Novel CASK mutations in cases with syndromic microcephaly
Funding information: Contract grant sponsors: National Institute of Mental Health (R01 MH106826); KU Leuven (PFV/10/016 SymBioSys, GOA/12/015); Belgian Science Policy Office Interuniversity Attraction Poles (BELSPO-IAP) program (IAP P7/43-BeMGI).
Communicated by Stephen Robertson
Abstract
Mutations in CASK cause a wide spectrum of phenotypes in humans ranging from mild X-linked intellectual disability to a severe microcephaly (MC) and pontocerebellar hypoplasia syndrome. Nevertheless, predicting pathogenicity and phenotypic consequences of novel CASK mutations through the exclusive consideration of genetic information and population-based data remains a challenge. Using whole exome sequencing, we identified four novel CASK mutations in individuals with syndromic MC. To understand the functional consequences of the different point mutations on the development of MC and cerebellar defects, we established a transient loss-of-function zebrafish model, and demonstrate recapitulation of relevant neuroanatomical phenotypes. Furthermore, we utilized in vivo complementation studies to demonstrate that the three point mutations confer a loss-of-function effect. This work endorses zebrafish as a tractable model to rapidly assess the effect of novel CASK variants on brain development.
1 INTRODUCTION
Microcephaly (MC) is a clinical finding defined as reduced occipitofrontal circumference (OFC) of at least 2/3 standard deviations (SD) below the mean for sex, age, and ethnicity-matched individuals. MC can be congenital (primary MC) when detected prior to 36 weeks of gestation, or develop after birth (secondary MC); in the latter case, it generally resembles dendritic or white matter disorders (Woods & Parker, 2013). MC may present as an isolated finding or present in association with other organ malformations and/or facial dysmorphisms in the syndromic forms (Von der Hagen et al., 2014). Despite the recent improvements in whole-exome/genome sequencing (WES/WGS) technologies and their growing application in research and diagnostic settings, a significant proportion of MC syndromes remain etiologically undefined. In addition, their phenotypic heterogeneity represents a diagnostic challenge (Rump et al., 2016). For example, heterozygous and hemizygous aberrations in CASK (MIM# 300172) are associated with a wide phenotypic spectrum, ranging from X-linked intellectual disability with MICrocephaly and disproportionate Pontine and Cerebellar Hypoplasia (MICPCH) syndrome (MIM# 300749); to mild-severe ID with or without nystagmus (MIM# 300422); and FG syndrome 4 (MIM# 300422), characterized by ID and variable dysmorphic features such as hypotonia and constipation. CASK mutations mainly occur de novo, although maternal inheritance has been described (Moog et al., 2015; Reinstein, Tzur, Bormans, & Behar, 2016; Saitsu et al., 2012). CASK mutations are detected typically in females. However, affected males have been reported, and they usually display a more severe phenotype than their female counterparts. The under-representation of males among CASK mutation-bearing individuals is probably due to reduced male viability or lethality in utero (Moog et al., 2015; Najm et al., 2008).
CASK was identified initially through yeast two-hybrid screening for intracellular molecules interacting with neurexins, highly polymorphic cell surface proteins involved in the formation of synaptic junctions (Hata, Butz, & Südhof, 1996). CASK is a member of a super-family of proteins known as MAGUKs (Membrane-Associated GUanylate Kinases), multi-domain proteins characterized by the presence of a common set of structural domains that bind both polypeptide and nucleotide ligands (Kim, 1995). CASK is composed of 3 MAGUK-specific protein domains: one PDZ (PSD-95, Discs-large, ZO-1), one SH3 (src homology 3), and a GUK (GUanylate Kinase) domain. In addition, CASK contains a calcium/calmodulin-dependent protein kinase-like domain (CaMK-like) at its N-terminus (Baines, 1996; Hata et al., 1996), followed by two L27 (LIN-2 and LIN-7 interaction) domains (Lee, Fan, Makarova, Straight, & Margolis, 2002). In neurons, CASK is involved in the regulation of several processes, and each of its domains has specific binding partners with which it forms protein complexes with discrete molecular functions. For instance, at pre-synaptic sites, CASK forms a complex with MALS/Mint-1/liprin α through its CaMK and L27A domains. This complex is involved in the organization of synaptic vesicle pools, thus regulating neurotransmitter release (Olsen, Moore, Nicoll, & Bredt, 2006). Through its PDZ and SH3 domains, CASK interacts with and regulates the synaptic targeting of neurexin-1 and ion channels in a CDK5-dependent fashion (Hsueh, 2009). At post-synaptic termini, CASK binds to syndecan-2 via its PDZ domain and contributes to the regulation of axon branching and dendritic outgrowth (Cohen et al., 1998; Ethell & Yamaguchi, 1999). In a SUMOylation-dependent manner, CASK connects plasma membrane proteins such as syndecan-2 to the actin cytoskeleton via protein 4.1 and spectrin, thus stabilizing dendritic spine morphology (Chao, Hong, Huang, Lin, & Hsueh, 2008). Thus, the known versatility of CASK is likely reflected in the variable clinical presentations associated with different gene mutations (Kuo, Hong, Chien, & Hsueh, 2010; Moog et al., 2011, 2015; Najm et al., 2008).
