore these hypotheses.
2.2.2 Functional assessment of PBX1 non-HD variations
Only one variation in the PBC domains was analyzed in a functional study by Slavotinek et al. (2017), with the protocol previously mentioned. The authors demonstrated that, as for variations in the HD, expression levels of the mutant PBX1 Arg227Pro were comparable to the wild-type (WT) protein. Mutant proteins also reduced transactivation properties in HEK293T cells but not in a Pbx1-KO murine cell line. The authors observed no change in nuclear levels in cells expressing the mutant protein compared to WT transfected cells.
The four other variations have not been extensively studied. One patient from a cohort of schizophrenic individuals was described with substitution of the threonine in position 88 by isoleucine (Fromer et al., 2014). This variation is located in PBX1 NES. PBX1 NES prevents PBX1 nuclear localization by interacting with its NLS (Saleh et al., 2000) and it is masked after PBX1 dimerization with PKNOX1 (Berthelsen et al., 1999). The substitution of threonine by a more hydrophobic amino acid could disrupt NES/NLS interactions. In fact, the NLS has an arginine-rich structure that could be destabilized by hydrogen bond modifications, but this hypothesis remains to be explored.
Two siblings were described with a variation substituting the arginine in position 107 for a tryptophan (Arts et al., 2020). This Arg107 is located just before one poly-alanine segment (Leu108-Tyr150) that plays an important role in PBX1 flexibility and DNA binding (Blasi et al., 2017). Deletion of this helix region (positions 105-113) mildly altered the ability of PBX1 to dimerize with PKNOX1, but did not influence PBX1 dimerization with MEIS1 (Bruckmann et al., 2020). However, in silico prediction tools (HOPE tool [Dunlavy et al., 2005; Venselaar et al., 2010]) suggest that this amino acid substitution would be damaging for the correct folding of the alpha-helical region (positions 103-116 [Bruckmann et al., 2020]). Arg107 is conserved in all species (Supporting Information: Figure S1).
One patient was reported with substitution of the arginine in position 184 by a proline (Alankarage et al., 2020; Slavotinek et al., 2017). The Arg184 was conserved up to Drosophila. This substitution could disrupt the alpha-helix structure implicated in interaction with MEINOX proteins ([Bruckmann et al., 2020] HOPE results). Moreover, this arginine is located in a MAP-kinase recognition motif (Calvo et al., 1999) and phosphorylation is a way for PBX1 to reach the nucleus (Kilstrup-Nielsen et al., 2003, see above).
Finally, another patient was reported with substitution of the methionine in position 224 by a lysine (Slavotinek et al., 2017). Met224 is conserved in all species (including Caenorhabditis elegans). This methionine is located in a potential “minimal inhibitory helix,” a domain that could play a role in preventing PBX1 monomers from binding DNA, an effect suppressed when PBX1 dimerizes (Calvo et al., 1999). However, the function of this domain has not been assessed in more recent studies.
Thus, missense variations in the PBC domains can lead to various deleterious effects: absence of interaction with MEINOX proteins, modification of DNA binding stringency, or alterations in nuclear import. However, more robust functional studies are needed to assess the role of these variants.
3 SHEDDING LIGHT ON HUMAN PBX1-INDUCED PHENOTYPES USING ANIMAL MODELS
Animal models of heterozygous PBX1 LOF are not helpful in explaining these developmental anomalies, since heterozygous mice for a Pbx1 null allele merely exhibit smaller size than their wild-type littermates, even if they express only half the normal level of the protein (L. Selleri et al., 2001). One notable exception concerns mice heterozygous for the p.(Arg184Pro) mutation that sometimes presents with a phenotype close to that of their KO littermates, although it tends to be less severe (Alankarage et al., 2020). However, PBX1-null mouse malformations clearly recall the developmental phenotype reported in humans (Figure 2C) (L. Selleri et al., 2001).
