Functional characterization of protein variants of the human multidrug transporter ABCC2 by a novel targeted expression system in fibrosarcoma cells†
Communicated by William S. Oetting
Abstract
The multidrug resistance-associated protein 2 (MRP2/ABCC2) is involved in the efflux of endogenous and xenobiotic substrates, including several anticancer and antiviral drugs. The functional consequences of ABCC2 protein variants remain inconsistent, which may be due to shortcomings of the in vitro assays used. To study systematically the functional consequences of nonsynonymous ABCC2 variants, we used a novel “Screen and Insert” (ScIn) technology to achieve stable and highly reproducible expression of 13 ABCC2 variants in HT1080 cells. Western blotting revealed lower (30–65%) ABCC2 expression for D333G, R1174H, and R1181L as compared with wild type (WT; 100%), whereas the linked variant V1188E/C1515Y resulted in higher expression (150%). R1174H caused mislocalization of ABCC2 to the cytoplasm with an endoplasmic reticulum-like distribution. Variants N1244K and R1174H decreased transport of glutathione–methylfluorescein (GS–MF) and glutathione–monochlorobimane (GS–MCB) by 80% and 50%, respectively, whereas R1181L and P1291L reduced only GS–MCB transport by 50% as compared with WT. Contrary to protein data, the double variant V1188E/C1515Y decreased specific transport activity for GS–MF and GS–MCB by 40%. The ScIn approach is a feasible and reliable method to functionally characterize systematically ABCC2 variants. D333G, R1174H, R1181L, N1244K, P1291L, and double variant V1188E/C1515Y have been identified as most promising for further clinical evaluation. Hum Mutat 33:750–762, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
Interindividual variability in drug response is well recognized in clinical practice and an increasing body of evidence indicates that this phenomenon may explain differences in the efficacy of a drug and/or the development of adverse drug reactions [Wang et al., 2011]. In addition to environmental and other nongenetic factors, variation in ADME (absorption, disposition, metabolism, excretion) genes is an important contributor to variability in drug response. As a result, testing for specific genetic variants in drug-metabolizing enzymes is recommended by the US Food and Drug Administration before initiating treatment with certain drugs (www.fda.gov/Drugs/ScienceResearch/ResearchAreas/ Pharmacogenetics/ucm083378.htm).
In addition to drug-metabolizing enzymes, membrane transporter proteins are increasingly recognized as an important determinant of drug response because they can affect therapeutic efficacy by influencing cellular uptake and the efflux of drugs [Giacomini et al., 2010]. Members of the ATP-binding cassette (ABC) superfamily are of particular interest. The most significant example is the multidrug resistance protein 1 (MDR1/ABCB1) [Deeley et al., 2006]. This influences chemoresistance by acting as a natural barrier to the entry of drugs, which can in part be explained by genetic variability [Schwab et al., 2003]. Another ABC transporter, the multidrug resistance-associated protein 2 (MRP2), encoded by the ABCC2 gene (MIM #601107), is a widely expressed human membrane transporter localized in the apical luminal membrane of polarized epithelial cells of excretory organs and in the blood–brain barrier [Keppler, 2011]. ABCC2 actively exports not only anionic drug conjugates (e.g., glucuronates, sulfates, and glutathiones) but also many unconjugated substances, thereby contributing significantly to detoxification in humans. Moreover, ABCC2 confers chemoresistance to common anticancer agents such as doxorubicin, cisplatin, irinotecan, vinblastin, and camptothecin derivatives [Jemnitz et al., 2010].
To date, more than 320 naturally occurring ABCC2 sequence variations have been identified in the single nucleotide polymorphism database (dbSNP), with substantially different frequency distributions between ethnic groups [Choi et al., 2007; Haenisch et al., 2008; Ito et al., 2001a; Itoda et al., 2002; Niemi et al., 2006; Sai et al., 2008]. At least 45 ABCC2 missense variants have been reported so far (Pharmacogenetics Research Network Website: http://www.PharmGKB.org; National Centre for Biotechnology Information [NCBI]: http://www.ncbi.nlm.nih.gov/SNP) [Nies and Keppler, 2007]. Of note, some rare ABCC2 missense variants are linked to the genetic disorder Dubin–Johnson syndrome, characterized by conjugated hyperbilirubinemia, and these variants affect the accumulation, localization, and function of the ABCC2 protein [Nies and Keppler, 2007]. Other naturally occurring ABCC2 missense variants alter the pharmacokinetics of ABCC2 substrates such as methotrexate, lopinavir, carbamazepine, doxorubicin, and talinolol [Elens et al., 2009; Haenisch et al., 2008; Hulot et al., 2005], contributing in part to the interindividual variability of drug response in patients with antiepileptic therapy (e.g., carbamazepine), and may explain doxorubicin-induced cardiotoxicity [Kim et al., 2010; Wojnowski et al., 2005]. Nevertheless, there is a need for systematic examination of the subcellular localization and functionality of ABCC2 variants in vitro because data on their functional consequences are conflicting or in part still missing.
In vitro transport studies measuring either the uptake of radiolabeled substrates into isolated membrane vesicles or the uptake and release of fluorescent compounds by intact cells [Hirouchi et al., 2004; Ryu et al., 2000] require the establishment of transient or stable transfection of expression plasmids. Transient transfection is advantageous for fast analysis of genes and small-scale protein production, whereas stable transfection allows large-scale protein production as well as long-term, reproducible, and defined gene expression [Colosimo et al., 2000]. Thus, a major application for stable transfection is the analysis of gene function and regulation. One of the experimentally important issues involving stably transfected cells is the vector-mediated introduction of complementary DNA (cDNA) into the host chromosome, which occurs at unpredictable sites. Depending on the site of integration, the flanking sequences are likely to modulate DNA expression.
We previously established a convenient “Screen and Insert” (or ScIn) method for isolating stably transfected clones [Brough et al., 2007]. Using this method, transgenes are reproducibly integrated by site-specific recombination at a particular target locus chosen for its ability to support transgene expression that is stringently regulated by tetracycline [Brough et al., 2007]. This approach is particularly attractive for undertaking functional comparisons of variants in membrane transporters such as ABCC2 because it eliminates the variations between cell clones caused by different chromosomal position effects on the integrated transgene.
Here, for the first time, we have systematically applied the ScIn method to the study of natural ABCC2 missense variants in a HT1080 (human fibrosarcoma) cell host. Known and unknown variants were identified by sequence analysis of genomic DNA samples from Korean and African-American subjects. We provide convincing evidence that several ABCC2 missense variants alter the transport activity and/or accumulation of ABCC2, demonstrating that ScIn is a promising method for further research, particularly in the field of human membrane transporter proteins.
Materials and Methods
Construction of Enhanced Green Fluorescent Protein (EGFP) Tagged and Untagged hABCC2(V1188E/C1515Y) Expression Plasmids
A 5.3-kB cDNA encoding the human ABCC2 variant V1188E/C1515Y (GenBank accession number U49248.1) was subcloned into the vector pGEM3 provided by Professor Dr Piet Borst, The Netherlands Cancer Institute, Amsterdam, The Netherlands [Evers et al., 1998]. A 1806-bp fragment of the insert of ABCC2.pGEM3 was amplified by polymerase chain reaction (PCR) using the oligonucleotides 5′-GAAGACGAAGA-ACTAGTGAAAGGAC-3′ (containing a SpeI site) and 5′-TAAC-CCATGGGGCCTCCCGGGAGAA-3′ (containing an artificially introduced NcoI site in the 3′-untranslated region (UTR) and an artificially introduced SmaI site that removes the stop codon) as sense and antisense primers, respectively. The PCR fragment was cloned into the SpeI/NcoI site of ABCC2.pGEM3. The insert of the resulting plasmid (ABCC2.pGEM3) was subcloned in frame into the HindIII/SmaI sites of pEGFP-N1 (Clontech, Heidelberg, Germany). The resulting plasmid was ABCC2(V1188E/C1515Y)–EGFP.pEGFP-N1.
