PCDH19-related infantile epileptic encephalopathy: An unusual X-linked inheritance disorder†
Communicated by Maria Rita Passos-Bueno
Abstract
PCDH19 encodes protocadherin 19 on chromosome Xq22.3. This 1,148-amino-acid protein, highly expressed during brain development, could play significant roles in neuronal migration or establishment of synaptic connections. PCDH19 is composed of six exons, with a large first exon encoding the entire extracellular domain of the protein. Heterozygous PCDH19 mutations were initially identified in epilepsy and mental retardation limited to females, a familial disorder with a singular mode of inheritance as only heterozygous females are affected, whereas hemizygous males are asymptomatic. Yet, mosaic males can also be affected, supporting cellular interference as the pathogenic mechanism. Recently, mutations in PCDH19, mostly occurring de novo, were shown to be a frequent cause of sporadic infantile-onset epileptic encephalopathy in females. PCDH19 mutations were also identified in epileptic females without cognitive impairment. Typical features of this new epileptic syndrome include generalized or focal seizures highly sensitive to fever, and brief seizures occurring in clusters, repeating during several days. Here, we present a review of the published mutations in the PCDH19 gene to date and report on new mutations. PCDH19 has become the second most relevant gene in epilepsy after SCN1A. Hum Mutat 33:627–634, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
Mutations in PCDH19 (MIM# 300460) were originally identified in 2008 in rare large families in which female patients had variable degrees of epilepsy and intellectual deficits. This disorder, first reported as epilepsy and mental retardation limited to females (or epilepsy, female restricted, with mental retardation) and recently renamed epileptic encephalopathy, early infantile, 9 (EIEE9 or Juberg-Hellman Syndrome, MIM# 300088), was transmitted via asymptomatic males, suggesting an unusual X-linked inheritance with selective involvement of females [Dibbens et al., 2008; Fabisiak and Erickson, 1990; Juberg and Hellman, 1971; Ryan et al., 1997].
Recently, PCDH19-related infantile epileptic encephalopathy turned out to be more frequent than first anticipated on the observation of families; PCDH19 becoming the second most clinically relevant gene in epilepsy after SCN1A (MIM# 182389). Most patients with PCDH19 mutations are sporadic cases or belong to families with few affected female patients, making the recognition of the inheritance pattern difficult. In addition, associated clinical features, including age at onset, seizure types and severity, and the degree of cognitive disability, are highly variable. However, clinical characteristics such as clustering of repeated seizures within short periods of time are now emerging as hallmark features that orient etiological diagnosis toward PCDH19. This mutation update summarizes the mutations identified in the gene and the recent findings in the field.
Background
Protocadherins (pcdhs) are transmembrane proteins primarily involved in calcium-dependent adhesion that constitute the largest subgroup of the cadherin superfamily. Mammalian genomes contain more than 70 Pcdh genes that are divided into two groups based on their genomic structure: clustered (∼58 genes, Pcdhα, β, γ) and nonclustered (∼13 genes, Pcdhδ and other Pcdhs). Pcdhs typically have six or more extracellular calcium-binding cadherin repeats or ectodomains (EC domains), which are required for cell–cell homophilic or heterophilic interactions, and a divergent cytoplasmic domain [Morishita and Yagi, 2007; Patel et al., 2003, 2006; Redies et al., 2005; Yagi and Takeichi, 2000]. Pcdhs, like many other cadherins, are predominantly expressed in the brain, where they play significant roles in neurodevelopment such as neuronal migration and synaptic plasticity [Frank and Kemler, 2002; Junghans et al., 2005; Morishita and Yagi, 2007; Redies et al., 2005]. The combinatorial expression of multiple cadherin and protocadherin genes could contribute to the molecular specification of the vast complexity of neurons in the cerebral cortex [Krishna et al., 2011].
PCDH19 (MIM# 300460), located on chromosome Xq22.3, encodes the 1,148-amino-acid protocadherin 19, and belongs to the δ2-subclass of nonclustered pcdhs that also includes PCDH8, PCDH10, PCDH17, and PCDH18 [Redies et al., 2005; Wolverton and Lalande, 2001]. The PCDH19 gene has six coding exons, the first exon being unusually large and encoding the entire extracellular domain, composed of six EC domains (Fig. 1, based on the NM_001184880.1 reference transcript corresponding to the longest isoform). PCDH19 expression is spatially and temporally regulated in the central nervous system during mammalian development and shows a unique expression profile among pcdhs. In particular, protocadherin 19 is highly expressed in the developing brain, including the subventricular zone, the intermediate zone, the subplate, specific layers (layers II, IV, V, and VI) of the cerebral cortex, the hippocampus, and the subiculum [Gaitan and Bouchard, 2006; Hertel and Redies, 2011; Kim et al., 2007, 2010; Krishna et al., 2009, 2011; Vanhalst et al., 2005]. Two other isoforms (NM_020766.1 and NM_001105243.1) resulting from the alternative splicing of exon 2 and the existence of two possible acceptor sites for intron 4 (adding a residue at the beginning of exon 5) exist in databases but their existence, distribution, and respective roles need to be confirmed and studied at the molecular level.
