Insight into vitamin B6-dependent epilepsy due to PLPBP (previously PROSC) missense mutations
Contract grant sponsors: Valencian Government (PrometeoII/2014/029); Spanish Government (BFU2014-58229-P and BFU2017-84264-P).
Communicated by David S. Rosenblatt
Abstract
Vitamin B6-dependent genetic epilepsy was recently associated to mutations in PLPBP (previously PROSC), the human version of the widespread COG0325 gene that encodes TIM-barrel-like pyridoxal phosphate (PLP)-containing proteins of unclear function. We produced recombinantly, purified and characterized human PROSC (called now PLPHP) and its six missense mutants reported in epileptic patients. Normal PLPHP is largely a monomer with PLP bound through a Schiff-base linkage. The PLP-targeting antibiotic d-cycloserine decreased the PLP-bound peak as expected for pseudo-first-order reaction. The p.Leu175Pro mutation grossly misfolded PLPHP. Mutations p.Arg241Gln and p.Pro87Leu decreased protein solubility and yield of pure PLPHP, but their pure forms were well folded, similarly to pure p.Pro40Leu, p.Tyr69Cys, and p.Arg205Gln mutants (judged from CD spectra). PLPHP stability was decreased in p.Arg241Gln, p.Pro40Leu, and p.Arg205Gln mutants (thermofluor assays). The p.Arg241Gln and p.Tyr69Cys mutants respectively lacked PLP or had a decreased amount of this cofactor. With p.Tyr69Cys there was extensive protein dimerization due to disulfide bridge formation, and PLP accessibility was decreased (judged from d-cycloserine reaction). A 3-D model of human PLPHP allowed rationalizing the effects of most mutations. Overall, the six missense mutations caused ill effects and five of them impaired folding or decreased stability, suggesting the potential of pharmacochaperone-based therapeutic approaches.
1 INTRODUCTION
Very recently a novel form of vitamin B6-dependent genetic epilepsy of recessive inheritance was reported (Darin et al., 2016; Plecko et al., 2017), associated to mutations in the PROSC gene (MIM# 617290; this gene is now renamed PLPBP, from pyridoxal phosphate binding protein; www.genenames.org). PLPBP is the human version of COG0325, a gene that is highly conserved and that is very widely distributed among all life forms, from bacteria to humans (Ito et al., 2013; Labella et al., 2017; Prunetti et al., 2016). As indicated by its name, PLPBP encodes a pyridoxal phosphate (PLP) containing protein of uncertain function that, having been proposed to be involved in PLP homeostasis (Darin et al., 2016; Ito et al., 2013; Plecko et al., 2017; Prunetti et al., 2016), is now called PLPHP (https://www.uniprot.org/uniprot/O94903; old name, PROSC). Although this protein has been found not to be essential for life in several organism (Ito et al., 2013; Labella et al., 2017; Prunetti et al., 2016), null mutations of the COG0325 gene associate in bacteria with pleiotropic phenotypes that include increased sensitivity to pyridoxine toxicity and to PLP-targeting antibiotics, as well as various amino-acid metabolism-related aberrations (Ito et al., 2013; Ito, Yamauchi, Hemmi, & Yoshimura, 2016; Kolodkin-Gal et al., 2010; Labella et al., 2017; Prunetti et al., 2016). In humans, of the 11 patients that have been reported thus far with vitamin B6-dependent epilepsy associated to PLPBP mutations (Darin et al., 2016; Plecko et al., 2017), four patients carried null mutations in homozygosis due to protein truncation at residues 71 or 78 (Darin et al., 2016), quite upstream in the 275-residue PLPHP polypeptide chain. Another patient was a compound heterozygote for two different splice-site changes that should have resulted in decreased levels of normal PLPHP (Darin et al., 2016). From these truncating or splicing mutations it is clear that the loss of PLPHP function triggers early in life nervous system pathology manifested as epilepsy with developmental delay if not effectively treated with vitamin B6-supplementation.
In addition to these patients carrying truncating or splicing mutations, six patients carried single amino acid substitutions of uncertain effects on PLPHP. Table 1 lists these mutations and includes the second mutant allele and a summary of the reported clinical data for each patient carrying one of these mutations (Darin et al., 2016, Plecko et al., 2017). Of these mutations, p.Leu175Pro was previously shown (Darin et al., 2016) to decrease PLPBP mRNA production by ∼75% in the fibroblasts from a patient carrying this mutation in homozygosity, allegedly because of the creation of a novel siRNA site. However, the effect of this mutation at the protein level was even more drastic, since no PLPHP could be detected in Western blots of these fibroblasts (Darin et al., 2016).