In this study, we report the identification of previously undescribed de novo CASK mutations in four female subjects by applying WES to a cohort of undiagnosed microcephalic individuals. Additionally, we have developed a physiologically relevant zebrafish model of cask depletion, and employ in vivo complementation (Davis, Frangakis, & Katsanis, 2014; Niederriter et al., 2013) to assess variant pathogenicity. As proof of concept, we investigated the effect of three CASK variants by focusing on MC and cerebellar phenotypic readouts analogous to those commonly observed in patients with MICPCH.
2 MATERIALS AND METHODS
2.1 Library preparation, exome enrichment, and massive parallel sequencing
Genomic DNA was extracted from whole blood according to standard procedures, quantified using the Qubit® dsDNA Broad Range Assay kit on a Qubit® Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and fragmented using a Diagenode Bioruptor®. Sequencing libraries were prepared using the TruSeq™ DNA Sample Preparation kit (Illumina, San Diego, CA, USA) following the gel-free Low Throughput protocol. The SeqCap EZ Exome Enrichment v.3.0 kit (Roche, Basel, Switzerland) was used for exome enrichment according to manufacturer's specifications. Sheared DNA, full-genomic and exome-enriched library quality was assessed with either Agilent DNA 1000 or DNA High Sensitivity chips run on an Agilent 2100 Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA, USA). The samples were then sequenced on an Illumina HiSeq2500 machine in rapid mode using a paired-end 2 × 100 bp protocol. Sequence reads were aligned to the human genome reference sequence (Genome Assembly GRCh37/hg19) with the Burrows-Wheeler Aligner (BWA v. 0.7.8). SAMtools (v. 0.1.19) was used for SAM to BAM file conversion, sorting and indexing alignments. Picard tools (v. 1.118) were used to compute quality metrics and mark PCR-generated duplicates. The Genome Analysis Toolkit (GATK v. 3.2.2) software package was used to perform local realignment around indels and base quality score recalibration. SNPs and small indels were called using GATK HaplotypeCaller (v.3.2.2). Variant annotation was performed with ANNOVAR (v. 11–0882013), including data sets from dbSNP137, the NHLBI 6500 Exome (v.October 2012) and 1000 Genomes (v.April 2012) projects for variant frequencies, amino acid change, functional predictions from SIFT, PolyPhen2, LRT, MutationTaster, PhyloP, and GERP++ conservation scores.
