SEPT12 mutations cause male infertility with defective sperm annulus†
Communicated by Ming Qi
Abstract
Septins are members of the GTPase superfamily, which has been implicated in diverse cellular functions including cytokinesis and morphogenesis. Septin 12 (SEPT12) is a testis-specific gene critical for the terminal differentiation of male germ cells. We report the identification of two missense SEPT12 mutations, c.266C>T/p.Thr89Met and c.589G>A/p.Asp197Asn, in infertile men. Both mutations are located inside the GTPase domain and may alter the protein structure as suggested by in silico modeling. The p.Thr89Met mutation significantly reduced guanosine-5′-triphosphate (GTP) hydrolytic activity, and the p.Asp197Asn mutation (SEPT12D197N) interfered with GTP binding. Both mutant SEPT12 proteins restricted the filament formation of the wild-type SEPT12 in a dose-dependent manner. The patient carrying SEPT12D197N presented with oligoasthenozoospermia, whereas the SEPT12T89M patient had asthenoteratozoospermia. The characteristic sperm pathology of the SEPT12D197N patient included defective annulus with bent tail and loss of SEPT12 from the annulus of abnormal sperm. Our finding suggests loss-of-function mutations in SEPT12 disrupted sperm structural integrity by perturbing septin filament formation. Hum Mutat 33:710–719, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
The Septin genes (Sept) were first identified in Saccharomyces cerevisiae. They encode a superclass of P-loop GTPase and their mutant strains are defective in cytokinesis [Hartwell, 1971; Leipe et al., 2002]. Septins (SEPT7/6/2/2/6/7; Sept5/7/11; Sept7/9b/11; Sept3/5/7) in mammals usually assemble into linear filaments [Fujishima et al., 2007; Joberty et al., 2001; Lukoyanova et al., 2008; Nagata et al., 2004; Sirajuddin et al., 2007; Xie et al., 2007]. They have been implicated in numerous cellular processes, including membrane association, cell movement, cell polarity, scaffold, morphogenesis, and so on [reviewed in Barral and Kinoshita, 2008; Hall and Russell, 2004]. Septins also play a role in the compartmentalization of the cell cortex in yeast [Barral et al., 2000; Luedeke et al., 2005; Takizawa et al., 2000]; indeed, this function was also demonstrated by the disorganized subcellular compartments of sperm in Septin 12 (Sept12) knockout mice [Lin et al., 2009]. Disruption of septin genes has been observed in different types of cancers and neurodegenerative diseases [Hall and Russell, 2004]. Point mutations in SEPT9 cause hereditary neuralgic amyotrophy [Kuhlenbaumer et al., 2005].
The role of septins in spermatogenesis was just beginning to be revealed. In sperm, SEPT4, 7, and 12 are located at the annulus, a submembrane ring structure demarcating the midpiece and the principle piece of sperm tail [Ihara et al., 2005; Kissel et al., 2005; Steels et al., 2007; Sugino et al., 2008]. Male Sept4 null mice are sterile due to immotile sperm with annulus disorganization and multiple defects [Ihara et al., 2005; Kissel et al., 2005]. Septin 12 (SEPT12; MIM# 611562) is exclusively expressed in the testis. By gene targeting, we found Sept12+/− chimeric mice were sterile with various sperm pathologies, including immotility, bent tail, acrosome break, and round head. Sept12 chimeric mice also harbored significant DNA damage in their sperm. Embryos generated by in vitro fertilization and intracytoplasmic sperm injection of the sperm failed to develop beyond the morula stage [Lin et al., 2009, 2011].
Proteins in the GTPase superfamily engineer molecular switch to promote guanosine-5′-triphosphate (GTP) binding and hydrolysis. The consensus GTPase domain exists in all septins, and GTP- or guanosine 5′-diphosphate (GDP)-bound septins are known to regulate a variety of physiological processes through GTPase signaling [Weirich et al., 2008]. Mutational studies of septin family showed that mutations in critical residues in the GTPase domain reduce GTP/GDP incorporation and hydrolysis, and cause temperature-sensitive defects in yeast. The guanine nucleotide binding by septins are vital for septin–septin interactions and filamentous complex formation, and may ensure septin structural integrity [Nagaraj et al., 2008; Sirajuddin et al., 2009; Vrabioiu et al., 2004]. Although mutations in septins have been shown to result in morphogenetic defects and different diseases by affecting filament assembly [Ihara et al., 2005; Kissel et al., 2005; Lin et al., 2009; Xie et al., 2007], the molecular and physiological bases for SEPT12 dysfunction have not been explored. Considering that haploinsufficiency of Sept12 results in severe sperm pathology in mice [Lin et al., 2009], it is reasonable to speculate that mutations in SEPT12 are accountable for abnormal spermatogenesis and male infertility in some human patients. In the present study, we identified two novel missense mutations of SEPT12, p.Thr89Met (SEPT12T89M) and p.Asp197Asn (SEPT12D197N), in infertile men. In silico modeling showed that SEPT12T89M had dramatically altered structure and was stripped off GTP hydrolysis ability. Although SEPT12D197N was only mildly affected in protein structure, its GTP binding ability was profoundly perturbed. These two mutants suppressed SEPT12WT filament formation in a dose-dependent manner. Finally, sperm of the SEPT12D197N patient had decreased motility, concentration, and multiple structural defects, notably a disruptive annulus with bent tail and loss of SEPT12 from the annulus of abnormal sperm. Our finding clearly demonstrates the importance of SEPT12 dysfunction in sperm annulus integrity.