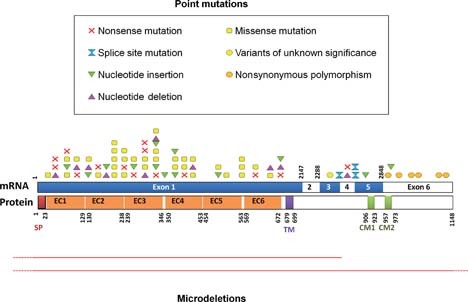
Schematic representation of the mutations, rearrangements, and polymorphisms identified in the PCDH19 gene on mRNA and protein. Point mutations (above): red crosses, nonsense mutations; yellow squares, missense mutations; hourglass, splice site mutations; green/pink triangles, small insertions/deletions leading to a frameshift; orange circles, nonsynonymous polymorphisms; yellow circles, variants of unknown significance. Microrearrangements (below): red lines indicate deletions. Dashed lines indicate that the deletion continues farther than the gene.
The precise function of protocadherin 19 remains currently unknown. Yet, other δ2-pcdhs were reported to mediate calcium-dependent cell–cell adhesion in vitro and cell sorting in vivo [Redies et al., 2005] and could regulate the establishment of neuronal connections during brain development and/or remodeling of selective synaptic connections during the early postnatal stage [Kim et al., 2007]. However, in contrast to cadherins, which associate through strong homophilic interactions, the extracellular domain of PCDH19 exhibits specific but weak homophilic adhesive properties, suggesting that the involvement of PCDH19 in cell adhesion could involve heterophilic interactions [Biswas et al., 2010; Tai et al., 2010].
Variants
Pathogenic Mutations
More than 60 different mutations in PCDH19 have been identified (Table 1 and Fig. 1). All types of DNA alterations are observed, including nonsense mutations, small nucleotide deletions and insertions, mutations altering the splice sites, missense mutations, and intragenic or whole gene deletions. Half of the pathologic allelic variants result in the appearance of premature termination codons (PTCs), either due to nonsense mutations (18.3%; 17/93 patients, based on review of the literature and personal data), small deletions or insertions leading to a frameshift (25.8%, 24/93 patients), or splice site mutations (3.2%, three of 93 patients). These mutation types are scattered along the PCDH19 gene with the exception of exon 2, in which no variants or mutations have been reported so far. Whole gene or intragenic deletions have been reported in only six patients (6.5%, six of 93) [Depienne et al., 2009, 2011; Vincent et al., 2011] but this mutation class has likely been underestimated because most studies used only direct sequencing, which misses heterozygous deletions, to screen PCDH19 (Table 3). Missense substitutions, which represent 46.2% (43/93) of the mutations, are, contrary to other mutation types, all clustered in exon 1, where they affect highly conserved amino acids in the large extracellular domain of protocadherin 19. Several recurrent mutations have been reported in patients, the most frequent being the Asn340Ser missense mutation (Table 1).