2.2 Variant filtering and validation by Sanger sequencing
Trio-based filtering of annotated variants collected on an Excel file was conducted manually using “knownGene”-based annotations. Exonic, splicing and nonsynonymous mutations with a minor allele frequency < 1% in 1000Genomes Project, ESP6500si_ALL and dbSNP137 were retained. Genotype predictions generated by the GATK UnifiedGenotyper tool were used to filter the remaining variants according to the hypothesized inheritance patterns. Candidate variants were queried manually in the Genome Aggregation Consortium (gnomeAD) database (https://gnomad.broadinstitute.org/), NHLBI Exome Variant Server (https://evs.gs.washington.edu/EVS/), Kaviar (https://db.systemsbiology.net/kaviar/cgi-pub/Kaviar.pl), and by the in-house developed NGS-Logistic software (https://ngsl.esat.kuleuven.be/) which collects anonymized exome sequencing data generated in 5 Belgian Human Genetics Centers (Ardeshirdavani et al., 2014). Confirmation of de novo variants was performed by standard capillary electrophoresis with mutation-specific primers designed with the Primer3 web application (https://primer3.ut.ee/). PCR products were purified using the ExoSAP-ITTM PCR Product Cleanup Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and Sanger sequencing reactions performed with the BigDye® Terminator v3.1 chemistry (Thermo Fisher Scientific, Waltham, MA, USA), following bidirectional DNA sequencing with an ABI 3500 Series Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Variants were reported to ClinVar under accession numbers: SCV000611616-SCV000611619 (https://www.ncbi.nlm.nih.gov/clinvar/submitters/506392/), and to the Leiden Open Variation Database (Individual IDs: 00163761-00163763, and 00163765).
2.3 Reverse transcription PCR
Epstein–Barr virus-immortalized lymphoblastoid cell lines (LCLs) were established from individuals UZL6032 and UZL7743. We extracted total RNA using the RNeasy Mini Kit (Catalog #74104; Qiagen, Hilden, Germany) according to the manufacturer's protocol. First-strand cDNA was synthesized with oligo-dT primers using the SuperScript III First Strand Synthesis System (Catalog #18080051; Invitrogen, Carlsbad, CA, USA) and used as template for PCR using mutation-specific primers. Bands corresponding to the canonical and alternatively spliced products were gel-excised and purified with the QIAquick Gel Extraction Kit (Catalog #28704; Qiagen, Hilden, Germany), prior to Sanger sequencing.
2.4 cDNA cloning, site-directed mutagenesis, and mRNA synthesis
The human full-length CASK cDNA was obtained from GeneCopoeia (Rockville, MD, USA) (Product ID I1396, corresponding to accession # NM_003688.3) and cloned into the pCS2+ vector using Gateway® Cloning Technology (Thermo Fisher Scientific, Waltham, MA, USA ). Site-directed mutagenesis for the p.Gln37*, p.Leu209Pro, and p.Arg639* variants was performed with an in-house developed method employing Phusion High-Fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and custom-designed primers bearing the mutations of interest (Supp. Table S3) as described (Niederriter et al., 2013). Capped mRNA was synthesized in vitro and purified using the mMessage mMachine SP6 kit (Catalog# AM1340; Thermo Fisher Scientific, Waltham, MA, USA). To evaluate size and integrity, purified mRNA was run on a 2100 Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA, USA) with a RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA).
2.5 Zebrafish care and embryo injection
Adult zebrafish (Danio rerio) were maintained at 28.5°C, on a 14/10 hr light/dark cycle under standard aquaculture conditions. All experiments were performed using embryos obtained from natural mating of wild-type (WT) (AB) adults. Embryos were maintained in embryo medium and staged by days post fertilization (dpf). Splice-blocking morpholinos (MOs) were designed and synthesized by GeneTools (Philomath, OR, USA) (Supp. Table S3). Diluted MOs and in vitro synthesized human mRNAs were injected into one-to-four cell stage embryos in a volume of 1 nl. Injected embryos were maintained at 28.5°C and analyzed at the appropriate stage. RT-PCR on a batch of injected embryos was performed to determine the splicing efficiency of each MO compared with uninjected controls. Both canonical and the resulting alternatively spliced products were gel-excised using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) prior to Sanger sequencing.
2.6 Αcetylated tubulin staining and imaging of zebrafish larvae
At 3 dpf, larvae were sacrificed using tricaine methane sulfonate overdose (MS-222 Cat#A5040; Sigma–Aldrich, St. Louis, MO, USA) followed by overnight fixation in Dent's fixative (80% methanol, 20% dimethylsulfoxide). Anti-acetylated tubulin staining was performed as described (Margolin et al., 2013). Image acquisition was performed using an Olympus BX51 fluorescence microscope equipped with a COHU 4912–5010 high performance CCD camera using an Olympus UPlan FI 10x objective. Image analysis and head size measurements were performed using ImageJ software (NIH). All experiments were repeated —two to three times; head area measurements and phenotype scoring were conducted blind to the injection cocktail on 50–60 larvae per category in each experiment.