| Mut. # | Sequence substitution | Effects on PLPHPc | Report/patientd | 2nd allele found in same patient | Seizures: onset age/ type at presentatione | B6 vitamer for seizure control | Developmental outcomee | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cDNAa | Proteinb | cDNA | Protein | Formf | mg/day | Response | |||||
| 1 | c.119C > T | p.Pro40Leu | ↓ thermal stability | 2/1 | c.722G > A | p.Arg241Gln | d7/myoclonia | PN | 300–400 | Good | Normal at y12.5 |
| 2 | c.206A > G | p.Tyr69Cys |
|
2/4 | c.206A > G | p.Tyr69Cys | d9/tonic head and eye deviation | PN | 300 | Good | Learning difficulties, y30 |
| 3 | c.260C > T | p.Pro87Leu | ↓ solubility | 2/3 | c.260C > T | p.Pro87Leu | d3/GTC | PN | 450 | Good | Marked delay at m27 |
| 1/ 7 | c.722G > A | p.Arg241Gln | m1/GTC | PN | 250 | Good | Normal schooling and life, IQ60, y17 | ||||
| 4 | c.524T > C | p.Leu175Pro | Misfolding | 1/4 | c.524T > C | p.Leu175Pro | d1/GTC | PLP | 45 kg−1 | Partial | Marked delay at y3.5 |
| 5 | c.614G > A | p.Arg205Gln | ↓ thermal stability | 2/2 | c.249_252del | p.Ser84Cysfs*21 | d6/cloni arms, legs | PN | 100 | Good | Normal at y15.5 |
| 6 | c.722G > A | p.Arg241Gln |
Protein lacks PLP |
|
|
|
|
||||
- a Respective GenBank reference sequences for the human PLPPB gene and for its mRNA, NC_000008.11 and NM_007198.3. Nucleotide numbering uses +1 as the A of the ATG translation initiation codon in the mRNA reference sequence, with the initiation codon as codon 1.
- b Protein reference sequence NP_009129.1; Uniprot KB O94903.
- c Results from this work. For each mutation, the effect that might have the most important negative impact is highlighted in bold-type. For mutation 3, the importance of the reported effect on disease causation is uncertain.
- d Patients are identified by their numbers in the original reports of Darin et al. (2016) (report 1) and Plecko et al. (2017) (report 2).
- e The letters preceding the figures denote days (d), months (m) or years (y). GTC, generalized tonic–clonic seizures. For further clinical details, see the original report in which each patient was described.
- f PN, pyridoxine. PLP, pyridoxal phosphate.
The effects of another two missense mutations (p.Pro87Leu and p.Arg241Gln) were studied in complementation assays in yggS− Escherichia coli (Darin et al., 2016). This mutant bacterial strain lacks the corresponding COG0325 protein, YggS, and exhibits a phenotype of decreased resistance to pyridoxine toxicity that can be restored to normal by complementation with wild-type PLPBP (Darin et al., 2016; Ito et al., 2013; Prunetti et al., 2016). However, the results of these complementation assays did not parallel the clinical severity inferred for these two mutations (Darin et al., 2016), what prompted us to introduce these mutations into the recombinantly expressed COG0325 gene of the cyanobacterium Synechococcus elongatus, using the produced protein, PipY, as a model of human PLPHP (Tremiño, Forcada-Nadal, Contreras, & Rubio, 2017).
We now produce recombinantly and purify human PLPHP, investigating the effects on protein properties of all the PLPBP missense mutations reported thus far in patients with vitamin B6-dependent epilepsy (Darin et al., 2016; Plecko et al., 2017), introduced by site-directed mutagenesis. Actually, we characterize here for the first time the properties of normal human PLPHP, determining its oligomeric state in solution, its spectroscopic properties reflecting PLP binding, its thermal stability using thermofluor assays, and its sensitivity to the PLP-targeting antibiotic d-cycloserine (DCS) (Neuhaus, 1967). This characterization has been essential for assessing experimentally the effects of these missense mutations introduced into the recombinant protein. A structural model of PLPHP based on the experimental structures of other COG0325 proteins (including PipY) has helped rationalize these effects. In this way, we have found for all the mutations differential traits relative to the wild-type protein that can account or contribute to disease causation.
2 MATERIALS AND METHODS
2.1 Recombinant production of wild-type and mutant forms of human PLPHP
The human PLPBP coding sequence (HGNC ID: 9457; GenBank gene reference sequence NC_000008.11; mRNA reference sequence NM_007198.3, numbering +1 the A of the ATG translation initiation codon, which is codon 1; protein reference sequence NP_009129.1; Uniprot KB entry O94903) was provided by GenScript (Piscataway, NJ) as a codon-optimized (for E. coli expression) synthetic gene inserted into the KpnI–BamHI sites of the pGS-21a plasmid. After biological amplification in DH5α cells, this plasmid was used for introduction of the desired PLPBP missense mutations by the Quickchange approach (Stratagene, La Jolla, CA) using Deep Vent DNA polymerase (New England Biolabs, Ipswich, MA) and the indicated (Table 2) forward and reverse mutagenic primers. After digestion of the parental plasmid by DpnI digestion, the mutant plasmids were used to transform E. coli DH5α cells, from which they were isolated and corroborated to host the correct mutant sequences (Sanger sequencing).