3.1 Head, face, and neck morphogenesis anomalies
The main defect observed in Pbx1-KO mice is a homeotic transformation of structures derived from the second branchial arch and the first pharyngeal groove into the first branchial arch derivatives (L. Selleri et al., 2001). PBX proteins are regulators of HOXA2 expression in rhombomere 4 (Lampe et al., 2008), which gives rise to the ectomesenchyme of the second branchial arch (Couly et al., 1996). However, the mouse phenotype is not completely reminiscent of Hoxa2-KO mice and Pbx1-deficient animals also present with delayed third pouch development, including thymus and parathyroid (Manley et al., 2004). Pbx1-KO mice also display external and inner ear anomalies that are not fully explained by Hox gene dysregulation. Hmx1, an HD TF, plays a role in lateral facial morphogenesis, notably in pinna formation, and possesses a downstream regulatory sequence that can be bound by the Hox-PBX-MEIS complex (Rosin et al., 2016). Deletions of this highly conserved regulatory region are responsible for the dumbo phenotype in mice and rats (Quina et al., 2012). Mutations in another homeobox gene, Emx2, which is known to be regulated by PBX1 in limb development (see below), also have a role in middle and inner ear formation. Mice mutated on this gene present ossicular chain malformations and abnormal hair cell development (Holley et al., 2010). Abnormal patterns or expressions of these two genes, combined with HOXA2 dysregulation, could be involved in the cranial and hearing phenotypes observed in patients, but this remains to be further explored. Finally, it has been demonstrated that PBX proteins control facial morphogenesis by promoting epithelial apoptosis through the Pbx-Wnt-p63-Irf6 network, and a compound loss of Pbx proteins in mice leads to cleft lip and palate (Ferretti et al., 2011). Interestingly, none of the PBX1-mutated patients previously reported presented oral clefts, but SNP in PBX1, WNT9B, and TP63 was significantly associated with nonsyndromic cleft lip and palate in humans (Maili et al., 2020).
3.2 Skeletal and limb deformities or hypoplasia
Mice KO for Pbx1 develops constant and severe skeletal anomalies compared to what is observed in humans. These anomalies mainly involve the cervical and thoracic vertebrae, the ribs and sternum, and proximal structures of the limbs (L. Selleri et al., 2001). The scapula, clavicle, and humerus, in particular, are hypoplastic and misshapen, resembling some malformations described in patients. In mice, Pbx1 and Pbx2 control both Polycomb and Hox TFs (namely Bmi, Eed, Hoxa4, Hoxb5, Hoxb8, Hoxc6, and Hoxd3) to establish hind limb positioning (Capellini et al., 2008). Pbx1, in association with Emx2, also controls the expression of scapula-specific genes such as Alx1 (Capellini et al., 2010). More recent studies have also suggested that PBX1 and PBX2 work together in regulating the pattern of the proximal limbs in a dose-dependent manner (Eyal et al., 2019). PBX1/PBX2 also interacts with GLI3, one of the main controllers of the global limb pattern regulatory network (Eyal et al., 2019) and an organizer of neural tube patterns. At cellular level, mice lacking Pbx1 display diminished chondrocyte proliferation with precocious ossification (L. Selleri et al., 2001). Pbx1 normally represses the ability of Hoxa10 to activate osteoblast-related gene programs (Gordon et al., 2010). This property could explain why Pbx1-KO mice display early abnormal osseous formation. The fact that heterozygous mice for a Pbx1-null allele are smaller than WT animals also suggests early halting of growth as a result of premature ossification. This late phenotype has not been reported in humans, but most of the patients reported to date were children and we cannot fully assess the impact of their PBX1 variant on their adult stature.