2.7 Statistical analysis
A Kolmogorov–Smirnov test (XLSTAT-free software) was used to evaluate whether the head area datasets were normally distributed; since the data distribution was not normal, the nonparametric Mann–Whitney's two-tailed test (alpha 0.05) was used to compare morphometric data from zebrafish larval batches following MO and mRNAs injections. Chi-squared tests were calculated using an online interactive tool (https://statpages.info/chisq.html) and used to evaluate differences in cerebellar phenotype proportions (alpha 0.01).
3 RESULTS
3.1 WES reveals novel CASK mutations
We evaluated three affected females who presented with sporadic postnatal progressive MC with profound ID and delayed psychomotor development (Table 1). Their ages ranged between 17 months to 25 years at last clinical follow-up. OFC at birth was within the normal range for three cases, but for the fourth OFC corresponded to −2 SD (31.5 cm). Birth length was also in the normal range for all four females (+0.6/−1.1 SD). At last assessment, OFC was significantly reduced in all individuals (−3.6/−6.6 SD), whereas length was mildly below average (−1.1/−3.1 SD). Brain imaging did not reveal clear abnormalities in two patients, whereas one had cerebellar hypoplasia. One patient presented with osteosynthesis material in the cervical spine, hence brain imaging could not be performed. Three of four affected individuals displayed neurological abnormalities such as severe hypotonia, tetraspasticity, and stiff gait. Only one girl showed a minor facial dysmorphism (hypotelorism and low-set ears). Furthermore, three of four cases also exhibited ophthalmologic anomalies (strabismus, nystagmus) and visual impairment.
| Patient | UZL6032 | UZL6596 | UZL4581 | UZL7743 |
|---|---|---|---|---|
| Mutation | chrX:g.41437788T > C c.1315-7A > G p.Met438_Ala439insHis* | chrX:g.41712431G > A c.C109T p.Gln37* | chrX:g.41524612A > G c.T626C p.Leu209Pro | chrX:g.41393958C > T c.2302+1G > A p.Gly741_His768delinsAsp |
| ClinVar Accession # | SCV000611616 | SCV000611617 | SCV000611618 | SCV000611619 |
| Gender | F | F | F | F |
| Ethnicity | European | European | European | European |
| OFC at birth | 33 cm (−1 SD) | 34 cm (0 SD) | 31.5 cm (term) (−2 SD) | 34 cm (0 SD) |
| Length at birth | 51 cm (+0.6 SD) | 47 cm (−1.1 SD) | 50 cm (0 SD) | 50.5 cm (0 SD) |
| Weight at birth | 3,500 g | 2,669 g | 3,240 g | 3,930 g |
| Age at last assessment (years) | 25 | 21 | 6 | 17 |
| OFC at last assessment | 47.3 cm (−6.6 SD) | 48 cm (−5.9 SD) | 45.2 cm (−3.6 SD) | 42 cm (−3.6 SD) |
| Length at last assessment | NA | 143 cm (−3.1 SD) | 110 cm (−1.1 SD) | 74.5 cm (−2.1 SD) |
| ID/DD | Profound | Severe | Severely delayed development | Delayed psychomotor development |
| Behavioral anomalies | No | No | No | No |
| Facial dysmorphism | No | Hypotelorism, low-set ears | No | No |
| Brain imaging | Small cerebellum | Not performed (osteosynthesis in spine) | No clear abnormalities | No clear abnormalities (age 11 months) |
| Neurological abnormalities | Tetraspasticity, dystonia | Stiff gait | Severe hypotonia | No |
| Skeletal abnormalities | No | Scoliosis | No | No |
| Gastrointestinal abnormalities | No | No | Feeding difficulties | No |
| Cardiovascular abnormalities | No | No | No | No |
| Visual impairment | No | Yes | Yes | Yes |
| Eye abnormalities | No | Strabismus | Strabismus, nystagmus | Hyperopia, nystagmus, infantile esotropia |
| Array-CGH result | Normal | Normal | Normal | Normal |
- DNA and protein numbering system follow the recommendations of the journal and Human Genome Variation Society (HGVS). Nucleotide numbering reflects DNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to the journal guidelines (www.hgvs.org/mutnomen). The initiation codon is 1. Abbreviations: CGH, comparative genomic hybridization; DD, developmental delay; F, female; ID, intellectual disability; OFC, occipitofrontal circumference; SD, standard deviation.