| Name | Sequence (5′- 3′) |
|---|---|
| PLPHP-P40L-F | CGATCCAGC TGCGTCTG |
| PLPHP -P40L-R | AGACGC AGCTGGATCGCC |
| PLPHP -Y69C-F | GTGAAAACT GTGTTCAAGAG |
| PLPHP -Y69C-R | TCTTGAACA CAGTTTTCACC |
| PLPHP -P87L-F | AGCCTGTGCC TGGAAATCAAG |
| PLPHP -P87L-R | GATTTCC AGGCACAGGCTCAG |
| PLPHP -L175-F | CCGAACC CGGAGTTC |
| PLPHP -L175-R | CTCC GGGTTCGGGC |
| PLPHP -R205Q-F | AGCCTGC AGGAGGAACTG |
| PLPHP -R205Q-R | AGTTCCTC CTGCAGGCTC |
| PLPHP -R241Q-F | AACGTTC AGATCGGTAGC |
| PLPHP -R241Q-R | ACCGAT CTGAACGTTGGT |
| PLPHP -1-F | aggagatataccatgTGGCGTGCGGGTAG |
| PLPHP -275-R | gtgatggtgatgtttGTGTTCCTGCGCAAC |
- The bases that are mutated are highlighted in bold type, and the codon is underlined. Sequences used for cloning on pOPINE are shown in low case.
For protein expression, the PLPBP coding sequences from the wild-type and the mutant pGS-21a plasmids were PCR-amplified (Deep-Vent DNA polymerase and primers PLPHP-1-F and PLPHP-275-R; Table 2) and cloned into NcoI/PmeI-digested pOPIN-E, using the In-Fusion technology (In-Fusion HD Enzyme Premix; Clontech, Madrid, Spain), followed by transformation into DH5α cells, plasmid isolation and verification (Sanger sequencing with primers PLPHP-1-F and PLPHP-275-R; Table 2). E. coli BL21 (DE3) cells (Invitrogen, Carlsbad, California, USA) transformed with the appropriate pOPIN-derived plasmid were grown at 37°C in aerated 0.5-l LB-ampicillin (0.1 mg/ml) to 0.6–0.8 OD600, then 1 mM isopropyl-β-d-1-thiogalactopyranoside was added and the culture was continued for 3 hr.
Wild-type and mutant PLPHP forms were purified in the same way, at 4°C. After the induction period, the cells were harvested by centrifugation, disrupted by sonication in 15 ml of lysis buffer [25 mM HEPES pH 7.5, 0.5 M NaCl, 1 mM dithiothreitol (DTT), and 1 mM phenyl methyl sulfonyl fluoride], and the suspension was clarified by 30-min centrifugation at 11,000 × g. The supernatant was applied to a 1-ml Ni-chelate HisTrap-HP column fitted in an ÄKTA-FPLC system (both from GE Healthcare, España, Barcelona, Spain). After a 30 ml-wash with column buffer (25 mM HEPES pH 7.5, 0.5 M NaCl, and 20 mM imidazole) a 80-ml 20 mM-to-500 mM linear imidazole gradient was applied in the same buffer, monitoring the optical absorption of the effluent at 280 nm, collecting fractions. PLPHP, eluted at about 120 mM imidazole, was pure (monitored by SDS-PAGE). It was concentrated to >2 mg protein/ml, placing it simultaneously in 20 mM HEPES pH 7.5, 16 mM NaCl, using centrifugal ultrafiltration (10 kDa cutoff membrane; Amicon Ultra, from Merck-Millipore, Merck Chemicals and Life Sciences, Madrid, Spain), and storing it at −20°C until use.
2.2 Electrophoretic techniques
SDS-PAGE (Laemmli, 1970) was performed in 12% polyacrylamide gels, using Comassie Blue staining to visualize proteins bands. When indicated, the 3% (v/v) β-mercaptoethanol (MSH) normally present in the sample buffer was omitted.
Western blotting was used to detect PLPHP in the supernatant and precipitate obtained after centrifugation of the initial crude cell extracts. Equal volumes of the supernatant and of the precipitate reconstituted in the original volume of lysis buffer were subjected to SDS-PAGE followed by Western blotting to nitrocellulose membranes and immunodetection as previously reported for PipY (Labella et al., 2017). The proper electrophoretic transfer to the membranes was verified by observing the similar protein banding pattern of wild-type and mutant proteins in the membranes after staining them with Poinceau red. The primary antibody (anti-PipY rabbit polyclonal antibody) was used at higher concentration (1:750 dilution) than for PipY (Labella et al., 2017), since although this antibody cross-reacts with human PLPHP, its potency for this protein in Western blots was ∼2-fold less than for PipY (data not shown).
2.3 Optical methods
Circular dichroism spectra were recorded at 21°C with a Jasco J-810 spectropolarimeter (Jasco, Easton, MD) on 0.1 cm-path cuvettes containing wild-type and mutant forms of PLPHP in 50 mM Na phosphate pH 7.5. Each spectrum was the average of five scans. All samples that were purified were analyzed at 5 and 10 μM concentration of PLPHP polypeptide chains. Closely similar results were obtained for these two concentrations for each protein form. Data were processed with the K2d server (available in DichroWeb, https://dichroweb.cryst.bbk.ac.uk).