| Exon/intron | DNA variant | Predicted effect on the protein | Type | Recurrent mutation | Transmission | References |
|---|---|---|---|---|---|---|
| Exon 1 | c.74T>C | p.Leu25Pro | Missense | Mosaic mother | Dibbens et al. (2011) | |
| Exon 1 | c.78delG | p.Lys26AsnfsX4 | Frameshift | De novo | Jamal et al. (2010) | |
| Exon 1 | [c.83C>A; c.90A>G] | p.Ser28X | Nonsense | De novo | Marini et al. (2010) | |
| Exon 1 | c.142G>T | p.Glu48X | Nonsense | Paternal inheritance | Depienne et al. (2009) | |
| Exon 1 | c.215T>G | p.Val72Gly | Missense | Unknown | Higurashi et al. (2011) | |
| Exon 1 | c.241dupC | p.Leu81ProfsX8 | Frameshift | De novo | This study | |
| Exon 1 | c.242T>G | p.Leu81Arg | Missense | Unknown | Depienne et al. (2011) | |
| Exon 1 | c.253C>T | p.Gln85X | Nonsense | Yes | Familial condition | Dibbens et al. (2008), this study |
| Exon 1 | c.352G>T | p.Glu118X | Nonsense | De novo | Depienne et al. (2009), this study | |
| Exon 1 | c.357delC | p.Lys120ArgfsX3 | Frameshift | Familial condition | Dibbens et al. (2008) | |
| Exon 1 | c.361G>A | p.Asp121Asn | Missense | Paternal inheritance | Depienne et al. (2009) | |
| Exon 1 | c.415_423dup | p.Ser139_Ala141dup | In-frame duplication | Maternal inheritance | Depienne et al. (2011) | |
| Exon 1 | c.424delG | p.Ala142ProfsX70 | Frameshift | Unknown | Depienne et al. (2011) | |
| Exon 1 | c.437C>G | p.Thr146Arg | Missense | Paternal inheritance | Depienne et al. (2011) | |
| Exon 1 | c.457G>A | p.Ala153Thr | Missense | Paternal inheritance | This study | |
| Exon 1 | c.462C>A | p.Tyr154X | Nonsense | Unknown | (Depienne et al. 2011) | |
| Exon 1 | c.506delC | p.Thr169SerfsX43 | Frameshift | De novo | (Depienne et al. 2009) | |
| Exon 1 | c.514dupG | p.Glu172GlyfsX54 | Frameshift | Unknown (not in the mother) | (Depienne et al. 2011) | |
| Exon 1 | c.569T>G | p.Leu190Arg | Missense | Unknown | This study | |
| Exon 1 | c.571G>C | p.Val191Leu | Missense | Unknown | (Higurashi et al. 2011) | |
| Exon 1 | c.595G>C | p.Glu199Gln | Missense | Unknown (not in the mother) | Depienne et al. (2009) | |
| Exon 1 | [c.608A>C; c.617T>G] | [p.His203Pro; Phe206Cys] | Missense | De novo | Marini et al. (2010) | |
| Exon 1 | c.617T>A | p.Phe206Tyr | Missense | Maternal inheritance (mother asymptomatic) | Depienne et al. (2011) | |
| Exon 1 | c.695A>G | p.Asn232Ser | Missense | Unknown | This study | |
| Exon 1 | c.697_700delinsTAAC | p.Asp233X | Nonsense | Unknown (not in the mother) | Depienne et al. (2011) | |
| Exon 1 | c.701A>G | p.Asn234Ser | Missense | De novo | This study | |
| Exon 1 | c.706C>T | p.Pro236Ser | Missense | De novo | Specchio et al. (2011) | |
| Exon 1 | c.729C>A | p.Tyr243X | Nonsense | De novo | Jamal et al. (2010) | |
| Exon 1 | c.730dupG | p.Ala244GlyfsX76 | Frameshift | De novo | This study | |
| Exon 1 | c.747A>T | p.Glu249Asp | Missense | Maternal inheritance (mother with FS) | Depienne et al. (2011) | |
| Exon 1 | c.772_773delAT | p.Ile258ProfsX61 | Frameshift | Unknown | Higurashi et al. (2011) | |
| Exon 1 | c.785C>A | p.Ala262Asp | Missense | Unknown | This study | |
| Exon 1 | c.826T>C | p.Ser276Pro | Missense | De novo | Hynes et al. (2010) | |
| Exon 1 | c.840C>G | p.Tyr280X | Nonsense | De novo | Higurashi et al. (2011) | |
| Exon 1 | c.859G>T | p.Glu287X | Nonsense | Yes | De novo (n = 1), unknown (n = 1) | Depienne et al. (2009) |
| Exon 1 | c.949C>T | p.Gln317X | Nonsense | Familial condition | Higurashi et al. (2011) | |
| Exon 1 | c.958dupG | p.Asp320GlyfsX22 | Frameshift | De novo | Specchio et al. (2011) | |