Molecular karyotyping was performed in all four affected individuals but no pathogenic copy number variations (CNVs) were found. We then performed WES on each case and their respective parents when possible (available for three of four cases). Trio-based filtering was conducted primarily under a de novo inheritance paradigm based on the known sporadic nature of the observed phenotypes. Parental WES was not performed for individual UZL7743, who was the product of a heterologous in vitro fertilization procedure; in this case, we screened for rare damaging variants in a literature-based list of genes known to be associated with MC, ID, and developmental delay (DD). After massive parallel sequencing, we obtained on average 87.5 million total reads per exome (between 65.1 and 112.8 million), and ∼90% of reads passed filter quality thresholds and mapped uniquely to the reference genome. We obtained an average of 20-fold enrichment of the baited region, with 61% of the target sites with a mean of 30x coverage and 88% of the target sites with 10× coverage (Supp. Table S1). We identified four de novo variants in CASK (GenBank IDs: NM_003688.3:NP_003679.2): one nonsynonymous (c.626T > C:p.Leu209Pro), one nonsense (c.109C > T:p.Gln37*), and two splicing mutations (c.1315-7A > G and c.2302+1G > A) (Figure 1A and Supp. Table S2). Except for the c.626T > C allele which is already described in ClinVar (Accession RCV000498072.1), these variants are absent from population databases [gnomAD, NHLBI Exome Variant Server (EVS), Leiden Open Variation Database, Kaviar, dbSNP147, and 1000Genomes Project], and from the in-house developed database NGS Logistics (Ardeshirdavani et al., 2014). Besides the two variants located in intronic regions near splicing sites for which damage prediction values were not available, both missense p.Leu209Pro and nonsense p.Gln37* mutations were assessed concordantly to be possibly or probably pathogenic by multiple in silico damage prediction tools (Supp. Table S2). Absence of the novel mutations in the respective biological parental couples was confirmed further by Sanger sequencing in all four cases (Supp. Figure S1).
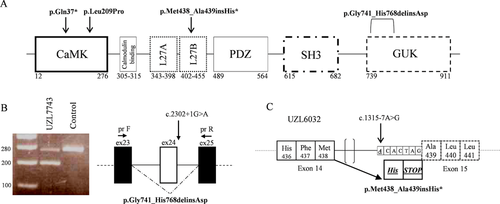
To determine the effect of the intronic variants (c.2302+1G > A and c.1315-7A > G) on mRNA splicing, we extracted total mRNA from patient-derived LCLs and monitored splicing products. We reverse transcribed LCL-derived mRNA, and used it as template for PCR amplification with primers located on exons surrounding each mutation site (Supp. Table S3). In contrast to control, the c.2302+1G > A variant resulted in two distinct PCR products as evidenced by agarose gel electrophoresis (Figure 1B). Gel purification and Sanger sequencing revealed that the larger product corresponded to the canonical transcript while the lower alternative band was the product of exon 24 skipping (NP_003679.2: p.Gly741_His768delinsAsp).
To determine the effect of the c.1315-7A > G variant, we first performed an in silico splicing analysis of both canonical and mutation-containing sequences using the Human Splicing Finder (HSF) v.3.0 tool (Desmet et al., 2009). Introduction of the de novo variant was predicted to disrupt an enhancer splice site and to generate a novel acceptor site. Experimental validation confirmed this prediction; RT-PCR amplification of LCL-derived cDNA resulted in a single product when visualized on gel, but Sanger sequencing on the amplified product showed a mixture of two different transcripts. Deconvolution of the mixed sequencing traces revealed the presence of both canonical and mutant transcripts (Figure 1C). The latter was generated by activation of an alternative acceptor splice site introduced by the c.1315-7A > G transition resulting in the insertion of a histidine and a premature stop codon (NP_003679.2: p.Met438_Ala439insHis*).