Optical absorption spectra over the range 220–748 nm were determined on 2 μl of protein solution using a NanoDrop™ 1000 V3.8.1 microspectrophotometer (Thermo Fisher Scientific, Wilmington, DE). The protein was dissolved in 20 mM HEPES pH 7.5 and 16 mM NaCl. When indicated, the solutions containing 5–7 mg/ml PLPHP (wild-type or mutant forms) and negative control solutions lacking the protein were incubated 20 min at 25°C with variable concentrations of DCS and then the spectra were determined. The spectra due to the protein was determined by difference of the spectra of the parallel protein-containing and protein-free incubations
2.4 Size-exclusion chromatography
Size-exclusion chromatography of purified PLPHP variants (0.1–0.3 mg; ≤50 μl injections) or of protein standards of known mass was carried out on a SuperdexTM 75 5/150 GL column (GE Healthcare) mounted on an ÄKTA FPLC system, monitoring the OD280 of the effluent. The solution used to equilibrate and to run the column at 25°C and at a flow rate of 0.4 ml/min contained 10 mM Na phosphate pH 7, 0.4 M NaCl, 5 mM MgCl2, 5 mM EDTA, and 0.2 mM DTT. The standards used (masses in kDa between parentheses) were rabbit phosphorylase b (97.4), bovine serum albumin (66.4), chicken ovalbumin (45), maltose binding protein (42), bovine erythrocyte carbonic anhydrase (29), soybean trypsin inhibitor (21.5), and horse heart cytochrome C (12.3).
2.5 Thermal stability assays
Thermofluor assays (Vedadi et al., 2006) were performed in triplicate on a real-time PCR instrument (7500 model from Applied Biosystems, Thermo Fisher Scientific, Alcobendas, Madrid, Spain), monitoring the increase in SYPRO Orange (from Invitrogen, Carlsbad, CA) fluorescence upon gradual (1°C/min) temperature increase of solutions of 15–20 μM PLPHP (wild-type or mutant forms) in 20 mM HEPES pH 7.5/16 mM NaCl and SYPRO Orange (a 1:1,000 dilution of the commercial preparation).
2.6 Structural model generation
An initial model for the PLPHP 3-D structure was generated with I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/) (Yang et al., 2015). Automated and manual refinement cycles with ModRefiner (https://zhanglab.ccmb.med.umich.edu/ModRefiner/) (Xu & Zhang, 2011) and COOT (Emsley, Lohkamp, Scott, & Cowtan, 2010) were used in succession to eliminate structural clashes and to improve the model. When the model appeared satisfactory and presented good stereochemistry (tested with RAMPAGE, Lovell et al., 2003), a PLP molecule was incorporated by superimposing this structure on the experimental structure of PipY [Protein Databank (PDB) entry 5NM8; www.rcsb.org] (Tremiño et al., 2017). Then refinement cycles were repeated again as above. The final model with PLP bound presented no clashes or bad contacts (tested with MolProbity; Williams et al., 2017) and exhibited excellent stereochemistry, with no outliers in the Ramachandran plot.
2.7 Other techniques
Pure PipY was prepared as previously reported (Tremiño et al., 2017).
The amount of pure PLPHP protein was determined from its optical absorption at 280 nm for sequence-deduced (EXPASY ProtParam tool, https://www.expasy.org) absorbance values for 0.1% solutions (1-cm optical path) of 0.636 (p.Tyr69Cys mutant) or 0.678 (all other PLPHP forms).
Plots, numerical calculations and curve fittings were performed with program GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Figures of protein structure were prepared with PyMOL (Pymol Molecular Graphics System, Version 1.6, Schrödinger, LLC, www.pymol.org).
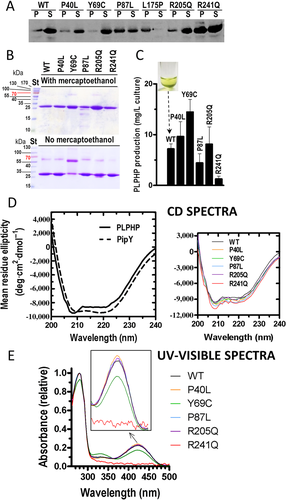
Preparations of wild-type and mutant proteins and experiments carried out with them were repeated at least twice. The electrophoretic and size-exclusion chromatography profiles shown in Figures 1 and 2 illustrate individual experiments but are representative of all the experimental repeats.

3 RESULTS AND DISCUSSION
3.1 Production and characteristics of pure wild-type human PLPHP
We produced recombinantly in E. coli human PLPHP with a C-terminal 6-His tag. Although many human proteins are not expressed well in E. coli, this was not the case for PLPHP, which was highly soluble, as shown by its recovery largely in the supernatant of the disrupted bacterial cells expressing it (Figure 1A, tracks labeled WT), and which could be purified to homogeneity (purity monitored by SDS-PAGE; Figure 1B, top panel, WT track) in quite a good yield (close to 10 mg/l of culture, Figure 1C, bar labeled WT). Its CD spectrum (Figure 1D, left panel, continuous line) closely resembled that of its cyanobacterial homologue PipY (Tremiño et al., 2017) indicating that PLPHP is well folded and agreeing with a similar composition of α helices and β strands of PLPHP and PipY, thus supporting the folding of PLPHP essentially as PipY. This last protein was shown crystallographically to present the modified TIM barrel fold of type III PLP proteins (Tremiño et al., 2017), a fold also exhibited by the structural model of PLPHP built here (see below Figure 3A).