3.3 Abnormal cardiac outflow tract development
Pbx-deficient mice present heart defects that mimic the malformations observed in humans, arising from abnormal cardiac outflow tract development (Stankunas et al., 2008). The anomalies observed in mice partly depend on which Pbx protein is deficient, but Pbx1 seems to be the main gene implicated in these malformations, with relatively minor roles for Pbx2 and Pbx3. These effects on the cardiac outflow tract are at least partially explained by the PBX1 requirement for correct expression of PAX3 in premigratory neural crest cells, a cell population highly implicated in heart development (review by Lescroart & Zaffran [2018]). We can also postulate a role of MEIS2 in this expression since MEIS2 mutations in humans lead to similar defects in neural crest-derived structures (Douglas et al., 2018; Fujita et al., 2016). At the cellular level, PBX1 controls both cardiac precursor proliferation in synergy with GATA4, and cell cycle arrest in mature cardiomyocytes by transcriptional activation of CDKN2a, with a suspected role of MEIS1 as a cofactor (Hatzistergos et al., 2019). Thus, PBX1 is a key factor in heart formation, and it is worth noting that cardiac anomalies are not very frequent in patients with PBX1 LOF, unlike patients carrying missense variants (26% vs. 55%). In fact, some authors (Stankunas et al., 2008) demonstrated that the severity of heart defects in various Pbx-deficient mice correlated with the total concentration level of PBX proteins. We can postulate that a decrease in PBX1 concentrations through nonsense-mediated decay (NMD) can be counterbalanced, at least partially, by other PBX proteins (Stankunas et al., 2008) in patients carrying PBX1 LOF variants. In fact, PBX proteins share the same HD sequence and are able to target the same binding sites (Longobardi et al., 2014; Penkov et al., 2013). Pbx1-KO mice have a more severe phenotype while they also carry a second hit in Pbx2, despite the fact that Pbx2-KO mice are viable and healthy (L. Selleri et al., 2001). This finding highlights the redundancy of PBX proteins (Capellini et al., 2006). In contrast, PBX1 missense variants can lead to defective PBX1 proteins retaining PBX1 cofactors (dominant-negative effect, see section 4 and Figure 3), preventing partial rescue by other PBX proteins and leading to severe cardiac phenotypes.

3.4 Lung hypoplasia and delayed maturation
PBX1 (and especially the PBX1b isoform) is expressed early in the pulmonary mesenchyme during the initial stages of budding and outgrowth of the bronchial tree. Pbx1b immuno-reactivity persists in the pulmonary interstitium until adulthood in mice (C. A. Schnabel et al., 2001). Pbx1-KO mouse embryos show severe lung hypoplasia (L. Selleri et al., 2001) and conditional KO in mouse lungs has demonstrated abnormal lung maturation with compact terminal saccules and severely reduced expression of surfactant genes (Li et al., 2014). The loss of Pbx1 in the lung mesenchyme also results in continuous vascular constriction and elastin matrix anomalies (McCulley et al., 2018). The role of Pbx1 in the developing lung resides, at least partly, in its ability to control the expression of Fgf10, a key factor for alveolar type-II cells and differentiation of lung epithelial progenitors, in collaboration with HoxB4 and Meis1 (Li et al., 2014). PBX1 interactions with HOXA5, HOXB5 and, more recently, the repression of TBX2, are also of importance in tracheal budding, mesenchyme induction, and lung maturation (Jeannotte et al., 2016; Lüdtke et al., 2021).
3.5 Diaphragmatic thinning and defects
A few patients carrying PBX1 missense variations presented diaphragmatic thinning and defects (Arts et al., 2020; Slavotinek et al., 2017). By studying the embryonic transcriptome in the search for genes implicated in diaphragmatic hernia, Russel et al. (2012) identified PBX1 as a candidate gene. In the Pbx1-KO mice they generated, the authors demonstrated that the homozygous mutant mice displayed diaphragmatic and muscle-pattern defects, with regions of the diaphragm reduced to a thin membrane with poor or absent musculature and abnormal muscle marker expression. The authors postulated that these defects originated from dysregulation of Hoxa5 and Hoxb5 through dysregulation of retinoic acid (RA) synthesis, since previous studies had demonstrated that PBX1 was able to control RA synthesis (Vitobello et al., 2011). Other signaling pathways, such as SHH (PBX1 interacts with GLI3 during limb formation, see above) could be implicated in the diaphragmatic defects highlighted in Pbx1-deficient mice and PBX1-mutated patients. The absence of PBX1 could lead to defects in the mesenchymal proliferation of the pleuroperitoneal folds, a structure from which the diaphragm originates. A recent study reported a decrease in Pbx1, Meis1, and Runx1 expression in rats with diaphragmatic defects and lung hypoplasia, suggesting reduced mesenchymal cell proliferation as a common mechanism for both diaphragmatic defects and abnormal bronchial budding in Pbx1-deficient rodents (Takahashi et al., 2020). Impaired mesenchymal cell proliferation has been previously demonstrated as a mechanism leading to renal anomalies (see below). Finally, PBX1 is also a cofactor for proteins implicated in myoblast differentiation, such as M-cadherin, (Y.-J. Lin et al., 2021).