3.2 In vivo analysis of CASK variants in developing zebrafish
To establish a rapid assay that could distinguish between hypomorphic and severely damaging CASK point mutations and to possibly identify more detailed genotype-phenotype correlations, we developed a transient zebrafish CASK suppression model. We employed this model to investigate two MICPCH characterizing traits in particular: MC and cerebellar defects. Both clinical features have been employed previously in the evaluation of candidate genes for neurocognitive disorders (Beunders et al., 2013; Borck et al., 2015). Previous genotype–phenotype observations hypothesized that females with hypomorphic CASK mutations display a milder phenotype compared with those bearing severely disrupting variants (Burglen et al., 2012; Moog et al., 2011, 2015). Two of our cases displayed point mutations, namely the missense CaMK domain p.Leu209Pro and the early truncating p.Gln37*. Notably, both females displayed similarly severe clinical features, however the typical cerebellar abnormalities observed in MICPCH cases were absent in the case with the p.Leu209Pro variant or could not be investigated in the p.Gln37* mutation-bearing individual due to spinal osteosynthesis.
Reciprocal BLAST of the longest human CASK isoform (NP_003679.2, 921 amino-acids) with D. rerio identified two orthologs, with 93% and 94% identity with zebrafish Caska (NP_694420.1, 920 amino-acids) and Caskb (NP_001135848.1, 921 amino-acids) proteins, respectively. Since both zebrafish cask proteins display > 90% similarity with the human ortholog, we designed splice-blocking morpholinos (sb-MOs) targeting both caska and caskb. We targeted caska exon 2 (E2I2) and exon 7 (E7I7); and caskb exon 20 (E20I20) donor splice sites. Splicing effect and efficiency of each MO were determined on reverse-transcribed cDNA derived from whole embryos injected with each MO using exonic primers flanking the targeted splicing junction (Supp. Table S3 and Supp. Figure S2A and C). Sequencing demonstrated that caska E2I2 MO caused skipping of exon 2 and introduction of a frameshift resulting in a premature termination codon (p.Gly21ProfsTer26), whereas the E7I7 MO produced a partial intron 7 retention with activation of a premature stop codon (p.Lys236_Met237insValValTer). For caskb, the E20I20 MO resulted in retention of intron 20 and a subsequent frameshift (p.Leu405Valfs) (Supp. Figure S2B and D).
To assess phenotypes relevant to MC, we first tested the effect of each MO on 3 dpf larval head-size by measuring the area between the eyes (Figure 2A–F). Compared with controls, we achieved a ∼10% reduction in head size with caska sb-MOs (8 ng, E2I2: 92% and E7I7: 91%, P = 0.0005 and <0.0001, respectively) and a 13% reduction for caskb knock-down (8 ng, P < 0.0001) (Supp. Figure S2E). Next, we established specificity of the phenotype by coinjection of either sb-MO with WT human mRNA (Figure 2G and H). For caska, we performed rescues using the E7I7 sb-MO which appeared to be more efficient on RT-PCR (Supp. Figure S2A) and with a more significant dose-dependent effect compared with the E2I2 MO (Supp. Figure S2E). We rescued the MC phenotype with 150 pg of WT human mRNA in both caska (95%, P = 0.052) and caskb (97%, P = 0.34) morphants such that they were indistinguishable from controls.

To further confirm specificity and to possibly observe phenotypic changes associated to the different type of variants, we performed in vivo complementation studies for both caska and caskb morphants with mRNA containing the point mutations of interest. We included a positive control for our zebrafish assays, the pathogenic nonsense p.Arg639* variant (rs137852815) (Najm et al., 2008), which should fail to rescue head size phenotypes for either MO. In vivo testing of all three mutant alleles resulted in head size defects comparable to the effect of both caska and caskb MOs alone, although p.Gln37* exerted a slightly more severe effect (P = 0.003 and 0.02 vs. caska and caskb MOs, respectively). We observed modestly different effects of the mutant alleles compared with WT for the caska MO rescue experiments (Figure 2G) with the p.Gln37* being the most significantly different from WT rescue (P < 0.0001), followed by the pathogenic p.Arg639* (P = 0.0026; positive control variant) and missense p.Leu209Pro (P = 0.019). However, for caskb MO rescues, the three mutant alleles were equally damaging compared with the WT allele rescue (Figure 2H, P < 0.0001).