As expected for a PLP-containing protein, PLP solutions had yellow color (picture inset in Figure 1C) and presented in the optical absorption spectrum the characteristic peak at about 425 nm (Figure 1E) that reflects the protonated Schiff base of protein-bound PLP (Fasella, 1967), thus justifying the inclusion of bound PLP in the structural model of PLPHP (see below Figure 3A).
Previously, PipY was found to be monomeric in solution as revealed by size exclusion chromatography (Tremiño et al., 2017). The same technique also supported that PLPHP is a monomer (Figure 2A, left). However, while with PipY only the monomer peak was observed (Figure 2A, left) (Tremiño et al., 2017), a minor peak was seen with PLPHP in the UV absorption profile of the column effluent, with the elution position of its maximum fitting the position expected for a dimer (Figure 2A, left). The monomer and the dimer peaks of PLPHP exhibited the same optical absorption spectrum (not shown) including the 425 nm peak, indicating that both of them had PLP bound. Rechromatography of fractions collected from each of the peaks gave (Figure 2A, right, top and middle panels) only the original peak in each case (except for minor amounts of the other peak possibly derived from contamination in the fractions used for rechromatography), excluding that the monomer and dimer were in rapid equilibrium. This suggested that the dimer was formed by covalent binding of two monomers, possibly by a disulfide bridge. This possibility appeared likely, since, in contrast with PipY, which has a single cysteine, PLPHP has five cysteine residues, three of them exposed in its 3D structural model (cysteines 15, 209, and 261, see below Figure 3A). Since no dimer was observed on standard SDS-PAGE of PLPHP (Figure 1B, top), which includes mercaptoethanol (MSH) in the sample solution, we repeated SDS-PAGE of this protein omitting the addition of this disulfide reducing agent, revealing a small amount of dimer (Figure 1B, bottom), agreeing with the size exclusion chromatography results and with the hypothesis of a disulfide bond being responsible for dimer formation. Similarly, brief preincubation of native PLPHP with 10 mM mercaptoethanol prior to size exclusion chromatography greatly reduced the amount of the early-eluting peak (Figure 2A, right, bottom panel). Therefore, although the majority of PLPHP is monomeric, it appears that this protein can dimerize stably by forming a disulfide bond between its exposed cysteines. As will be seen, dimerization is much more prominent for the p.Tyr69Cys mutant.
3.2 Effects of the disease-associated PLPHP amino acid substitutions on folding, absorption spectra, and oligomeric state of the protein
Site-directed mutagenesis was used to introduce each one of the six missense PLPHP mutations reported in patients with vitamin B6-induced epilepsy (Table 1). Only one of these mutations, p.Leu175Pro, caused gross misfolding, judged from the complete insolubility of the mutant protein when it was expressed in E. coli (Figure 1A). This was revealed by specific detection of the protein in Western blots using a polyclonal rabbit antibody against PipY that cross-reacts specifically with PLPHP. Clearly, the p.Leu175Pro mutant was in the precipitate of the initial cell extract, with no detection of any PLPHP in the corresponding supernatant. With the other five mutants, soluble protein was always found, although the data (Figure 1A) revealed lower solubility of the p.Pro87Leu and p.Arg241Gln mutants compared with wild-type PLPHP. Nevertheless, the decreased solubility of these last two mutants did not prevent their purification, although in lower yield than the wild-type protein or the other mutants (Figure 1C). Circular dichroism spectra of the purified mutant proteins were very similar to that for the wild-type protein, showing that these purified mutant forms were well folded (Figure 1D, right panel).
Naked-eye observation of the preparations of the purified mutants showed that all of them, similarly to the wild-type enzyme, were colored yellow, with the exception of the p.Arg241Gln mutant, which was colorless. In addition, the p.Tyr69Cys mutant appeared to have a fainter tone than preparations of wild-type PLPHP having similar protein concentration. These observations were confirmed by examining the absorption spectra of these mutant proteins (Figure 1E). While the spectra were essentially identical for wild-type PLPHP and for the p.Pro40Leu, p.Pro87Leu, and p.Arg205Gln mutants, there was no peak at 425 nm in the spectrum of the p.Arg241Gln mutant, indicating lack of bound PLP, strongly suggesting an important decrease in the affinity of this mutant apoprotein for PLP. In turn, the p.Tyr69Cys mutant showed a decrease of ∼30% in the height of the 425 nm peak (Figure 1E). This indicates a decrease in the amount of the protonated Schiff base of protein-bound PLP formed by the reaction of PLP with Lys46 (residue identified from sequence alignment with COG0325 proteins of known structure, see below Figure 3B). Thus, this mutant protein has a reduction in the amount of normally bound PLP, what could have a negative impact on PLPHP function.