| Exon 1 | c.1019A>G | p.Asn340Ser | Missense | Yes | De novo (n = 7), inherited from the mother (n = 1), mosaic mother (n = 1), unknown (n = 1) | Depienne et al. (2009), Marini et al. (2010), Specchio et al. (2011), Dibbens et al. (2011), Higurashi et al. (2011), this study |
| Exon 1 | c.1023C>G | p.Asp341Glu | Missense | De novo | Depienne et al. (2011) | |
| Exon 1 | c.1026_1027delinsAA | p.Asn342_Pro343delinsLysThr | Frameshift | Unknown | This study | |
| Exon 1 | c.1031C>G | p.Pro344Arg | Missense | Unknown | This study | |
| Exon 1 | c.1036_1040dup | p.Asn347LysfsX23 | Frameshift | Familial condition | Depienne et al. (2009) | |
| Exon 1 | c.1091dupC | p.Tyr366LeufsX10 | Frameshift | Yes | De novo (n = 1), familial condition (n = 1), paternal inheritance (n = 1) | Dibbens et al. (2008), Higurashi et al. (2011), this study |
| Exon 1 | c.1129G>C | p.Asp377His | Missense | De novo | Marini et al. (2010) | |
| Exon 1 | c.1131C>A | p.Asp377Glu | Missense | De novo | This study | |
| Exon 1 | c.1143dupT | p.Gly382TrpfsX19 | Frameshift | Unknown | This study | |
| Exon 1 | c.1192G>T | p.Glu398X | Nonsense | Paternal inheritance | Marini et al. (2010) | |
| Exon 1 | c.1211C>T | p.Thr404Ile | Missense | De novo | Marini et al. (2010) | |
| Exon 1 | c.1240G>C | p.Glu414Gln | Missense | Paternal inheritance | Marini et al. (2010) | |
| Exon 1 | c.1298T>C | p.Leu433Pro | Missense | De novo | Specchio et al. (2011) | |
| Exon 1 | c.1300_1301delCA | p.Gln434GlufsX11 | Frameshift | De novo | Specchio et al. (2011) | |
| Exon 1 | c.1322T>A | p.Val441Glu | Missense | Familial condition | Dibbens et al. (2008) | |
| Exon 1 | c.1521dupC | p.Ile508HisfsX15 | Frameshift | Unknown | Marini et al. (2010) | |
| Exon 1 | c.1537G>C | p.Gly513Arg | Missense | De novo | Specchio et al. (2011) | |
| Exon 1 | c.1628T>C | p.Leu543Pro | Missense | Paternal inheritance | Depienne et al. (2009) | |
| Exon 1 | c.1671C>G | P.Asn557Lys | Missense | Familial condition | Dibbens et al. (2008), Hynes et al. (2010) | |
| Exon 1 | c.1682C>G | p.Pro561Arg | Missense | Yes | Paternal inheritance (n = 1), unknown (n = 1) | Depienne et al. (2011) |
| Exon 1 | c.1700C>T | p.Pro567Leu | Missense | Maternal inheritance (mother asymptomatic) | Depienne et al. (2011) | |
| Exon 1 | c.1804C>T | p.Arg602X | Nonsense | De novo | This study | |
| Exon 1 | c.1852G>A | p.Asp618Asn | Missense | Maternal inheritance (mother asymptomatic) | Depienne et al. (2011) | |
| Exon 1 | c.1924G>A | p.Val642Met | Missense | Unknown | This study | |
| Exon 1 | c.1956_1959delCTCT | p.Ser653ProfsX6 | Frameshift | Unknown | This study | |
| Exon 1 | c.2012C>G | p.Ser671X | Nonsense | Familial condition | Dibbens et al. (2008) | |
| Exon 1 | c.2019delC | p.Ser674LeufsX2 | Frameshift | De novo | Depienne et al. (2011) | |
| Exon 1 | c.2030dupT | p.Leu677PhefsX41 | Frameshift | Familial condition | Dibbens et al. (2008) | |
| Intron 3 | c.2617–1G>A | p.? | Misplicing (abolition of exon 4 acceptor site) | De novo | Marini et al. (2010) | |
| Exon 4 | c.2631_2634delTTTT | p.Phe878ThrfsX5 | Frameshift | De novo | Jamal et al. (2010) | |
| Exon 4 | c.2656 C>T | p.Arg886X | Nonsense | Yes | Familial condition (n = 1), unknown (n = 1) | Depienne et al. (2011), this study |
| Intron 4 | c.2675+1G>C | p.? | Misplicing | Unknown (not in the mother) | This study | |
| Intron 4 | c.2676–6A>G | p.? | Misplicing (creation of a new acceptor site) | De novo | Marini et al. (2010) | |
| Exon 6 | c.2697dupA | p.Glu900ArgfsX8 | Frameshift | De novo | Marini et al. (2010) | |
| Exon 6 | c.2903dupA | p.Asp968GlufsX18 | Frameshift | De novo | Marini et al. (2010) |
- a Mutation nomenclature is based on the PCDH19 cDNA reference sequence (NM_001184880.1). Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence.