A 2.4-kb fragment of the insert of ABCC2.pGEM3 was amplified by PCR using the oligonucleotides 5′-GAAGACGAAGA-ACTAGTGAAAGGAC-3′ (containing a SpeI site) and 5′-CTACAG-GCGGCCGCCCATAGTAGTACTGCTTAT-3′ including the ABCC2 stop codon (containing a NotI site). The PCR fragment was cloned into the SpeI/NotI site of ABCC2(V1188E/C1515Y)–EGFP.pEGFP-N1 obtaining ABCC2(V1188E/C1515Y).pEGFP-N1 without an EGFP tag.
In Vitro Site-Directed Mutagenesis of ABCC2
Site-directed mutagenesis in ABCC2 was performed using the QuikChange mutagenesis kit (Stratagene, Heidelberg, Germany) and the oligonucleotides 5′-GAAACACAATGAGGtGAGGATT-GACACCAA-3′ and 5′-GAAGATTATAGAGTgCGGCAGCCCTGA-AGA-3′. The variants c.3563A>T and c.4544A>G were removed from ABCC2 cDNA to obtain ABCC2(WT)–EGFP.pEGFP-N1 and ABCC2(WT).pEGFP-N1. All other ABCC2 variant forms were created using ABCC2(WT)–EGFP.pEGFP-N1 as a template. Mutagenesis primers are reported in Supp. Table S1. For all ABCC2 protein variants, the correct cDNA sequences were verified by sequence analysis.
Insertion Plasmids
The cytomegalovirus (CMV) promoter was removed from ABCC2(WT, variants)–EGFP.pEGFP-N1 and ABCC2(WT).pEGFP-N1 as a PciI/NheI fragment, and a lox66 site (annealed oligonucleotides 5′-CATGATAACTTCGTATAGCATACATTATAC-GAACGGTAGAAT-3′ and 5′-CTAGATTCTACCGTTCGTATAATG-TATGCTATACGAAGTTAT-3′) was cloned into the PciI/NheI sites to generate the promoterless insertion constructs lox66–ABCC2(WT, variants)–EGFP.pEGFP-N1 and lox66–ABCC2(WT).pEGFP-N1.
Cell Culture and Stable Transfection
Conditions used for the culture of HT1080 cells and derivatives (Rht14-10) have been described previously [Brough et al., 2007]. When required, the medium was supplemented with one or more of the following drugs: G418 (750 µg/ml; Biochrom, Berlin, Germany), doxycycline (1 µg/ml; Sigma–Aldrich GmbH, München, Germany), and zeocin (200 µg/ml; InvivoGen, San Diego, CA). Metafectene Pro (Biontex Laboratories GmbH, München, Germany) was used for delivery of insertion constructs lox66–ABCC2(WT, variants)–EGFP.pEGFP-N1 and lox66–ABCC2(WT).pEGFP-N1 in Rht14-10 cells and in transient expression of Cre-recombinase. For Cre-mediated insertion, 2 µg of each insertion construct and 2 µg of pMC-Cre15 (kindly donated by H. Gu, University of Köln) were used per well (2 × 105 cells per six-well plate), respectively. The following day, the cells were seeded into 10 cm plates and the appropriate drug selection (+ G418) was added after a further 24 hr. After 2 weeks, G418-resistant colonies were picked for further analysis (PCR, immunoblotting, confocal fluorescence microscopy, fluorescence-activated cell sorting [FACS], and transport activity).
Cell Culture and Transient Transfection
Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco's modified eagle medium (Lonza Verviers Spl, Verviers, Belgium) supplemented with 10% fetal bovine serum (PAA Laboratories GmbH, Pasching, Austria), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine (Invitrogen GmbH, Karlsruhe, Germany) at 37°C. For transient transfections, cell cultures were set up 24 hr prior to transfections, either in six-well plates (Nalge Nunc International, Rochester, NY) at 2 × 105 cells/well or in four-well chamber slides (Nalge Nunc International) at 4 × 104 cells/well. HEK 293 cells were transfected with ABCC2(WT, variants)–EGFP.pEGFP-N1 and ABCC2(WT).pEGFP-N1. Transfections were carried out using Metafectene Pro (Biontex Laboratories GmbH). Two days after transfection, proteins were either extracted from the cells for immunoblotting or the ABCC2(WT, variants)-EGFP expression was analyzed under the confocal laser scanning fluorescence microscope.
PCR
Genomic DNA was isolated from Rht14-10 (negative control) and Rht14-10/lox66-ABCC2-EGFP.pEGFP-N1 cells by standard method using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). PCR reactions were conducted in a reaction volume of 50 µl with 50 ng of genomic DNA, 5 µl 10× PCR buffer, 200 µM dNTPs, 10 pmol of each primer (P1 [pTARG4_f]: 5′-AGAACGTATGTCGAGGTAGG-3′, P2 [d2EGFP_r]: 5′-CGGACTGGGTGCTCAGGTAG-3′, and P3 [ABCC2_r_1]: 5′-GCAAAACCAGGAGCCATGTG-3′) (Biomers, Ulm/Donau, Germany), and 2.5 units of Taq DNA polymerase (Qiagen). PCR fragments were generated in a MJ Research PTC-200 thermo cycler (Bio-Rad, München, Germany) with an initial denaturation step of 3 min at 94°C, followed by 32 cycles of denaturation at 94°C for 60 sec, annealing for 60 sec at 60°C, and extending for 1 min at 72°C.
Preparation of Crude Membrane Fractions
Crude membrane fractions from stably (Rht14-10) and transiently (HEK 293) transfected cells were prepared as described previously [Cui et al., 1999]. Cells were disrupted by sonication in hypotonic buffer (0.5 mM sodium phosphate, pH 7.3). After centrifugation (20,800 g, 4°C, 1 hr) using a E1175 rotor (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany), pellets were resuspended in hypotonic buffer (0.5 mM sodium phosphate, pH 7.3) and stored at −80°C until use. All membranes were prepared in the presence of proteinase inhibitor (0.1 mM phenylmethylsulfonyl fluoride).
Immunoblotting
On the day of immunoblot analysis, crude membrane fractions were diluted with Laemmli sample-loading buffer after determining the protein concentration by the Smith method [Smith et al., 1985]. Same amounts of protein (10 µg of crude membrane lysates) from WT and 12 variant ABCC2 samples were separated by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then semidry-blotted onto nitrocellulose membranes (Whatman GmbH, Dassel, Germany). Staining of the ABCC2 protein was achieved by sequential incubations in blocking buffer (5% nonfat milk powder, 0.05% TBS-T) for 1 hr at room temperature; a 1:500 dilution of mouse anti-ABCC2 monoclonal antibody (M2III-6; Alexis, San Diego, CA) in TBS-T (10 mM Tris–HCl, pH 8.0; 150 mM NaCl; 0.05% Tween-20) containing 1% nonfat milk powder and 0.05% Tween-20 overnight at 4°C; three times in TBS-T for 10 min at room temperature; a 1:5,000 dilution of peroxidase-conjugated goat antimouse immunoglobulin G (IgG) secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in TBS-T containing 1% nonfat milk powder and 0.05% Tween-20 for 1 hr at room temperature; three times washed in TBS-T; and finally detected in Super Signal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Rockford, IL) for 5 min. The membranes were then immediately analyzed with the AIDA image analyzer (Raytest GmbH, Straubenhardt, Germany), up to two different exposure times were used for quantification. For standardization, membranes were stained with anti-β-tubulin monoclonal antibody (1:10,000; Sigma–Aldrich, St. Louis, MO) and peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5,000; Santa Cruz Biotechnology). Protein expression levels were analyzed semiquantitatively by densitometry with AIDA 2.31 software (Raytest GmbH).