Next, we used the morphant model to investigate the cerebellar phenotype associated with CASK mutations. We performed anti acetylated tubulin staining on 3 dpf larvae to mark axon tracts (Figure 3A) and scored them according to the organization and integrity of the cerebellar structure as normal (symmetric and intact corpus cerebelli, with dense and intact axon tracts across the midline), moderately affected (intact corpus cerebelli with reduction or absence of axon tracts across the midline), and severely affected (hypoplastic corpus cerebelli with absent axon tracts across the midline, agenesis) (Margolin et al., 2013). caska and caskb sb-MOs caused marked cerebellar defects in 48.7% and 61.1% of larvae, respectively, compared with less than 5%–7% of uninjected controls (Figure 3B and C). This effect was specific since coinjection of WT human mRNA rescued the cerebellar phenotype induced by caska or caskb knock-down significantly (17.6% and 14.5% abnormal larvae, respectively). Moreover, coinjection of the mutant alleles was not effective in rescuing the cerebellar phenotype in either of caska or caskb morphants. For caska, nonsense mutations exerted a slightly more severe effect than the sb-MO alone (p.Gln37* rescue = 67.0%; p.Arg639* rescue = 60.0% of abnormal larvae compared with 48.7% in caska morphants, P = 0.002 and 0.003, respectively). However, for caskb all mutant mRNAs were equally not significantly different from MO. For both zebrafish cask paralogues, all variants were significantly worse compared with WT rescue.
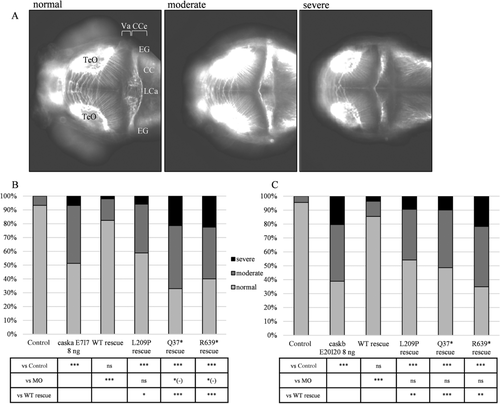
4 DISCUSSION
In a whole exome screen of cases with syndromic MC, we identified four novel CASK mutations. To confirm the role and the functional effect of these variants, we developed a transient loss-of-function zebrafish cask model. Our complementation assays revealed that caska and caskb morphants display a head area reduction between 10% and 13%, respectively, compared with controls, and this decrease is rescued by coinjection with WT human CASK mRNA in both caska and caskb morphants. On the contrary, the missense p.Leu209Pro and the two nonsense p.Gln37* and p.Arg639* mutations do not rescue the microcephalic phenotype. In addition, either caska or caskb MO injection results in 50%–60% larvae with a variable degree of cerebellar abnormality. For both cask paralogs, human WT mRNA is able to rescue the cerebellar anomalies significantly, whereas the mutant alleles fail to rescue when coinjected with either MO. Hence, for both phenotypes, complementation assays indicate that all three mutant alleles are functional null variants. In summary, we demonstrate that a zebrafish morphant model can be used to evaluate the functional effect of CASK variants on the development of MC and cerebellar defects.