| Exon/intron | DNA variant | Predicted effect on the protein | Type | Recurrent mutation | Transmission | References |
|---|---|---|---|---|---|---|
| Exons 1–6 | c.1-?_3447+?del | Absence of protein synthesis | Whole gene deletion | Recurrent gene deletion with different BP | De novo (n = 3), unknown (n = 2) | Depienne et al. (2009), Depienne et al. (2011), Vincent et al. (2011) |
| Exons 1–3 | c.1-?_2616+?del | Absence of protein synthesis | Deletion of exons 1, 2, and 3 | De novo | Depienne et al. (2011) |
- a BP, breakpoint.
| Exon/intron | Nucleotide change | Protein consequence | rs number | Frequency in European population (other populations) | References |
|---|---|---|---|---|---|
| Exon 1 | c.6G>A | p.Glu2Glu | <2% | Hynes et al. (2010), Higurashi et al. (2011) | |
| Exon 1 | c.402C>A | p.Ile134Ile | rs41300169 | 7% | Hynes et al. (2010), Depienne et al. (2011), Higurashi et al. (2011) |
| Exon 1 | c.531G>A | p.Glu177Glu | <1% (Japanese population) | Higurashi et al. (2011) | |
| Exon 1 | c.655C>T | p.Leu219Leu | <1% | Hynes et al. (2010) | |
| Exon 1 | c.1137C>T | p.Gly379Gly | rs56277715 | 4% | Hynes et al. (2010), Depienne et al. (2011) |
| Exon 1 | c.1627C>T | p.Leu543Leu | 20% | Tarpey et al. (2009), Hynes et al. (2010), Depienne et al. (2011) | |
| Exon 1 | c.1683G>A | p.Pro561Pro | <1% | Tarpey et al. (2009), Hynes et al. (2010) | |
| Intron 3 | c.2617-27C>A | This study | |||
| Intron 5 | c.2849-28A>C | This study | |||
| Exon 6 | c.2873G>A | p.Arg958Gln | <1% | Tarpey et al. (2009), Hynes et al. (2010) | |
| Exon 6 | c.2938C>T | p.Arg980Cys | rs3764758 | <1% (Asian population) | Hapmap data (phase II), Higurashi et al. (2011) |
| Exon 6 | c.2994T>C | p.Thr998Thr | <1% (Japanese population) | Higurashi et al. (2011) | |
| Exon 6 | c.3018C>T | p.Asp1006Asp | rs16983426 | <1% (17% African population) | Tarpey et al. (2009), dbSNP |
| Exon 6 | c.3280C>G | p.Leu1094Val | <1% | This study, Higurashi et al. (2011) | |
| Exon 6 | c.3319C>G | p.Arg1107Gly | 1% (2–3% in Japanese population) | Depienne et al. (2009) | |
| Exon 6 | c.3400A>C | p.Asn1134His | <1% (>10% in Japanese population) | This study, Higurashi et al. (2011) | |
| 3′UTR | c.3447+8T>C | <1% | This study |
Mutations have been reported in the Leiden Open Variation Database (X chromosome gene database, PCDH19: http://www.lovd.nl/PCDH19).
Polymorphisms and Variants of Unknown Significance
The PCDH19 gene contains few known polymorphisms (Table 4). The most frequent natural variations reported correspond to synonymous substitutions located in exon 1. Rarer, nonpathogenic missense polymorphisms, all clustered in the intracellular domain of protocadherin 19 (exon 6), were occasionally found in patients and in ethnically matched control populations, suggesting that this domain, contrary to the extracellular domain, can tolerate some missense changes. One missense variant located in exon 3 has been identified in a patient with mental retardation but the pathogenic effect of this variant remains uncertain (Table 2) [Tarpey et al., 2009].
| Exon/intron | DNA variant | Predicted effect on the protein | Type | Phenotype of the patient (gender) | Transmission | Reference |
|---|---|---|---|---|---|---|
| Exon 3 | c.2454C>G | p.His817Gln | Missense | Mental retardation (unknown) | Unknown | Tarpey et al. (2009) |
Biological Relevance
Mutations in PCDH19 : A Loss of Function at the Cellular Level
The mutation spectrum in PCDH19 is compatible with a loss of function of the mutated allele. Messenger RNAs (mRNAs) with mutations introducing PTCs have indeed been shown to be degraded via the nonsense-mediated mRNA decay surveillance system of the cell in patients' fibroblasts [Dibbens et al., 2008]. The identification of whole gene deletions in five patients [Depienne et al., 2009, 2011; Vincent et al., 2011] also supports the notion that loss of function is the main consequence of the mutations. Missense mutations located in the extracellular domain could alter the adhesive properties of protocadherin 19 and also lead to a loss of function. In particular, the Asn340Ser and Glu414Gln mutations could specifically alter amino acids involved in calcium binding [Marini et al., 2010; Patel et al., 2006]. However, these hypotheses have yet to be formally tested.