Confocal Fluorescence Microscopy
Fluorescence analysis of stably (Rht14-10) and transiently (HEK 293) transfected cells with ABCC2(WT, variants)-EGFP constructs was performed in four-well chamber slides. Images were taken with a confocal laser scanning microscope (model LSM510; Carl Zeiss, Oberkochen, Germany) equipped with a Plan-Appochromat 63×/1.4 oil objective.
Flow Cytometry
Cells containing doxycycline (or tetracycline)-regulated ABCC2–EGFP (approximately 2 × 105 cells) were plated into medium with or without doxycycline 0/16/24/32/48/72/96 hr prior to FACS analysis in 10 cm plates, and HT1080 cells were used as reference cells. Cells, generally no more than 80% confluent on the day of FACS analysis, were first washed in phosphate-buffered saline (PBS), trypsinized, and then resuspended in 500 µl (per 1/2 million cells) of cold PBS before storing on ice. Prior to flow cytometry, the samples were mixed by pipetting up and down. On a high-flow-rate setting, 10,000 cells were counted per sample, using an Argon ion laser turned to 488 nm (fluorescence channel FL-1, with fluorescence channel FL-3 as a negative control) on a Becton Dickinson FACScan machine (Becton Dickinson, Franklin Lakes, NJ). The analysis of data was carried out using CellQuest software (Becton Dickinson), and plots are displayed as FL-1 (log scale) versus the number of counts.
Glutathione–Methylfluorescein and Glutathione–Monochlorobimane Transport Assay
Excretion measurements were made 48 hr after seeding stable ABCC2(WT, variants)-EGFP and ABCC2(WT)-expressing Rht14-10 cells. The excretion assay medium used was PBS containing 0.1 mmol/l Ca2+ and 1 mmol/l Mg2+ (PBS-CM, Hanks Buffer) (PAA Laboratories GmbH, Pasching, Austria) supplemented with 10 mM HEPES, pH 7.4 (PAA Laboratories GmbH). Cells were washed twice with ice-cold excretion assay medium and then incubated at 4°C for 1 hr with ice-cold excretion assay medium containing 5 µM 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen GmbH) or 100 µM monochlorobimane (MCB, Sigma–Aldrich GmbH). Thereafter, the cells were washed twice with ice-cold excretion assay medium and incubated with or without doxycycline either at 4°C or 37°C for different time points in excretion assay medium. In a cyclosporine A-directed (Sigma–Aldrich GmbH) inhibition assay, cells were incubated with culture medium containing 10 µM cyclosporine A at 37°C for 60 min before preloading of CMFDA or MCB. Cyclosporine A, 10 µM, was also included in the preloading and excretion period. At designated time points, 100µl aliquots of the medium were taken, and the fluorescence of glutathione–methylfluorescein (GS–MF) and glutathione–monochlorobimane (GS–MCB) was determined by measuring the fluorescence at excitation of 490 nm and emission of 520 nm and at excitation of 355 nm and emission of 460 nm in a Wallac 1420 Victor2 multilabel plate counter (Wallac, Turku, Finland), respectively. At the end of the experiment, the cells were solubilized by adding 1 ml of 0.1% Triton X-100 in PBS, and the GS–MF and GS–MCB fluorescence of cell lysate was measured. In addition, cells not treated with CMFDA and MCB were solubilized by adding 1 ml of 0.1% Triton X-100 in PBS, and the EGFP background fluorescence of cell lysate was measured and substracted. The excreted amount of GS–MF and GS–MCB was normalized by the amount of GS–MF and GS–MCB that remained in the cells after excretion measurement.
DNA Samples
Anonymous blood samples for DNA extraction were taken from 48 Korean and 47 African-American healthy volunteers. The African-American DNA samples were purchased from Genomics Collaboratives Inc. (Cambridge, MA), and Korean samples were provided by Dr. Jae-Gook Shin, Department of Clinical Pharmacology, Pusan Paik Hospital, Inje University College of Medicine, Korea. Genomic DNA was isolated by standard methods using the Qiagen blood isolation kit (QiaAmp DNA Blood BioRobot 9604 Kit; Qiagen) on a Qiagen 9604 biorobot.
DNA Sequencing Strategy
The genomic DNA of all Korean and African-American subjects were sequenced for genetic variations in ABCC2 including all 32 exons, adjacent intronic regions, and 1.5 kB of the 5′-UTR region. The sequences of purified PCR fragments were analyzed on an ABI3700 capillary sequencer (ABI, Weiterstadt, Germany) and assembled using the Phred-Phrap, Consed, and Polyphred software (University of Washington, Seattle, WA). The sequences were inspected for deviations from ABCC2 genomic sequence (Reference sequence number NT_030059.13), which was defined as reference. Details regarding the primers, optimized PCR conditions and subsequent purification and sequencing of the fragments are available upon request.
Mutation Nomenclature
The indicated cDNA numbers are relative to the ATG site starting with +1 corresponding to the A of the ATG translation initiation codon and based either on the cDNA sequence from GenBank accession number NM_000392.3 or U49248.1. The promoter sites are relative to the A of the ATG translation initiation codon starting with the 5′-following base being −1. Numbering of intronic variants is relative to the corresponding exon. Intronic variants are designated with (+) or (−) for variants located upstream or downstream of an exon, respectively. Nucleotide changes in the intronic and promoter region are from the accession number NT_030059.13.
Computational Haplotype and Structural Analysis
ABCC2 haplotypes and their frequencies were statistically inferred in each ethnic group using PHASE, version 2.1 (University of Washington) [Stephens et al., 2001] and included all identified single-nucleotide polymorphisms (SNPs), respectively. Analyses were run five times, and all haplotypes estimated in five out of five runs are given. Prediction of functional effects of ABCC2 missense variants was calculated by PolyPhen 2 (http://genetics.bwh.harvard.edu/pph2/).
Statistics
Data are expressed as means ± standard deviation (SD) or means ± standard error of the mean (SEM) of at least three independent experiments. Data comparisons among groups were performed by unpaired t-test or one-way analysis of variance (ANOVA) followed by Dunnett's post test. For all calculations, the GraphPad Prism program (version 4.03) was used (GraphPad Software Inc., San Diego, CA). P ≤ 0.05 was considered statistically significant.