3.6 Pancreas hypoplasia and splenic anomalies
Pbx1-KO mice are also characterized by hypoplastic pancreas and asplenia (L. Selleri et al., 2001). Intriguingly, only two patients (siblings) presented asplenia or small spleen associated with accessory spleens. No pancreatic phenotype (pancreas hypoplasia, maldigestion, diabetes, etc.) was reported among PBX1 patients. In Pbx1 mouse mutants, Tlx1 and Nkx2.5, early markers for splenic progenitor cells, are not expressed (Brendolan et al., 2005). PBX1 is able to bind to TLX1 promoter, alone and in cooperation with TLX1 itself, underlining the fact that PBX1 is a key element in spleen development. During embryogenesis, both PBX1 and PBX2 are expressed in the developing pancreas. PBX1 is mainly expressed in the pancreas mesenchyme, unlike PBX2, which is more broadly expressed, including in the pancreatic epithelium (Zhang et al., 2006). Both PBX proteins, in association with PKNOX1, bind to the pancreatic PAX6 enhancer (Zhang et al., 2006). Thus, in Pbx1-deficient mouse embryos, Pax6 expression is strongly reduced in the pancreas, which is not the case for Pdx1, although this TF is also a cofactor of Pbx1 (Asahara et al., 1999). Finally, as demonstrated in the limbs (Capellini et al., 2006), the pancreatic defects observed in Pbx1-KO mouse embryos are more severe when a second Pbx2 hit is present (Zhang et al., 2006). In the adult pancreas, the expression of Pbx1 isoforms differs in exocrine and endocrine tissues, Pbx1b being expressed in acinar cells and Pbx1a preferentially in Langherans islets (Asahara et al., 1999). The absence of pancreatic phenotypes among patients could be explained by partial rescue of PBX1 function by PBX2, as suggested for heart development (see above). Finally, mild pancreatic features could be underreported by patients or clinicians, or appear later in life (since most of the PBX1-mutated patients described were young children).
3.7 CAKUTHED
In KO mice, renal anomalies are similar to the human CAKUTHED phenotype, with hypoplastic caudally positioned and ventrally rotated kidneys. Renal hypoplasia is linked to oligonephronia, due to a reduced nephrogenic zone and nephronic differentiation (Schnabel, Godin, et al., 2003). Schnabel et al. (Schnabel, Godin, et al., 2003) demonstrated that Pbx1 was expressed in the nephrogenic mesenchyme, mainly in the stroma. Renal defects in KO mice rely on both deficient ureteric branching and an abnormal extension of the induced nephrogenic mesenchyme. These anomalies could be the consequence of abnormal interactions between Pbx1 and other TFs, such as Hoxd genes (Patterson & Potter, 2004). The Hoxd gene cluster has been shown to be implicated in the regulation of ureteric branching response and the maintenance of the structural integrity of the tubular epithelia (Di-Poï et al., 2007). However, the Hoxd genes are known to interact with Pbx1 and Meis1 during limb bud pattern development (Capdevila et al., 1999). Similar interactions could explain the renal phenotype observed in Pbx1-KO mice and PBX1-mutated patients, but this hypothesis remains to be explored. Moreover, PBX1 also has a prominent role in the establishment of renal vascular patterns: conditional Pbx1-KO in mouse renal vascular mural cells (VMC) leads to a loss of Pdgfrb downregulation, premature VMC differentiation, vascular pattern defects, and thus early kidney dysfunction (Hurtado et al., 2015). These findings suggest a prominent role of PBX1 in the induction and differentiation of the renal mesenchyme and especially of vasculature progenitors, essential for organ development and function.