Cellular Interference: A Gain of Function at the Tissue Level
Males with hemizygous PCDH19 mutations show normal cognitive function and do not have seizures, although a subtle psychiatric phenotype was evoked in some asymptomatic carriers [Dibbens et al., 2008]. The absence of major symptoms in hemizygous males indicates that the constitutive loss of function of protocadherin 19 (i.e., the absence of functional protein in all the cells of an individual's body) is not pathogenic. Hence, although protocadherin 19 could be essential for early brain morphogenesis in other species [Emond et al., 2009], it is a nonessential protein in humans, its absence likely being compensated for or buffered by other proteins and pathways.
In contrast, females with heterozygous PCDH19 mutations present with early intractable seizures and a variable degree of mental retardation. PCDH19 is located in a region submitted to X inactivation in females [Dibbens et al., 2008]. Random X inactivation in mutated females is expected to lead to tissue mosaicism; that is, coexistence of cells that have inactivated the mutated PCDH19 allele and express the normal protein, and PCDH19-negative cells that have inactivated the normal allele [Depienne et al., 2009]. This mosaicism could account for the pathogenesis by altering cell–cell interactions [Depienne et al., 2009; Dibbens et al., 2008; Ryan et al., 1997]. The loss of function of protocadherin 19 at the level of the cell would thus result in a gain of function at the tissue level because of abnormal interactions between “mutated” and “normal” cells. A mechanism of this type was termed “cellular interference” [Wieland et al., 2004] in reference to the “metabolic interference” concept developed many years ago by Johnson (1980). According to this theory, mosaic males would be affected like mutated females. The identification of an affected male with a mosaic PCDH19 deletion in his fibroblasts strongly supports cellular interference as the pathogenic mechanism associated with PCDH19 mutations [Depienne et al., 2009]. To definitely establish that cellular interference is the pathogenic mechanism, it would however be necessary to demonstrate that females homozygous for PCDH19 mutations are also unaffected like hemizygous males. In the absence of human cases, the development of a PCDH19-deficient mouse model will be crucial to confirm this pathogenic mechanism, assuming that the pathogenic mechanisms are identical in both species.
Interestingly, another human disorder, craniofrontonasal syndrome (CFNS; MIM# 304110), caused by mutations in EFNB1, the gene encoding Ephrin B1, a ligand for Eph receptors (EphRs) on chromosome Xq12, has the same unusual X-linked pattern of inheritance [Wieland et al., 2004]. Ephrin B1/EphR signaling plays a role in cell migration and pattern formation during developmental morphogenesis [Klein, 2004], reminiscent of the possible function of protocadherin 19 in brain development. Cellular interference has been confirmed as the pathogenic mechanism in CFNS. Female mice heterozygous for mutations in Ephrin B1 have a mosaic expression of Ephrin B1 resulting in ectopic interactions between the Ephrin B1 ligand and EphB receptors that are sufficient to induce the skeletal defects [Compagni et al., 2003].
Clinical Relevance
Clinical Features of Patients with PCDH19 Mutations
Female patients with heterozygous PCDH19 mutations have epileptic phenotypes ranging from mild to severe in terms of seizure type and severity. Seizures usually begin in infancy or early childhood (mean age at onset: 12.9 months, median age: 10 months; range: 4–60 months; n = 86 patients from the literature and unpublished personal data) and are highly sensitive to fever. Febrile seizures (FSs) are the initial manifestation in approximately half of the cases and seizures are triggered or worsened by fever in approximately 90% of the patients [Depienne et al., 2009, 2011; Marini et al., 2010; Scheffer et al., 2008]. Seizure types mostly consist in generalized tonic, clonic or tonic–clonic, and/or focal seizures with or without secondary generalization. Atypical absences, atonic seizures, and myoclonic jerks may also be part of the clinical picture, although they are rarely observed [Depienne et al., 2009, 2011; Marini et al., 2010; Scheffer et al., 2008; Specchio et al., 2011]. Status epilepticus, which can be inaugural, and prolonged seizures are reported in PCDH19-positive patients but the most characteristic feature is the presence of brief seizure clusters lasting 1–5 min and repeating up to or more than 10 times a day during several days [Depienne et al., 2009, 2011; Marini et al., 2010; Specchio et al., 2011]. Seizures are resistant to treatment in most cases, especially during infancy and childhood, but their frequency and intractability tend to decrease naturally over time, some patients being sometimes free of seizures during adolescence or adulthood on monotherapy [Depienne et al., 2011; Scheffer et al., 2008; Specchio et al., 2011].