We also assessed the oligomeric nature of the different mutants by size exclusion chromatography (Figure 2B, top). Excepting the p.Tyr69Cys mutant, all the mutants were eluted as wild-type PLPHP, as monomers with a minoritary dimer content. The dimer peak appeared somewhat smaller in the case of the p.Arg241Gln mutant, possibly reflecting conformational changes between the PLP-containing and PLP-free forms (as reported with PipY, Tremiño et al., 2017) that could influence the accessibility in the PLP-free form of the exposed cysteines involved in disulfide bridge formation. However, the largest difference between mutant and wild-type was observed with the p.Tyr69Cys mutant. The fresh protein containing this mutation was eluted from the gel filtration column as two large peaks, one corresponding to the dimer and the other to the monomer (Figure 2B, top). When a part of the monomer peak was reinjected, it was predominantly monomeric (Figure 2B, middle), although there was an important amount of dimer that could have arisen from contamination of the original sample with dimer and/or from spontaneous dimer formation from the monomer. To evaluate how fast dimers were generated from monomers, we left a fraction of the monomer peak at 4°C for 12 hr. After this period, the majority of the protein was dimeric (Figure 2B, middle). Thus, it appears that the dimer and the monomer are not in rapid equilibrium, and that the dimer increases with time, as expected from dimerization due to disulfide bridge formation by the novel cysteine introduced by the mutations. This was confirmed by the elution of the protein as a monomer from the gel filtration column when the protein had been treated with 10 mM MSH (Figure 2B, bottom). Further confirmation was provided by SDS-PAGE of the p.Tyr69Cys mutant without or with treatment with MSH (Figure 1B). When subjected to this reducing treatment (Figure 1B, top) all the protein moved as a monomer, whereas when not treated with MSH (Figure 1B, bottom) much of the p.Tyr69Cys mutant migrated as a dimer. This contrasts with the behavior of all other mutants and of wild-type PLPHP, which, when not treated with MSH, migrated largely as monomers, with only a minority as dimers (Figure 1B, bottom). Also in agreement with the gel filtration results, SDS-PAGE without MSH confirmed that the dimer was less abundant in the p.Arg241Gln mutant than with the other mutants or with wild-type PLPHP (Figure 1B, bottom). It is to be noted that the dimer of the p.Tyr69Cys mutant moved in SDS-PAGE slightly faster than the dimers for the other forms (Figure 1B, bottom), whereas the movement of the monomer was identical for all forms. Thus, even in the presence of SDS there appear to be structural differences between the p.Tyr69Cys dimer and the dimers observed with the other forms of PLPHP. These differences may reflect the formation with the p.Tyr69Cys mutant of a novel type of dimer involving a disulfide bridge between two monomers mediated by the newly created cysteine found in the mutant.
3.3 Influence of the mutations on PLPHP thermal stability
We compared in thermofluor assays the thermostability of the different mutants with that of wild-type PLPHP (Figure 2C). For wild-type PLPHP the change in fluorescence with heating attained 50% of its maximum at about 56°C (Tm value) (Figure 2C). The same Tm temperature was observed with the p.Pro87Leu mutation, while the P40L, R201Q, and particularly the R241Q mutants exhibited decreased thermal stabilities, corresponding to Tm values of approximately 50°C, 49°C, and 42°C, respectively (Figure 2C). These reductions in Tm values are quite large, making it likely that the decrease in thermal stability could be an important disease-causing element for these mutants. In contrast with the observations with the p.Pro40Leu, p.Arg205Gln, and p.Arg241Gln mutations, the p.Tyr69Cys mutant was substantially more stable in the thermofluor assay than the wild-type protein (Tm, 60.7°C, nearly 5°C higher than the Tm of wild-type PLPHP) (Figure 2C). Apparently, the dimerization stabilizes the protein, protecting it from thermal unfolding.
3.4 Decreased PLP accessibility in the p.Tyr69Cys mutant, assessed using DCS
The existing evidence supports a role of PLPHP in PLP homeostasis (Darin et al., 2016; Ito et al., 2013; Plecko et al., 2017; Prunetti et al., 2016; Tremiño et al., 2017), whereby PLPHP could deliver PLP to target PLP-binding proteins. Dimerization of PLPHP could hamper such delivery and thus could be an important determinant of disease causation. Since the antibiotic DCS targets protein-bound PLP, we used DCS to monitor the degree of accessibility of PLP, since such accessibility could be an important factor for PLP delivery. Thus, we compared the dependency on the concentration of DCS of the reaction of this antibiotic with the PLP of wild-type PLPHP or of the p.Tyr69Cys mutant (Figure 2D). We also tested in the same assays the p.Pro87Leu mutant (not shown), a mutant for which no ill-effect of the mutation had been found other than a decrease in protein solubility. In these assays, we monitored the decrease in the PLP absorption peak after 20-min reaction with variable concentrations of DCS. While in these assays the wild-type and the p.Pro87Leu mutant reacted with DCS to similar degrees, the reaction of the PLP in the p.Tyr69Cys mutant was approximately 2.5-fold slower (Figure 2E), indicating that the p.Tyr69Cys substitution decreases the availability of PLP for reaction with DCS. Therefore, it appears that PLP donation by PLPHP could be importantly hampered by the p.Tyr69Cys mutation.