3.8 Genital organs and genitalia anomalies
Analyses of mice KO for Pbx1 showed that the anomalies observed for kidneys were part of a larger spectrum of defects of the urogenital ridge mesodermal derivatives (Schnabel, Godin, et al., 2003). PBX1 is expressed early in the coelomic epithelium, nephrogenic cord, and adrenogenital precursors, it is maintained in the somatic cells of the undifferentiated gonad and finally in the interstitium of the mature gonad (Schnabel, Godin, et al., 2003). Gonads of Pbx1-KO animals initiate sexual differentiation but remain rudimentary due to reduced cell proliferation rate, and the expression of the steroid hydroxylase is barely detectable in gonads of male mutant mice. Interestingly, the authors also demonstrated that, in addition to gonadal anomalies, the mice completely lacked adrenal formations, due to the absence of Nr5a1 expression (SF-1, a key TF implicated in steroidogenesis and sexual differentiation, review by Tremblay & Viger [2003]). However, SF-1 expression appeared to be retained in gonadal cells, suggesting differential regulation of Nr5a1 expression depending on the tissue (Schnabel, Selleri, et al., 2003). These results demonstrate that Pbx1 does not interfere in the establishment of the urogenital ridge intermediate mesoderm but plays a prominent role in the establishment of the bipotential gonad (Schnabel, Selleri, et al., 2003) and is active upstream from Nr5a1 only in adrenal development. Moreover, Pbx1-KO mice lack Müllerian ducts, but not Wollfian ducts which are normally formed and joined. These findings have led some authors to explore the role of PBX1 in Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome. Burel et al. (2006) did not find morbid variations in the HOX genes or PBX1 in MRKH patients, but Ma et al. (2015) found an increased risk of MRKH syndrome in association with rare SNP in PBX1 and WNT9B, which are known to interact together in facial morphogenesis (see above and Ferretti et al. [2011]; Maili et al. [2020]). More recently, Chen et al. (2021) also found MRKH candidate variants in WNT9B, HOXA10, and EMX2. These last two genes are known to interact with or be recruited by PBX1 during limb formation and osteoblast activation (Capellini et al., 2010; Gordon et al., 2010). It is also worth noting that Hoxa10-KO male mice present bilateral cryptorchidism (Satokata et al., 1995), a phenotype also observed in some 46,XY patient carriers of PBX1 truncating variants. Thus, we can hypothesize that a partial disruption of PBX1 interaction with these effectors, especially HOXA10, could explain certain mild phenotypes observed in PBX1-mutated patients. However, the discrepancies between mouse models and PBX1-mutated patients, especially for adrenal agenesis and sex-dependent gonadal/genital malformations, are not well known. These differences recall the variations observed between NR5A1-mutated patients and Nr5a1-KO mice: XY mice are sex-reversed and present adrenal agenesis, unlike patients who can either have isolated adrenal deficiency or 46,XY DSD (Biason-Lauber, 2000). The phenotypes of Nr5a1-KO mice are known to vary depending on the strain studied, suggesting a prominent role of other genetic modifiers. This phenomenon could also play a partial role in the phenotypic variability observed in PBX1-mutated patients, where DSDs are not fully penetrant. These results demonstrate that the precise role of PBX1 in human gonadal and genital development has not yet been fully understood.