We and others have shown that the clinical spectrum associated with PCDH19 mutations can overlap that of Dravet syndrome (DS, previously named severe myoclonic epilepsy of infancy or SMEI), a stereotyped epileptic encephalopathy also associating FS and epilepsy but due in 75% of cases to a de novo mutation in the SCN1A gene [Depienne et al., 2009, 2011; Higurashi et al., 2011; Marini et al., 2010; Nabbout et al., 2011]. However, DS-like patients with PCDH19 mutations slightly differ on average from SCN1A-positive classical DS patients: age at onset is slightly older (12.5 months [range: 4–60] vs. 6.3 months [range: 0.5–14]), status epilepticus and occurrence of myoclonic jerks are less frequent, and long-term outcome is better in PCDH19-positive patients than in SCN1A-positive patients; most patients with PCDH19 mutations fitting the definition of borderline SMEI [Fukuma et al., 2004]. Furthermore, photosensitivity, frequently reported in classical DS, is exceptional in PCDH19-positive patients [Depienne et al., 2009; Marini et al., 2010].
Behavioral disturbances are frequent in patients with heterozygous PCDH19 mutations and essentially manifest as autistic, obsessive, or aggressive features [Depienne et al., 2009, 2011; Marini et al., 2011; Scheffer et al., 2008]. In some patients, social withdrawal or personality disorders are even the most prominent and disabling feature when the patient becomes older [Depienne et al., 2011].
Intellectual outcome ranges from normal intellect (27.7%, 23/83) to mild (36.1%, 30/83), moderate (21.7%, 18/83), or severe (14.5%, 12/83) cognitive impairment [Depienne et al., 2009, 2011; Marini et al., 2011; Scheffer et al., 2008; Specchio et al., 2011]. Interestingly, the cognitive prognosis does not appear to be clearly related to the severity of epilepsy [Depienne et al., 2011; Specchio et al., 2011]. Language delay is frequently associated with cognitive deficit.
Finally, neurological features such as ataxia can also be observed in some patients and are reminiscent of those observed in DS patients.
Sporadic Versus Familial Cases
Although PCDH19 was first identified in large families wherein the mutations were inherited over several generations, PCDH19-related epileptic encephalopathy is more commonly sporadic or encountered in families with few affected females, making the recognition of the unusual pattern of inheritance difficult. De novo mutations account for most isolated cases (72%, 32/44) and represent 56% (32/57) of all mutations reported so far, in which transmission could be investigated [Depienne et al., 2009, 2011; Hynes et al., 2010, Jamal et al., 2010, Marini et al., 2010; Specchio et al., 2011]. The remaining mutations found in sporadic cases were inherited by asymptomatic fathers (18%, eight of 44), asymptomatic mothers (7%, three of 44), or by a mother who has had only FS (2%). In addition, parental mosaicism leading to the recurrence of the disease was demonstrated in two mothers (one being asymptomatic and one being affected) of unrelated families [Dibbens et al., 2011], a result reminiscent of mosaicism in DS caused by SCN1A mutations [Depienne et al., 2010].
Genotype–Phenotype Correlations
Because the clinical features (age at onset, severity of the epilepsy, and cognitive outcome) are highly variable for a given mutation even within the same family, genotype–phenotype correlation studies will likely be uninformative. Strikingly, Higurashi et al. (2011) reported monozygotic twin sisters in whom the p.Asn340Ser mutation was associated with different clinical pictures, confirming the existence of nongenetic modifiers. An expected source of phenotypic variability is the status of X inactivation in females. Interestingly, skewing of X chromosome inactivation can occur in normal females and increases in tissues with age [Bolduc et al., 2008; Chagnon et al., 2005]. A totally skewed X inactivation situation would theoretically reproduce a nonpathogenic situation. Partially skewed X inactivation would represent intermediate situations in which cellular interference could be limited compared to balanced X inactivation, where it would be expected to be the highest. In this setting, the severity of the epilepsy and/or the intellectual disability could be correlated with the relative amount of inactivated neurons for each chromosome, and the female mutation carriers with a totally skewed pattern of inactivation in the brain would be asymptomatic. However, so far, no correlations between the X-inactivation status in blood cells and the phenotypic expression have been found in support of this hypothesis [Marini et al., 2010]. Nonetheless, the X-inactivation status in lymphocytes does not reflect that of the neural tissues, and studies investigating directly cerebral tissues, in mouse models for example are important to further investigate this hypothesis.