Results
Generation of Stable and Stringently Tetracycline-Regulated ABCC2(WT)–EGFP Expressing Rht14-10 Cell Clones
We first established that ABCC2(wild type, WT) was properly tetracycline-regulated and inserted into the plasma membrane following site-specific integration of an ABCC2–EGFP fusion cassette in Rht14-10 cells. An outline of the basic insert approach for ABCC2 in Rht14-10 cells is shown in Figure 1A. Rht14-10 cells are stably transfected with tetracycline transactivator protein and with a single integration of tetracycline-regulated d2EGFP reporter integrated into HT1080 cells (Supp. Fig. S1) [Brough et al., 2007]. Rht14-10 cells were cotransfected with a Cre-expression plasmid (pMC-Cre) and a promoterless ABCC2(WT)–EGFP insertion construct, which was linked to a lox66 site, positioned upstream of ABCC2 (lox66–ABCC2–EGFP.pEGFP-N1). Cre-mediated recombination placed ABCC2(WT)–EGFP successfully under the control of the tetracycline-responsive promoter (TRP) as assessed by PCR (Fig. 1B). Rht14-10 cells lost the typical cytosolic d2EGFP expression and showed a clear ABCC2(WT)–EGFP fluorescence in the plasma membrane and in intracellular membranous structures as detected by confocal laser scanning microscopy (Fig. 2A). As shown in Figure 2B, immunoblot analysis using the monoclonal antibody M2III-6 confirmed ABCC2(WT)–EGFP to be a mature glycoprotein of ∼220 kDa, which is not normally expressed at detectable levels in Rht14-10 cells. Furthermore, ABCC2–EGFP expression disappeared after 72 hr exposure to doxycycline (1 µg/ml) (Fig. 2A and B). FACS analysis revealed the kinetics of ABCC2–EGFP regulation by doxycycline (1 µg/ml) or its analogue tetracycline (1 µg/ml) (Supp. Fig. S2A–D). It took up to 120 hr for ABCC2–EGFP expression to return to background level (Supp. Fig. S2A–C) and ≥96 hr to reestablish ABCC2–EGFP expression (Supp. Fig. S2B and C). As demonstrated elsewhere [Rennel and Gerwins, 2002], we found that tetracycline is more easily and reliably removed from the cells than doxycycline to reinduce expression from the TRP. Down-regulated background fluorescence was as low as fluorescence in parental HT1080 cells, whereas noninhibited fluorescence was approximately 800-fold higher. Supp. Figure S2D compares the results of Western analysis for ABCC2–EGFP protein expression with various FACS profiles. While ABCC2–EGFP protein disappeared after 48 hr of exposure to doxycycline, ABCC2 protein-dependent EGFP fluorescence was still present in ABCC2–EGFP cells and approximately 60-fold higher than in nonfluorescing cells. Thus, at least for the antibody used in this analysis, immunoblotting was less sensitive than measurement of EGFP by FACS.
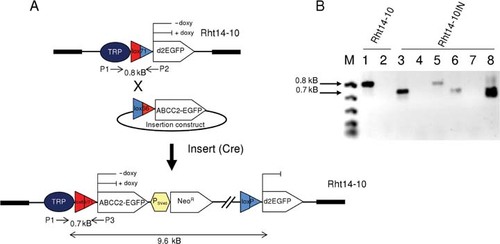
Cre-mediated insertion of lox66–ABCC2(WT)–EGFP.pEGFP-N1 in the Rht14-10 cell line. A: Schematic of the target locus in clone Rht14-10 before and after Cre-mediated insertion of lox66–ABCC2(WT)–EGFP.pEGFP-N1. PCR primers and expected products are indicated. B: Agarose gel of electrophoresed PCR products of DNA isolated from clone Rht14-10 (lanes 1and 2) and three selected Rht14-10 ABCC2(WT)–EGFPG418r clones (lanes 3–8). PCR products are generated either with the primer pairs P1/P2 (lanes 1, 4, 5, and 7) or with P1/P3 (lanes 2, 3, 6, and 8). All clones were positive for the 700-bp PCR product diagnostic for the expected integration. Two clones (lanes 3/4 and 7/8) were of high purity and one clone (lanes 5/6) showed an additional 800-bp PCR product, indicating an Rht14-10 impurity. For further analyses, only the high-purity clone was used.
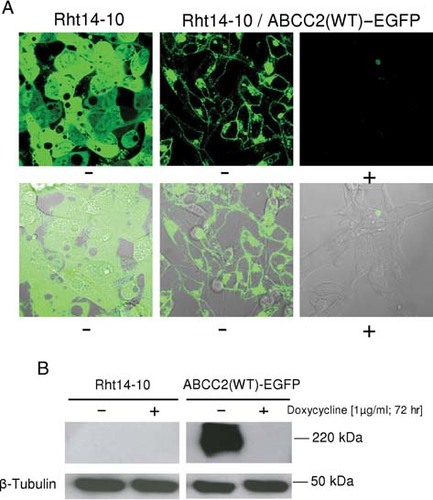
Localization and expression of ABCC2(WT)–EGFP in Rht14-10 cells. A: Using confocal laser scanning microscopy, EGFP fluorescence and overlapping fluorescence/bright-field images of ABCC2(WT) in stably transfected Rht14-10 cells are shown. Cells were grown with (+) or without (−) doxycycline (1 µg/ml) for 72 hr. B: Stably ABCC2(WT)–EGFP expressing Rht14-10 cells grown with (+) or without (−) doxycycline (1 µg/ml) for 72 hr were analyzed by immunoblotting using the monoclonal antibody M2III-6 and crude membrane fractions protein.
Carboxylfluorescein and MCB Transport Assay of ABCC2 Activity
To assess the transport activity of ABCC2, excretion of the fluorescent substrates GS–MF and GS–MCB from stably ABCC2–EGFP-expressing Rht14-10 cells was examined [Ryu et al., 2000]. The nonfluorescent CMFDA was used as a prodrug for ABCC2. CMFDA is well absorbed by the cells at a low temperature (4°C), and the green fluorescent metabolite GS–MF is formed intracellularly by the action of esterase and glutathione S-transferase [Roelofsen et al., 1997]. Like GS–MF, GS–MCB is also a fluorescent glutathione conjugate synthesized from MCB within the cells. Cells preloaded with 5 µM CMFDA or 100 µM MCB were incubated in medium without these fluorescent substrates, and the excreted GS–MF or GS–MCB in the medium was determined to be time dependent and temperature dependent in the presence and absence of doxycycline or the ABCC2 inhibitor cyclosporine A (Fig. 3A–D). GS–MF and GS–MCB was not excreted by the cells at 4°C, whereas at 37°C, both ABCC2 substrates were actively excreted by ABCC2 (Fig. 3A and B). The excretion rate of GS–MF and GS–MCB into the medium was approximately 4.5-fold and 3.5-fold higher, respectively, than that seen with control Rht14-10 cells (t = 15 min) (Fig. 3A). Following extracellular addition of doxycycline (1µM) for 3 days, substrate efflux was reduced by up to 100% due to downregulation of ABCC2 expression (Fig. 3C). In addition, efflux could be significantly reduced in the presence of the ABCC2 inhibitor cyclosporine A (Fig. 3D). Taken together, the ScIn method clearly demonstrated that ABCC2–EGFP is functionally expressed and regulated with high stringency following its targeted integration (Figs. 1–3, Supp. Fig. S2). Additional control experiments in tetracycline-regulated and stably ABCC2-expressing Rht14-10 cells using GS–MF and GS–MCB as substrates showed that an N-terminal EGFP tag does not alter the expression or function of ABCC2 (Supp. Fig. S3).
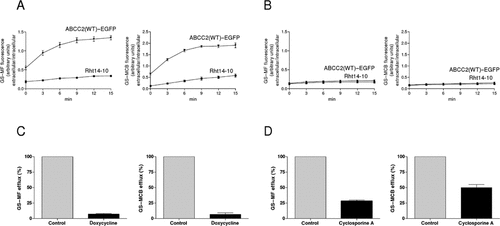
Efflux of GS–MF and GS–MCB by ABCC2(WT)–EGFP from Rht14-10 cells. Efflux of the intracellular formed ABCC2 substrates GS–MF and GS–MCB measured in stably transfected ABCC2(WT)–EGFP-expressing Rht14-10 cells. A: Time dependent over 15 min at 37°C. B: Time dependent over 15 min at 4°C. C: After exposure to doxycycline (1 µM) for 3 days at 37°C. D: After exposure to the ABCC2 inhibitor cyclosporine A (10 µM) at 37°C. The fluorescence of GS–MF and GS–MCB was measured before and after lysis of cells, resulting in the GS–MF and GS–MCB efflux (shown as %). Experiments were performed in triplicate (mean ± SD). The results indicate ABCC2-specific transport.