4 GENOTYPE-PHENOTYPE VARIATIONS IN PBX1-MUTATED PATIENTS
Two distinct phenotypes can be identified for PBX1-mutated patients (as suggested by Slavotinek et al. (2017), Figure 2a,b). First, a phenotype of syndromic CAKUTHED with skeletal anomalies, deafness, ear dysplasia, neurodevelopmental delay, and cryptorchidism is observed for patients carrying a LOF mutation or a gene deletion. A more severe phenotype is observed for patients with missense variations, with gonadal and genital anomalies that can lead to complete sex reversal in 46,XY individuals and lung hypoplasia sometimes associated with diaphragmatic thinning. This discrepancy has already been discussed by Slavotinek et al. (2017). Genotype-phenotype correlations are of importance to further assess protein function but above all to provide appropriate genetic counseling and suggest the most suitable management for the patients.
Phenotypic variation depending on the mutation type is a mechanism previously described for other TFs. For example, GLI3 mutations cause Greig syndrome (MIM# 175700) in case of inactivating variations or haploinsufficiency (through gene deletion), unlike truncating variants in the middle third of the gene, which are responsible for Pallister–Hall syndrome (MIM# 146510). Similarly, we can postulate different mechanisms to explain the phenotypic variability observed in PBX1.
4.1 Nonsense-mediated decay (NMD)
Stop or frame-shift mutations in PBX1 are thought to lead to mRNA NMD, as described for other TFs such as PAX6 (Lima Cunha et al., 2019). The NMD of PBX1 mRNA could lead to a decrease in PBX1 concentration, but the WT allele and other PBX proteins (Figure 3) could counterbalance this loss, at least partially. There is partial functional redundancy between PBX proteins (Stankunas et al., 2008). However, total concentration levels of all PBX proteins are also of importance for normal cellular functions, as explained above for cardiac development. Dose-dependency has been previously reported for other TALE TFs, such as MEIS1 (Marcos et al., 2015). Haploinsufficiency has been described in numerous diseases caused by mutations in TFs (Seidman & Seidman, 2002). Haploinsufficiency could be the only pathogenic mechanism reported for the gene (e.g., MYRF, HAND2, or NFIB [Cohen et al., 2020; Rossetti et al., 2019]), or it could be associated with various gene function disruptions caused by missense mutations (e.g., PAX6). However, these later mutations are often responsible for phenotypes that are milder than haploinsufficiency (Lima Cunha et al., 2019), unlike what is observed in PBX1-mutated patients.
4.2 mRNA instability and posttranslational protein modifications
Mechanisms other than NMD can alter mRNA stability or translation, leading to a drastic decrease in the total protein levels. mRNA secondary structures are known to alter protein expression through changes in mRNA half life (Mauger et al., 2019). Any nucleotide alteration of the secondary structure of mRNA can lead to premature mRNA decay and haploinsufficiency-like phenotypes in patients carrying PBX1 missense variations. Micro-ARNs (miRNA) are also of importance in regulating mRNA levels: 3′UTR variations affecting miRNA binding sites are implicated in human diseases such as Tourette's syndrome (review by Kawahara [2014]). Very recently, an SNP in the 3′UTR sequence of PBX1 was linked to poor prognosis in breast and gastric cancers, by alteration of the regulatory affinity of miR-522-3p (Mohammadi et al., 2021). Thus, the occurrence of aberrant miRNA binding sites in PBX1 could also play a role in the milder phenotypes caused by missense variants. It is worth noting that PBX1 3′UTR variants are rare in the general population (64 variants reported in the gnomAD database, of which only 11 had an allele count >10). However, 3′UTR variants are not fully explored by exome sequencing, a routine first-tier technique, and they may be under-diagnosed in CAKUTHED patients.
Posttranslational changes such as ubiquitinylation or phosphorylation are of importance for correct protein function or degradation. At least 14 phosphorylation sites are reported in PBX1 (https://www.phosphosite.org/). Only the sites located in the PBC-B domain have been studied in vivo, with proven effect on PBX1 nuclear localization (Kilstrup-Nielsen et al., 2003), and no germline variation affecting, or creating, a phosphorylation site has been reported to date.