Diagnostic Relevance
The identification of mutations in the PCDH19 gene provides a definite diagnosis in female patients with infantile epilepsy. This result also makes it possible to calculate the risk of recurrence of the disorder in the family, which is markedly different if the pathogenic mutation is de novo or if it has been inherited from a parent. Although diagnosis is made clinically, differentiating one epileptic condition from another is sometimes difficult, especially when the first symptoms appear. The molecular confirmation of the genetic defect underlying the epilepsy and an analysis of the parents' status are crucial to be able to give families appropriate genetic counseling.
Molecular testing of PCDH19 should be considered in females with early-onset FS and/or epilepsy with or without cognitive impairment and family history. Some clinical features could help to prioritize the analysis of PCDH19, such as female patients presenting with seizure clusters, the presence of multiple affected females in the family with obligate male carriers, and, more generally, the presence of generalized and/or focal seizures beginning in infancy or early childhood, resistant to treatment and sensitive to fever. With regard to DS, screening for PCDH19 mutations should be performed for female patients when analysis of SCN1A is negative. Molecular testing of PCDH19 should include sequencing of the coding sequence of the gene as well as a method (quantitative polymerase chain reaction, multiplex ligation-dependent probe amplification or equivalent) able to identify heterozygous deletions. The percentage of PCDH19-positive cases in females with FS and epilepsy has been shown to range from 5% to 37% depending on the clinical criteria [Depienne et al., 2009, 2011; Marini et al., 2010]. Molecular testing of PCDH19 in sporadic affected males can be considered with the same indication, although the somatic mosaicism expected in this case can easily be missed if the analysis is performed from genomic DNA extracted from blood cells, which considerably complicates the interpretation and decreases the reliability and significance of the result.
Given the unusual mode of inheritance and the wide phenotypic variability associated with PCDH19 mutations, genetic counseling appears delicate. In the case of mutations inherited from an asymptomatic father, all the daughters are expected to be affected. Considering the frequent poor outcome (mild-to-severe cognitive impairment in about 70% of PCDH19-positive females) of PCDH19-related epileptic encephalopathy, it is feasible to offer a prenatal diagnosis that could simply be based on fetal sex determination from maternal blood [Wright and Burton, 2009]. Female patients with PCDH19 mutations have a 50% risk of transmitting the mutation but, as only the females would be affected, the overall risk would be 25%. In cases with de novo mutations, the risk of recurrence is expected to be low but the possibility of germinal mosaicism in one parent is still possible [Dibbens et al., 2011].
Future Prospects
Although the frequency and clinical features of PCDH19-related epileptic encephalopathies have become more precisely determined during the past 3 years, several challenges remain to fully understand the functional consequences of the mutations and the mechanisms by which they contribute to epileptogenesis and cognitive impairment.
Several steps were recently made toward elucidating the function of protocadherin 19 in zebrafish or chicken models. The zebrafish ortholog of protocadherin 19 (pcdh19) was shown to be crucial for early steps of brain morphogenesis. Partial depletion of the protein with morpholino oligonucleotides impairs the convergence cell movements of the anterior neural plate, where pcdh19 is specifically expressed, a phenotype reminiscent of the n-cadherin (ncad) mutants [Emond et al., 2009]. Interestingly, the Pcdh19 and Ncad proteins directly interact in vitro and in vivo in this model [Biswas et al., 2010]. Together with the observation that Pcdh19 exhibits weak homophilic adhesive properties [Biswas et al., 2010; Tai et al., 2010], these results suggest that protocadherin 19 could preferentially interact with other members of the cadherin superfamily to regulate cell adhesion, neuronal migration, or synapse formation. The development of mouse models is an important step to confirm these hypotheses as well as the cellular interference theory, and to investigate the genetic or epigenetic factors underlying the phenotypic variability observed in the human disorder.
So far, although mammalian genomes contain over 70 pcdh genes, only two, PCDH19 and PCDH15 (causing autosomal recessive Usher syndrome), have been related to a human Mendelian disorder. Recent studies have suggested that defects in the expression or function of some other pcdhs may be related to neurodevelopmental disorders such as autism, schizophrenia, and mental retardation [Bray et al., 2002; Dean et al., 2007; Morrow et al., 2008]. These findings suggest that pcdhs are likely to play many roles that have yet to be discovered in human disorders.
Acknowledgements
We thank the families for their participation, the clinicians who referred their patients to our laboratory, and the DNA and cell bank for DNA extraction and cell culture of research samples.
Disclosure Statement : The authors declare that they have no conflicts of interest and no financial interest in this study.