ABCC2 Variants and Haplotypes in Korean and African-American Subjects
Sequencing of DNA samples from 48 healthy Korean and 47 African-American subjects revealed 15 and 36 genetic variants, respectively, in the ABCC2 gene. The frequency distribution of all known SNPs in both cohorts was similar to public data (e.g., NCBI dbSNP database, http://www.ncbi.nlm.nih.gov/snp/) and no variant showed a deviation from the Hardy–Weinberg equilibrium. The added allele frequency of the 13 ABCC2 missense variants in African-Americans, including one novel nonsynonymous SNP, was significant higher (64%) than in Koreans (four missense variants, one novel nonsynonymous SNP, 12%). On average, each African-American subject carried at least one ABCC2 missense variant. A summary of all ABCC2 variants, their sequence context, and frequency distribution for both ethnic groups are given in Supp. Table S2.
ABCC2 haplotypes were inferred by PHASE for Koreans and African-Americans separately. A total of 15 and 40 haplotypes, respectively, were constructed resulting in seven common haplotypes for both ethnic groups (KO_ABCC2_1,2,3,4,5,6,7; AA_ABCC2_1,7,14,16,30,33,38) (Supp. Table S3 and Supp. Table S4).
Prediction of Functional Effects of ABCC2 Protein Variants by Evolutionary Conservation and Structural Features
On the basis of our sequence analyses and public SNP databases (NCBI, PharmGKB), 13 ABCC2 missense variants were prioritized for functional analysis based on the following three criteria: (1) evolutionary conservation, (2) structural features, and (3) allele frequency ≥1% (Table 1). Computational comparative genomic studies using PolyPhen 2 predicted that nine of these ABCC2 variants (F39Y, D333G, I670T, I1036T, R1174H, R1181L, V1188E, N1244K, and P1291L) would be located within the transmembrane regions, close to the ATP-binding domain of ABCC2 or close to two missense variants (I1173F, R1150H) causing Dubin–Johnson syndrome [Keitel et al., 2003; Mor-Cohen et al., 2001]. Five of the variants (D333G, R1174H, R1181L, N1244K, and P1291L) were predicted to have a functional effect. A summary of all 13 selected ABCC2 missense variants is presented in Table 1 and Supp. Figure S4.
| rs# NCBI | Genetic variationa | Amino acid | Ethnicity | Allele frequency (%) | Nb | Data source | Predicted phenotypec |
|---|---|---|---|---|---|---|---|
| rs927344 | c.116T>A | F39Y | AA | 2.3 | 86 | Current study | Benign |
| AA | 2 | 200 | pharmGKB | ||||
| KO | 0 | 94 | Current study | ||||
| rs17222674 | c.998A>G | D333G | AA | 0 | 88 | Current study | Probably damaging |
| AA | 1 | 200 | pharmGKB | ||||
| KO | 0 | 94 | Current study | ||||
| rs7080681 | c.1058G>A | R353H | AA | 6.7 | 90 | Current study | Benign |
| AA | 3.5 | 200 | pharmGKB | ||||
| KO | 0 | 94 | Current study | ||||
| rs17222589 | c.1457C>T | T486I | AA | 0 | 84 | Current study | Benign |
| KO | 3.7 | 82 | Current study | ||||
| JA | 5 | 20 | pharmGKB | ||||
| JA | 2.3 | 144 | Itoda et al. (2002) | ||||
| rs17222632 | c.2009T>C | I670T | AA | 1.1 | 92 | Current study | Benign |
| AA | 1.5 | 200 | pharmGKB | ||||
| KO | 0 | 92 | Current study | ||||
| rs41318029 | c.2761G>A | G921S | AA | 0 | 84 | Current study | Benign |
| KO | 0 | 92 | Current study | ||||
| CA | 1 | 120 | NCBI dbSNP | ||||
| rs45441199 | c.3107T>C | I1036T | AA | 0 | 96 | Current study | Benign |
| AA | 0.5 | 200 | pharmGKB | ||||
| KO | 0 | 94 | Current study | ||||
| CA | 0.5 | 198 | pharmGKB | ||||
| CA | 1 | 120 | NCBI dbSNP | ||||
| rs139188247 | c.3521G>A | R1174H | AA | 2.3 | 86 | Current study | Probably damaging |
| KO | 0 | 94 | Current study | ||||
| JA | 1 | 144 | Itoda et al. (2002) | ||||
| rs8187692 | c.3542G>T | R1181L | AA | 5.8 | 86 | Current study | Probably damaging |
| AA | 8.5 | 200 | pharmGKB | ||||
| KO | 0 | 96 | Current study | ||||
| rs17222723 | c.3563T>A | V1188E | AA | 5.8 | 86 | Current study | Benign |
| AA | 6.5 | 200 | pharmGKB | ||||
| KO | 0 | 96 | Current study | ||||
| CA | 7.5 | 200 | pharmGKB | ||||
| c.3732T>G | N1244K | JA | 1.5 | 144 | Itoda et al. (2002) | Possibly damaging | |
| rs17216317 | c.3872C>T | P1291L | AA | 4.7 | 86 | Current study | Probably damaging |
| AA | 2 | 86 | pharmGKB | ||||
| KO | 0 | 86 | Current study | ||||
| CA | 0.5 | 196 | pharmGKB | ||||
| rs8187710 | c.4544G>A | C1515Y | AA | 13 | 92 | Current study | Benign |
| AA | 19.6 | 194 | pharmGKB | ||||
| KO | 0 | 94 | Current study | ||||
| CA | 8 | 198 | pharmGKB |
- AA, African-American; CA, Caucasian; KO, Korean; JA, Japanese.
- aNucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference ABCC2 sequence NM_000392.3.
- bNumber of chromosomes analyzed.
- cPrediction was calculated by PolyPhen 2 (http://genetics.bwh.harvard.edu/pph2/), pharmGKB (www.pharmGKB.org), NCBI dbSNP (http://www.ncbi.nlm.nih.gov/snp/).
Expression Levels of ABCC2 Missense Variants in HEK 293 Cells
To investigate the effect of the selected missense variants on ABCC2 protein expression, expression vectors (ABCC2–EGFP.pEGFP-N1) for these variants were generated by site-directed mutagenesis and transiently transfected into HEK 293 cells. Because V1188E and C1515Y are linked, only the double variant V1188E/C1515Y was investigated. The Dubin–Johnson syndrome-causing variant ABCC2 I1173F served as a positive control for reduced ABCC2 expression. Endogenous expression of ABCC2 was not evident in HEK 293 cells. Immunoblots of the variants F39Y, R353H, T486I, I670T, G921S, I1036T, N1244K, and P1291L showed similar expression compared with WT (range 85–110% of WT). Staining of ABCC2 was weaker for D333G, I1173F, R1174H, and R1181L (range 30–55% of WT, P < 0.01), whereas higher expression was found for the double variant V1188E/C1515Y (150% of WT, P < 0.01) (Supp. Fig. S5).