In our cohort, only the individual with the intronic c.1315-7A > G mutation displayed cerebellar hypoplasia. This variant results in a protein lacking PDZ, SH3, and GUK domains. The affected female carrying the c.2302+1 G > A variant, resulting in exon 24 skipping, did not display clear brain abnormalities at 11 months. Interestingly, a few other individuals carrying splicing mutations proximal to the same position have been reported previously (Supp. Table S4). We demonstrated by RT-PCR that the c.2302+1 G > A mutation results in the partial loss of the C-terminal GUK domain of CASK (p.Gly741_His768delinsAsp). The same consequence was demonstrated in an individual carrying a de novo c.2302+5 G > A variant (Burglen et al., 2012). For the other three individuals, the splicing effect was not determined (Hayashi et al., 2017; Rump et al., 2016; Takanashi et al., 2012), but we suspect it to be identical to the other two cases. Besides two individuals for which clinical data were scarce, the other two presented severe ID/DD, brain defects, spasticity and ophthalmologic abnormalities. The affected female from our cohort did not present such a severe phenotype. Considering her young age though, her clinical course might deteriorate, although a wide variability in clinical phenotype has been observed even among cases with variants predicted to have similar effects on protein functionality (Dunn et al., 2017).
In our cohort, three girls showed ophthalmologic anomalies and two of them specifically displayed nystagmus. Previous reports have indicated that congenital nystagmus is strongly associated with mutations localized in the GUK domain of CASK (Dunn et al., 2017; Hackett et al., 2010; Watkins et al., 2013). This domain interacts with FRMD7, a plasma membrane-cytoskeleton coupling protein, mutations of which cause X-linked congenital nystagmus (Tarpey et al., 2006). Nystagmus was present in the individual with the p.Gly741_His768delinsAsp mutation, involving the initial part of GUK domain. However, this feature was also present in the girl carrying the p.Leu209Pro variant, located in the CaMK domain of the protein. We cannot exclude that this missense mutation might affect the protein structure. For example, it has been shown that the p.Arg28Leu variant alters the splicing machinery, resulting in an out-of-frame exon 2-skipped transcript as a consequence of an altered recognition of Exonic Splicing Enhancer motifs (Piluso et al., 2008). Nystagmus has not been observed in individuals with CASK nonsense mutations or gene-disrupting CNVs, and was absent in our two cases carrying pre-GUK terminating mutations. This observation suggests that the absence of the GUK domain may not be a sufficient condition for the development of nystagmus in these affected individuals, as could however be the aberrant FRMD7/CASK interaction caused by mutations altering GUK structure and functionality.
There are a few modest discrepancies in the severity of the cerebellar phenotype observed in caska and caskb complementation assays (Supp. Table S5). This is possibly due to different effects of the MOs employed in these assays, which may not cause a reduced protein expression via nonsense mediated decay, but rather generate shorter alternative proteins with novel functions. Our aim though was not to investigate the different functions of caska and caskb in zebrafish. Nonetheless, we propose that the two genes are not functionally redundant given that microcephalic and cerebellar phenotypes were evident in both caska and caskb morphants individually. Comparing our results with those resulting from coinjecting both caska and caskb MOs could elucidate a putative functional divergence between the two paralogs. In addition, the generation of caska and caskb mutants using genome editing would be beneficial to strengthen our findings.
In conclusion, we identified four novel mutations in CASK as the cause of syndromic MC and used the zebrafish model to evaluate differences in cerebellar phenotype and head size by complementation assays using different types of point mutations, thus highlighting the possibility to establish a tractable pipeline to screen novel CASK variants in the future. Since CASK interacts with several proteins through its different protein domains, the generation of constructs bearing mutations interrupting a specific protein–protein interaction would be useful to elucidate their individual physiological significance on brain development and synapse maintenance (Huang & Hsueh, 2009). In addition, comparison of rare variants both within the CASK locus as well as the genomic background across affected individuals will prove critical toward elucidating phenotype-genotype correlations for CASK-associated disorders.
ACKNOWLEDGMENTS
The authors wish to thank the patients and family members involved in this study. Furthermore, the authors thank the Aquatic Facility of KU Leuven for adult zebrafish maintenance and care. F.C. is PhD aspirant and H.V.E. is Clinical Investigator of the Research Foundation-Flanders (Fonds Wetenschappelijk Onderzoek, FWO, Belgium).
DISCLOSURE STATEMENT
The authors declare no conflict of interest.
STATEMENT OF ETHICS
Genetic studies were approved by the Institutional Review Board of the University Hospitals of Leuven, and informed consent was obtained from the parents of each affected child for WES. The animal care and experimental procedures were carried out in accordance with the ethical committee guidelines for laboratory animal experimentation at KU Leuven.