3.5 Overall considerations
All the missense mutations studied here caused ill effects on PLPHP (Table 1, 4th column), strongly suggesting that the epilepsy resulted from decreased PLPHP function. This agrees with the results of in silico predictions that predominantly suggested probably damaging effects for the mutations p.Pro40Leu, p.Tyr69Cys, p.Arg205Gln, and p.Arg241Gln (Plecko et al., 2017) as well as for the p.Leu175Pro mutation (our own analysis with PolyPhen-2 and MutPred servers, http//genetics.bwh.harvard.edu/pph2/, https://mutpred.mutdb.org/; Adzhubei et al., 2010; Li et al., 2009). Only for the p.Pro87Leu mutation the in silico predictions were ambiguous (Plecko et al., 2017).
Misfolding/decreased stability appear to be the major pathogenic determinants (Table 1, highlighted in bold-type) for the mutations p.Pro40Leu, p.Leu175Pro, p.Arg205Gln, and p.Arg241Gln. Gross misfolding is reflected in the complete lack of solubility of the p.Leu175Pro mutant. The p.Arg241Gln mutations also appeared to cause substantial misfolding, reflected in an important increase in the fraction of insoluble PLPHP protein (Figure 1A), which was translated in our in vitro expression assays into low recovery of pure soluble PLPHP protein (Figure 1C). In addition, the thermal stability of the p.Arg241Gln mutant was found to be drastically decreased, as it also was the case (although to a lesser extent) for the p.Pro40Leu and p.Arg205Gln mutants (Figure 2C). The p.Pro87Leu mutation also increased the fraction of the protein that was insoluble, decreasing the yield of pure PLPHP. Therefore, for five of the six reported PLPHP missense mutations the structural stability of the protein fold was decreased, indicating that PLPHP deficiency due to these mutations could be a disorder of folding/stability. Thus, a search for pharmacological chaperones (Gámez et al., 2018) should be considered as a potential alley towards therapy of destabilizing PLPHP missense mutations. PLP could be such a chaperone at least in the case of the p.Arg241Gln mutation, since this mutation primarily hampered PLP loading of PLPHP (Figure 1E and Table 1) and the apo form of PLP-proteins can be less stable than the holoproteins (Relimpio et al., 1981). Perhaps the increased PLP levels observed in these patients and their fibroblasts upon administration of pharmacological doses of pyridoxine (Darin et al., 2016; Plecko et al., 2017) can overcome the PLP loading deficiency of the p.Arg241Gln mutant, increasing in vivo the level of this mutant protein by a chaperoning effect, improving the ability of this mutant to carry out its PLP-homeostatic role. This could contribute to the relatively good outcomes of the two patients found to carry this mutation (patients 2/1 and 1/7, Table 1; both were compound heterozygotes of two missense mutations), particularly in the case of patient 1/7 in whom the second mutation, p.Pro87Leu, is considered to be very severe given the presentation observed in an homozygous patient for this last mutation (Table 1, patient 2/3, Plecko et al., 2017). This severity of the p.Pro87Leu mutation is hard to explain on the bases of our present experimental results, which only documented a substantial but incomplete decrease in the solubility and the yield of E. coli-produced recombinant mutant protein. The fact that the p.Pro87Leu mutant protein was produced in substantial level in E. coli, together with its normal loading with PLP, explain the complementation by this mutant of E. coli yggS− for its pyridoxine toxicity phenotype (Darin et al., 2016), which might depend on buffering down the intrabacterial PLP level (Prunetti et al., 2016). This buffering effect in E. coli would not be possible with the p.Arg241Gln mutant because of the intrinsic and importantly decreased affinity for PLP of this mutant. The severity of the p.Pro87Leu mutation might reflect selective loss in the human of the function of this mutant, perhaps because of accelerated mutant protein destruction by an animal-specific degradative process that would not be active against this mutant in E. coli. Alternatively, Pro87 might be important for interaction of PLPHP with its human protein targets, so that the p.Pro87Leu mutation might deprive PLPHP of at least part of its PLP homeostatic function.
As in the case of the p.Arg241Gln mutation, but to a much lesser extent, the p.Tyr69Cys mutation also hampered PLP loading of PLPHP. Perhaps a more significant effect of this mutation was the fact that the novel cysteine also caused covalent dimer formation of the p.Tyr69Cys mutant, resulting in decreased PLP accessibility, judged from the reaction of this mutant with DCS (Figure 2C and E). This decreased accessibility might be a key factor in disease-causation by this mutant form of PLPHP (Table 1, highlighted in bold-type). In any case, the results with p.Arg241Gln and p.Tyr69C support the importance of PLP in the function of PLPHP, as would be expected if this function were PLP homeostasis (Darin et al., 2016, Ito et al., 2013; Labella et al., 2017, Prunetti et al., 2016, Tremiño et al., 2017). For this function, difficulties in loading PLPHP with its PLP cargo, most prominent in the case of the p.Arg241Gln mutant, or hampering of the release of this cargo as it appears to occur with the p.Tyr69Cys mutant are likely causes of decreased PLPHP function.