Cellular Localization of ABCC2 WT and Variants in HEK 293 Cells
The variable ABCC2 expression in HEK 293 cells carrying the D333G, R1174H, R1181L, and V1188E/C1515Y variants suggests that processing or protein stability of ABCC2 could be affected by these variants. Consequently, we determined the intracellular localization of ABCC2 variants by confocal laser scanning microscopy (Supp. Figs. S6 and S7). For all variants except D333G and R1174H, ABCC2 protein was localized to intracellular membranous structures, indicating that processing and targeting of these variants seems to be comparable to WT. In contrast, the transmembrane variant D333G showed a punctuated localization in the plasma membrane. Moreover, sparse intracellular distribution of the variant R1174H was found by examination of several fields in separate transfection experiments.
Establishment of Rht14-10 Cell Lines Stably Expressing ABCC2 with Missense Variants
The CMV promoter of all ABCC2(variants)–EGFP.pEGFP-N1 constructs was replaced with a lox66 site to generate the promoterless insertion constructs lox66-ABCC2(variants)–EGFP.pEGFP-N1. Following the transfection step for isolating stably ABCC2(variants)–EGFP-expressing Rht14-10 cell clones involved 14 days selection in G418, followed by the analysis of colonies using PCR and UV microscopy for EGFP fluorescence, immunoblotting, and FACS. Pure clones positive for the 700-bp PCR product diagnostic for the expected integration (Supp. Fig. S8) were selected. For additional investigations, clones were grown in the presence or absence of doxycycline for 72 hr and subsequently analyzed by FACS (Supp. Fig. S9). All ABCC2(variant)–EGFP-expressing clones down-regulated their EGFP expression upon the addition of doxycycline to the medium. Interestingly, the variable noninhibited EGFP value measured by the peak channel was approximately 800-fold for ABCC2 (WT) and approximately 500-fold (R1174H) and 1500-fold (V1188E/C1515Y) for ABCC2 variants compared with nonfluorescing cells (HT1080). With the exception of V1188E/C1515Y, the degree of downregulation after 72 hr did not seem to depend upon the type of variant construct used and the particular clone analyzed. The EGFP value was approximately 10-fold for ABCC2 (WT and 11 variants) compared with 20-fold for the ABCC2 double variant V1188E/C1515Y. Those clones that demonstrated uniform expression profiles and negligible inhibited expression (+dox, 72 hr) were chosen for a comparative analysis of ABCC2 transport activity, expression, and localization.
Protein Expression of ABCC2 Variants
To elucidate the effect of ABCC2 variants on protein expression, we investigated Rht14-10 cell lysates from stably expressing ABCC2(variants)–EGFP by immunoblotting 3 days after seeding out (Fig. 4). All variants revealed ABCC2 protein expression but with significant differences. D333G, R1174H, and R1181L exhibited a 40%, 70%, and 35% reduction in protein expression, respectively, whereas the double variant V1188E/C1515Y resulted in a 150% increase in protein expression compared with WT (P < 0.01). All remaining variants showed no significant changes.
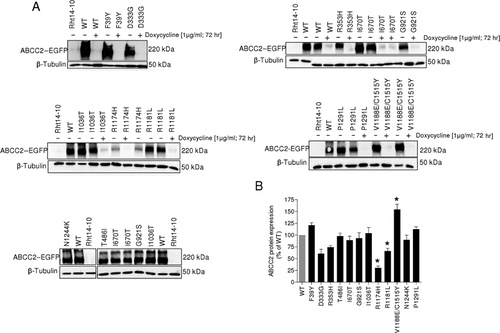
Expression of ABCC2 variants in Rht14-10 cells. A: Western blot results of Rht14-10 cells stably expressing ABCC2(WT, variants)–EGFP. Cell experiments were performed with or without doxycycline treatment (1 µg/ml, for 72 hr). Subsequently, crude membrane fractions were isolated and the specific monoclonal antibody (M2III-6) was used for detection of ABCC2. B: Protein expression was analyzed semiquantitatively by densitometry (AIDA image analyzer). For standardization, ABCC2 protein levels were normalized by β-tubulin expression. ABCC2(WT) expression was set as 100%. At least three experiments for each variant were performed (mean ± SD). *indicates a P value < 0.01 compared with ABCC2(WT) expression.
Functional Analysis of ABCC2 Variants
To determine whether the variants affected ABCC2 transport activity, efflux of GS–MF and GS–MCB from Rht14-10 cells was determined (Fig. 5A and B). Data revealed a high variability in efflux activity for both substrates. The N1244K variant showed a significant decrease of transport activity, with a 15% and 26% efflux of GS–MF and GS–MCB, respectively, compared with ABCC2 WT (100%) (P < 0.01, one-way ANOVA followed by Dunnett's post test). The activity of R1174H was reduced by approximately 50% for both substrates compared with WT (GS–MF, 56%; GS—MCB, 54%; P < 0.01). Interestingly, the variant R1181L resulted in an increased efflux of GS–MF (137%, P < 0.01) and a decreased efflux of GS–MCB compared with WT (59%, P < 0.01). The D333G, R353H, T486I, G921S, and P1291L variants showed a lower transport activity for GS–MCB (71%, 60%, 75%, 63%, and 51% of WT, P < 0.01) but not for GS–MF, whereas the variants F39Y, I1036T, and V1188E/C1515Y did not alter ABCC2-mediated transport for either substrate.
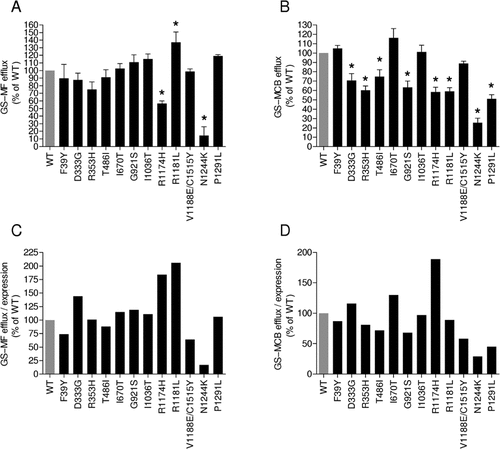
Efflux of GS-MF and GS-MCB by ABCC2(variants)-EGFP from Rht14-10 cells. A and B: Time-dependend efflux of the intracellular formed GS-MF and GS-MCB measured in stably transfected ABCC2(WT)-EGFP expressing Rht14-10 cells at 37°C. The fluorescence of GS-MF and GS-MCB was measured before and after lysis of cells resulting in the GS-MF and GS-MCB efflux (shown as %). Each result represents the mean ± SEM (n = 3–5) at 15 min. *indicates a P value < 0.01 compared with the efflux of GS-MF and GS-MCB from ABCC2(WT). C and D: Specific transport activity of ABCC2 variants for GS-MF and GS-MCB in Rht14-10 cells. Mean efflux values (see A and B) were divided by the respective mean protein expression values obtained by immunoblotting (see Fig. 4). Data are shown as a percentage of the specific transport activity of ABCC2 WT (100%).
Comparison of Transport to Protein Expression
To determine the specific activity of ABCC2 variants, we studied the consequences for protein expression and transport activity (Fig. 5C and D). Only N1244K caused a major decrease of ABCC2-specific activity (up to 80% for GS–MF and GS–MCB when compared with WT), whereas protein expression was similar to WT. In contrast, R1174H (30% of WT) and R1181L (65% of WT) caused a twofold increase in specific GS–MF and/or GS–MCB efflux compared with WT. The P1291L variant caused a 50% decrease in specific GS–MCB transport activity without alteration of ABCC2 protein. Notably, the double variant V11888E/C1515Y showed the highest protein expression level but caused a 40% decrease in specific efflux of GS–MCB and GS–MF.