4.3 Dominant-negative or gain-of-function (GoF) effects
Missense variations can also lead to dominant-negative or GoF effects, especially when proteins are engaged in multimeric complexes. This type of mechanism is well known in TP53-linked pathologies, for example, where TP53 tetramers formed by both normal and mutant proteins (Gencel-Augusto, 2020) affect the stability and localization of the complex. These mechanisms were also observed in complexes composed of different subunits, such as the chromatin-remodeling switch/sucrose non-fermentable complex. Mutations in genes encoding subunits of this complex are known to cause Coffin–Siris syndrome (MIM# 135900): depending on the protein affected, both dominant-negative effects and GoF have been described (Kosho et al., 2014). Since PBX1 forms heterodimers or trimers with various cofactors, we can postulate that PBX1 missense variations can generate the expression of abnormal PBX1 proteins that can compete with normal PBX1 TF, either for cofactor dimerization or DNA binding (Figure 3).
4.4 Second genetic hit and epigenetic alterations
However, since PBX1 can interact with various cofactors with pleiotropic modalities, a second genetic hit (such as a rare SNP) in one of these cofactors, in another TALE protein, or in one of PBX1 DNA-binding sites, could play a role in this phenotypic variability. The potential role of the second variation in another TALE protein has been partially discussed above: Pbx1 KO mouse models have a more severe phenotype when they carry a heterozygous variant in another Pbx protein (Capellini et al., 2008). We can postulate that partial disruption of a MEIS or PKNOX partner could worsen the phenotype. The hypothesis of a second genetic hit has already been reported for other diseases linked to TF mutations, such as SHH, for example, some of the intrafamilial variability observed for SHH-induced holoprosencephaly has been linked to a second pathogenic variation in another causative gene (Nanni et al., 1999). Animal models support this hypothesis of a second hit, with a more severe phenotype when there are additive variants in partner or target genes (e.g., otocephaly and otx2 in zebrafish [Chassaing et al., 2012] or double mutations in MECP2 and CDKL5 in Rett-like syndrome [Jdila et al., 2020]). In contrast, TF binding variations induced by rare SNPs in TFs binding sites have been recently described, especially in common diseases such as thalassemia (Cheng et al., 2020; Savinkova et al., 2013). Despite growing knowledge in this field, in silico predictions of variations in TF binding sites remain challenging and require experimental validation (Moradifard et al., 2020). Finally, variations in sequence or level of expression of miRNAs targeting PBX1 could partly explain both intra- and interindividual variability by modulating the impact of PBX1 variations from one tissue (or individual) to another. Recent studies have linked the roles of miRNAs to PBX1 function and degradation in both development processes and cancer (Cao et al., 2022; N. Liu et al., 2020).
Shedding light on these mechanisms would be of help to better understand PBX1 function and regulation, and also refine genetic counseling.
5 CONCLUDING REMARKS
First discovered in cancer, PBX proteins, and especially PBX1, have proved to be essential in organism development, from the early gastrulation stages to adulthood. As a transcription cofactor, PBX1 interacts with numerous proteins, including those of the HOX family, and reaches dozens of regulating sequences, even in the heterochromatin state, suggesting that PBX proteins could act as pioneer factors. This pleiotropy explains the polymalformative phenotypes observed in both Pbx1-deficient mice and patients carrying heterozygous PBX1 variations. By altering PBX1 cellular localization and its interaction with its partners or DNA binding, these variations impact the wide network under control of PBX1. However, despite a growing number of studies about this particular family of PBC/MEINOX proteins, questions are still emerging: the mechanisms behind the phenotypic variability among PBX1-mutated patients, the discrepancies between human phenotypes and animal models (such as sexual development anomalies), the whole landscape of PBX protein redundancy, or the key elements developed by organisms to compensate for PBX1 deficiency. The end of the TALE has not yet been written.
ACKNOWLEDGMENTS
Authors would like to thank AVIESAN (Plan Cancer). Figures 2 and 3 were created with the help of BioRender.com tool. This study did not receive any specific grant from commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.