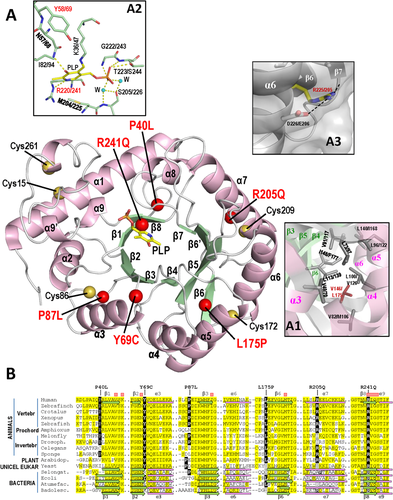
3-D structural modeling yielded for PLPHP (Figure 3A and insets therein) the expected modified TIM barrel fold that has been observed in other COG0325 members of known structure (Eswaramoorthy et al., 2003; Tremiño et al., 2017; and PDB files 1W8G, 3CPG, and 3R79), and allowed characterization of the mutation's effects for the p.Leu175Pro, p.Arg241Gln, and p.Tyr69Cys mutations. The structural model of PLPHP suggests (Figure 3A, inset A1) that the p.Leu175Pro mutation is structurally very disturbing because it causes a very drastic change (Pro is smaller than Leu and it does not have a ramified hydrophobic side-chain; its imino acid nature imposes very important conformational restrictions to the polypeptide chain; Creighton, 1993) of a virtually constant (only substituted by Ile or Val; Figure 3B) Leu in a tightly packed and crowded hydrophobic nest that glues the core β sheet of the barrel with the outlying helix layer. The lack of PLPHP in the fibroblasts of a patient that was homozygous for this mutation (Darin et al., 2016) strongly suggests that this misfolding, with removal of the protein by the proteostatic machinery, is the reason for this lack of the mutant protein despite the fact that the fibroblasts still contained substantial amount of the mRNA for PLPBP (Darin et al., 2016). The absolute lack of PLPHP in this patient fits the severity of the presentation, which required PLP instead of pyridoxine for seizure control (Table 1) as was found in patients with obligatorily null mutations of both PLPBP alleles (Darin et al., 2016).
The lack of PLP in the p.Arg241Gln mutation fits the fact that this mutation affects an invariant arginine (Figure 3B) shown by the structures of COG0325 proteins to be involved in PLP binding (Figure 3A, inset A2), since its side chain runs along the buried border of the PLP pyridoxal ring, making with it extensive van der Waals contacts and a N-H-N hydrogen bond (Tremiño et al., 2017). This explains the failure of this mutant to complement the pyridoxine toxicity phenotype of yggS− E. coli (Darin et al., 2016) since this complementation is known to depend on proper PLP binding (Ito et al., 2013). The somewhat decreased PLP content of the p.Tyr69Cys mutation may reflect the fact that Tyr69 is adjacent to an invariant PLP-binding residue (Asn68, Figure 3A, inset A2; and Figure 3B), covering it as a lid with its flat and exposed phenolic side chain. This lid also covers partially the extended side-chain of the invariant lysine that anchors covalently the PLP to the protein (Lys47 in PLPHP, Figure 3A, inset A2). Concerning the covalent dimer formation with the p.Tyr69Cys mutant, such dimerization is understandable on the basis of the introduction of a highly exposed cysteine that most likely reacts with the same neo-cysteine of another subunit. As already indicated, this dimer formation might explain the decrease in the availability of the PLP for reaction with external agents, such as DCS, possibly reflecting also hampering of donation of PLPHP-bound PLP to its potential physiological partner PLP-requiring proteins.
For the other three missense mutations, p.Pro40Leu, p.Pro87Leu, and p.Arg205Gln, the structure supports their proper binding of PLP, as observed, given their normal visible absorption spectra (Figure 1E), since these mutations do not affect residues of the PLP site. The structure could suggest some destabilizing effect of the p.Arg205Gln mutation, which maps in helix 7 of the barrel scaffold and thus could destructure somewhat this helix (Figure 3A, inset A3). Effects for the p.Pro40Leu and p.Pro87Leu mutations are more difficult to infer from the structure, since these changes, although drastic at the amino acid level (Creighton, 1993), map in loops closely preceding β strands (β1 and β3, respectively), on the N-edge of the β-sheet, the opposite side of the barrel with respect to the side where PLP sits. Further structural studies involving X-ray crystallography may be necessary to fully unveil the structural consequences of these two mutations.
ACKNOWLEDGMENTS
We thank A. García and M. Orzáez (CIPF-Valencia) for CD facilities, and Silvia Ventas for technical help. This work was supported by grants from the Valencian (PrometeoII/2014/029) and Spanish (BFU2014-58229-P and BFU2017-84264-P) governments.
DISCLOSURE STATEMENT
The authors declare no conflict of interest.