Localization of ABCC2 Variants
The subcellular distribution of stably expressing WT and the selected missense variant ABCC2–EGFP in Rht14-10 cells were examined using confocal laser scanning microscopy (Fig. 6 and Supp. Fig. S10). Consistent with the findings in HEK 293 cells, ABCC2 WT and all variants except R1174H were predominantly localized to the cell surface. The R1174H variant was localized primarily in the cytoplasm with an endoplasmic reticulum (ER)-like pattern distribution. The D333G variant, which showed punctuated localization in HEK 293 cells, was predominantly localized to the Rht14-10 cell surface.
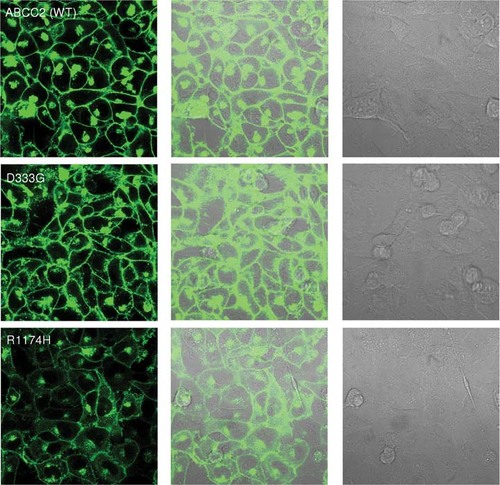
Subcellular localization of ABCC2 variants D333G and R1174H in Rht14-10 cells. ABCC2 localization was analyzed by confocal laser scanning microscopy using the EGFP as fluorescent (green).
EGFP Kinetics of the ABCC2 Variants D333G, R1174H, R1181L, and V1188E/C1515Y
To elucidate whether protein stability will affect degradation of ABCC2 after adding of doxycycline (1 µg/ml), we investigated EGFP kinetics of the ABCC2 D333G, R1174H, R1181L, and V1188E/C1515Y variants expressed by Rht14-10 cells. The mean GFP fluorescence of all cells at each time point was measured by FACS analysis before and after adding of doxycycline. No differences in EGFP kinetics for the ABCC2 variants D333G, R1174H, R1181L, and V1188E/C1515Y were detected, indicating no changes in protein stability (Supp. Fig. S11).
Discussion
The expression of genes depends not only on their constitutional behavior and regulatory phenomena (e.g., induction or inhibition) but also upon epigenetic factors that may be significantly altered by chromosomal position effects at the site of transgene integration. We recently described the ScIn method, a two-step protocol, to isolate stable cell clones for genes of interest that are stringently regulated by tetracycline [Brough et al., 2007]. This system takes chromosomal position effects into account and has the advantage of enabling the study of genetic variants by simultaneous elucidation of protein expression, cellular localization, and/or functionality. The method is highly accurate and sensitive, as well as labor saving and time saving.
We employed the ScIn technique in an analysis of the functional consequences of genetic variants in an important human transporter protein, ABCC2, which contributes to the energy-dependent efflux of both endogenous and exogenous compounds. ABCC2 was selected as the gene of interest due to its relevance for the disposition of several therapeutic agents (e.g., lopinavir, carbamazepine, and talinolol) [Elens et al., 2009; Haenisch et al., 2008; Kim et al., 2010] and effect on drug response. Data on the functionality of previously identified nonsynonymous variants in ABCC2 are incomplete. Using the ScIn method, we generated stable clones in fibrosarcoma cells (HT1080) that stringently regulate the expression of ABCC2 (WT, variants) in order to perform systematic analyses of 13 selected ABCC2 missense variants on protein expression, localization, and function. The preselected cell clone Rht14-10, derived from HT1080 cells with stringently tetracycline-regulated d2EGFP expression, was applied [Brough et al., 2007].
Stably integrated ABCC2(–EGFP) in HT1080 cells synthesizes an intact surface membrane protein with the molecular mass of glycosylated ABCC2(–EGFP) that is not endogenously expressed. Transport studies using two chemically distinct and well-established ABCC2 fluorescent substrates (GS–MF and GS–MCB) displayed robust ABCC2 activities similar to those observed in other mammalian cell lines (COS-7, CHO, HEK 293) [Hashimoto et al., 2002; Mor-Cohen et al., 2001; Ryu et al., 2000].
Compared with the ABCC2 reference sequence, the expression of ABCC2 was reduced for the three missense variants D333G, R1174H, and R1181L (range 30–65% of WT), whereas the double variant V1188E/C1515Y revealed a significantly higher expression (150% of WT). Altered protein expression of the ABCC2 variants D333G, R1174H, R1181L, and V1188E/C1515Y seems not to be the direct consequence of impaired or increased protein stability (Supp. Fig. S11), thereby suggesting alteration in synthesis or stability of the mutant ABCC2 mRNA.
No other variant altered the expression of ABCC2. Regarding the localization of ABCC2 in the HT1080 cells, ABCC2–EGFP could be detected in the plasma membrane by confocal fluorescence microscopic analysis for each of the ABCC2 variants except R1174H. Here, ABCC2–EGFP was mislocalized and detected mainly in the cytoplasm with an ER-like distribution, a phenomenon that was confirmed in HEK 293 cells. Of note, mislocalization of this type has already been described for another ABCC2 variant, R1173F, which causes the Dubin–Johnson syndrome in humans [Keitel et al., 2003; Mor-Cohen et al., 2001]. Moreover, D333G plasma membrane localization was reduced in HEK 293 cells, and showed a striking punctiform pattern. This matches the protein data but is in contrast to the localization findings in HT1080 cells. Because the cell type may influence localization of ABCC2 and polarized cells may be a more physiological system, it remains still open whether the alteration in membrane localization of the two ABCC2 variants D333G and R1174H in HEK 293 and/or HT 1080 cells is due to missing proteins, which are required for sorting or a result of an incomplete polarity of the HEK 293 and HT1080 cells in culture.
Generally, GS–MCB efflux was affected to a greater extent by the ABCC2 variants than GS–MF transport. N1244K was associated with a maximal decrease of approximately 80% in overall efflux of GS–MF/MCB compared with WT, in contrast to the protein data. Moreover, the R1174H variant resulted in a decrease of overall transport activity of approximately 50% for both the substrates, GS–MF and GS–MCB, whereas only GS–MCB efflux was reduced with R1181L and P1291L. In the case of R1174H and R1181L, altered transport capacity was associated with a lower protein expression. As it is well known that transport activities may differ among various substrates when using the same variant, it is not surprising that the R1181L variant resulted in a twofold increase in efflux for GS–MF but not for GS–MCB. Interestingly, although the double variant V1188E/C1515Y showed the greatest expression of ABCC2 protein (150% of WT), this did not result in an increased specific efflux of GS–MF and GS–MCB because transport activities were virtually similar to WT. Finally, GS–MF and/or GS–MCB efflux for all other ABCC2 variants (F39Y, D333G, R353H, T486I, I670T, G921S, and I1036T) showed only minor alterations compared with WT based on our definition of at least 50% change in transport activity. We investigated the prototypic ABCC2 substrates GS–MF and GS–MCB. Transport activities for other ABCC2 substrates such as glucuronide-conjugated substrates may be affected differently. Because the data on ABCC2 expression in HT1080 cells are comparable to those in HEK 293 cells, we suggest that stably transfected HT1080 cells provide a suitable cell model for further in vitro studies of membrane transporters such as ABCC2.
The results of our functional characterization of various ABCC2 variants suggest several conclusions but may also provide a springboard for further research activities. The selected variants R1174H, R1181L, and V1188E (linked to C1515Y) from the present study, in addition to two recently identified variants that cause Dubin–Johnson syndrome (R1150H, I1173F), are located at the same gene region between the transmembrane helices TM15 and TM16. As it has been shown that ABCC2 expression, localization, and/or transport activity in vitro are altered by each of these variants [Keitel et al., 2003; Mor-Cohen et al., 2001], this cytoplasmic region appears to be important for the correct processing of ABCC2, that is, expression and transport activity. In vitro mutagenesis studies of artificially generated ABCC2 transmembrane variants to identify determinants of substrate specificity and affinity have so far focused primarily on transmembrane helices TM6 through TM17 [Ryu et al., 2000]. Ryu et al. (2000) indicated the involvement of TM6, TM9, TM16, and TM17 as critical substrate binding sites of ABCC2 [Ryu et al., 2000]. Three of the four transmembrane variants studied (F39Y [TM1]), D333G [TM6]), I1036T [TM13], and N1244K [TM17]) were localized in the TM6 to TM17. The impact of TM6 and TM17 on the transport function of ABCC2 is consistent with our observation for D333G (TM6) and N1244K (TM17), showing a decreased transport activity and/or expression. Moreover, a substitution of W1254 by conserved and nonconserved amino acids (e.g., W1254A, W1254Y) in TM17 seems to be critical for substrate binding and transport activity [Ito et al., 2001b]. In contrast, TM13 appears to be of minor importance for ABCC2 function, which is supported by our data that the I1036T variant, located at TM13, did not induce any alteration in proteins or functionality [Ryu et al., 2000].
The total allele frequency for all functionally relevant ABCC2 protein variants (D333G, R1174H, R1181L, V1188E/C1515Y, and P1291L) was highest in the African-American subjects, at approximately 20%. As shown by the haplotype analysis of our sequencing data, the functional variants D333G, R1174H, R1181L, and P1291L were not linked to each other or to other variants in African-Americans. The double variant V1188E/C1515Y has been associated with differences in tissue expression by several in vivo studies, resulting in possible consequences for disease susceptibility and/or drug response, underscoring the clinical importance of ABCC2 V1188E/C1515Y [Elens et al., 2009; Grisk et al., 2009; Meier et al., 2005; Ni et al., 2010; Sookoian et al., 2009; Wojnowski et al., 2005]. Because the R1174H, R1181L, and P1291L variants impaired transport activity by approximately 50%, considerably alteration of the pharmacokinetics of ABCC2 substrates may be expected, resulting in potential clinical consequences such as adverse drug reactions. Of note, a frequency distribution of these variants in African-Americans with 2.3% up to 8.5% was found, which is not rare. In contrast, the D333G and N1244K variants, which also significantly impaired ABCC2 transport activity, are extremely rare. Because the Dubin–Johnson syndrome follows a recessive trait typically caused by rare loss of function ABCC2 variants, one can assume that only homozygous variant carriers may be at risk to develop clinical consequences (e.g., drug-induced liver injury). Further clinical studies are warranted to elucidate this issue in more depth.
Evolutionary conservation of the polymorphic amino acids among different ABCC orthologs and homologs is shown in Table 2. The human ABCC2 WT residue was highly conserved only for D333G, R1174H, R1181L, and P1291L compared with sequences of ABCC2 orthologs and other ABCC homologs. This was consistent with the observation that conserved amino acids have stronger effects compared with those that are less conserved. With the exception of N1244K, these data are in line with our in vitro results.
| Protein | Speciesa | F39Y | D333G | R353H | T486I | I670T | G921S | I1036T | R1174H | R1181L | V1188E | N1244K | P1291L | C1515Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCC2 | Human | F | D | R | T | I | G | I | R | R | V | N | P | C |
| Mouse | F | D | P | K | V | S | I | R | R | K | N | P | Y | |
| Rat | F | D | S | N | V | S | I | R | R | K | N | P | Y | |
| Rabbit | F | D | P | N | V | G | L | R | R | I | N | P | Y | |
| Rhesus | F | D | R | T | M | S | I | R | R | V | N | P | Y | |
| ABCC1 | Human | Y | D | T | T | V | G | I | R | R | L | Y | P | Y |
| Mouse | Y | D | R | T | V | G | A | R | R | L | Y | P | C | |
| Rat | Y | D | R | T | V | V | V | R | R | L | Y | P | C | |
| Macaque | Y | D | T | T | V | G | I | R | R | L | Y | P | Y | |
| Dog | Y | D | K | T | V | G | I | R | R | L | Y | P | C | |
| ABCC3 | Human | Y | D | P | A | V | G | F | R | D | T | Y | E | F |
| Mouse | Y | N | P | T | V | V | L | R | D | T | Y | P | F | |
| Rat | Y | D | P | T | V | G | L | R | D | A | Y | P | F | |
| ABCC4 | Human | – | E | Y | S | V | R | L | R | R | A | Y | P | Y |
| ABCC5 | Human | – | Q | A | Y | C | P | I | H | E | E | Y | P | E |
| ABCC6 | Human | Y | D | P | H | V | K | I | R | P | A | A | P | S |
| ABCC11 | Human | – | C | E | K | C | T | C | H | D | R | I | Q | F |
- Multiple sequence alignment was performed using ClustalW (www.ebi.ac.uk/clustalw). Bold text indicates the ABCC2 reference amino acid sequence for human, mouse, rat, rabbit, and rhesus.
- aSwiss-Prot accession numbers: human ABCC2, Q92887; mouse Abcc2, Q8VI47; rat Abcc2, Q63120; rabbit Abcc2, Q28689; rhesus Abcc2, Q4U3V2; human ABCC1, P33527; mouse Abcc1, O35379; rat Abcc1, Q8CG09; macaque Abcc1, Q864R9; dog Abcc1, Q95M36; human ABCC3, O15438; mouse Abcc3, B2RX12; rat Abcc3, O88563; human ABCC4, O15439; human ABCC5, O15440; human ABCC6, O95255; human ABCC11, Q96J66.
When PolyPhen 2 (http://genetics.bwh.harvard.edu/pph) was used to evaluate the ABCC2 missense variants in this study, D333G, R1174H, R1181L, N1244K, and P1291L were predicted as deleterious (Table 1). Interestingly, these functional predictions were in accordance with our experimental results. All amino acid substitutions causing Dubin–Johnson syndrome were correctly classified in this database as “probably damaging” [Nies and Keppler, 2007].
In summary, for the first time, our study provides evidence that the ScIn method permits reliable and valid functional characterization of ABCC2 protein variants in the fibrosarcoma fibroblast HT1080 cells by stringently tetracycline-regulated and stably expressed ABCC2 variants. The ScIn approach has at least three advantages over the standard approach of immunoblot screening of multiple clones with randomly integrated ABCC2. First, the preselected cell clone Rht14-10 requires no further screening to identify a cell clone with optimal ABCC2 expression characteristics. Second, tetracycline regulation of ABCC2 is likely to be far more stringent and lacking in cellular heterogeneity. Third, the same protocol can be used repeatedly for different ABCC2 variants to generate clones whose phenotypes can be compared without the complication of differential chromosomal loci responsible for altered expression and activity. Moreover, convincing evidence of highly variable GS–MF and GS–MCB transport activity and ABCC2 expression is provided by the functional characterization of ABCC2 nonsynonymous variants. The D333G, R1174H, R1181L, N1244K, P1291L, and V1188E/C1515Y variants showed the greatest alterations in function and/or expression in the ScIn in vitro system. Further studies are needed to elucidate the clinical consequences of this altered function.
Acknowledgements
We gratefully acknowledge the expert technical assistance of Sabine Rekersbrink and Ute Gödtel-Armbrust. We would also like to thank Jae-Gook Shin for providing us with anonymous blood samples from healthy Korean volunteers.
Disclosure Statement: The funding organizations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflict of interest.