Materials and Methods
Clinical Information
The study was approved by the Institutional Review Board of National Cheng Kung University Hospital and Kuo General Hospital. From January 2005 to July 2007, 160 infertile men with abnormal semen parameters and 200 fertile men with normal semen parameters were enrolled into the study. All infertile men were presented with at least one of the parameters: sperm concentration <20 × 106/ml, motile sperm <50% [WHO, 1999], or sperm with normal morphology <14% based on strict Kruger criteria [Kruger et al., 1986]. All patients underwent a comprehensive examination, including a detailed history, physical examination, hormone profilings, and a molecular test for Y-chromosome microdeletions as described previously [Lin et al., 2002]. The control subjects were recruited from husbands of women who received regular prenatal care at the University Hospital. All control subjects had fathered at least one child within 2 years without assisted reproductive technologies. The control subjects underwent semen analysis and only those with normal semen parameters were enrolled for the study. All study and control subjects were Han Taiwanese, the major ethnic group in Taiwan (making up more than 95% of the country's population).
Mutation Analysis
Genomic DNA was extracted from lymphocyte or saliva using a Puregene DNA isolation kit (Gentra, Minneapolis, MN). Specific primers were designed to amplify each coding region of the human SEPT12 gene (GenBank accession no. NM_144605.3). The polymerase chain reaction (PCR) products were directly sequenced with the same primer sets. The end point TaqMan genotyping assays using probes labeled with the fluorophores FAM and VIC were purchased from Applied Biosystems (Carlsbad, CA), and allelic discrimination was read automatically with the SDS2.2 software by using an ABI PRISM 7900HT Sequence Detector (Applied Biosystems). TaqMan reactions were carried out according to the instruction manual of manufacturers.
Plasmids Construction and Transfection
The wild-type SEPT12 full-length complementary DNA (cDNA) was generated by PCR amplification of human testis cDNA (Human Total RNA Master Panel II; Clontech, Mountain View, CA, USA) and cloned into pFLAG–CMV-2 plasmid (Sigma–Aldrich, St. Louis, MO) and pEGFP-N1 plasmid (Clontech). Mutant construction of pFLAG–SEPT12T89M, pFLAG–SEPT12D197N, pEGFP–SEPT12T89M, and pEGFP–SEPT12D197N were generated by the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the instruction manual of manufacturers. For the production of recombinant proteins in bacteria, the wild-type and mutant SEPT12 cDNA were subcloned into pET32a plasmid (Novagen, Madison, WI) to generate His–SEPT12WT, His–SEPT12T89M, and His–SEPT12D197N. For transient transfection, human testis malignant pluripotent embryonal carcinoma NT2D1 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the instruction manual of manufacturers. Forty-eight hours later, cells were harvested for immunofluorescence staining.
GTP Binding and Hydrolysis Assay
For GTP-binding studies, 200 µg of purified His–SEPT12WT, His–SEPT12T89M, His–SEPT12D197N, or His-only were incubated with GTP–agarose beads (Innova Biosciences, Babraham, Cambridge, UK) at 4°C, agitated for overnight. The beads were then washed seven times with the washing buffer (20 mM Tris–HCl pH 6.0, 500 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1% Triton X-100 (Sigma-Aldrich). The wild-type and mutant His–SEPT12–GTP–agarose complexes were eluted with an elution buffer containing 8.5 mM GTP (Promega, Madison, WI) at 4°C, agitated for 3 hr. The supernatants containing wild-type and mutant His–SEPT12–GTP were analyzed by Western blot. Quantification of the GTP–agarose-bound proteins was performed with the SPOT DENSO software on an AlphaImager2200 instrument (Alpha Innotech Corporation, San Leandro, CA). GTP-hydrolysis activities of His–SEPT12WT, His–SEPT12T89M, and His–SEPT12D197N were determined using a colorimetric GTPase assay kit (Innova Biosciences) according to the manufacture's instruction. In brief, the wild-type and mutant His–SEPT12 were diluted to an appropriate protein concentration. Hydrolysis assays were carried out at 37°C in a substrate buffer containing 0.5 mM of GTP for 30 min. Finally, the released phosphate was read at optical density at 635 nm using a SpectraMax M2e Microplate Reader (Molecular Devices, Sunnyvale, CA).
Western Blot Analysis and Immunofluorescence Staining
Equal amounts of purified recombinant His–SEPT12 proteins were separated on sodium dodecyl sulfate-polyacrylamide gels as described previously [Cheng et al., 2006]. The blots were incubated with an anti-His antibody (1:3000; AbD Serotec, Kidlington, Oxford, UK). For immunofluorescence assay, human spermatozoa were stained with an anti-SEPT12 antibody (1:100; Abnova, Taipei, Taiwan) or Mito Tracker Red 580 (Molecular Probes, Eugene, OR) according to procedures as described [Lin et al., 2009]. For immunofluorescence staining, transfected NT2D1 cells were incubated with an anti-GFP antibody (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), followed by Alexa Fluor 488-labeled antibody staining (1:200; Molecular Probes). The nuclei were counterstained with 4,6-diamidino-2-phenylindole, and visualized with a fluorescence microscopy (Olympus, Tokyo, Japan) under identical settings.
Atomic Force Microscopy System
All the sperm images in this study were obtained by the JPK NanoWizard® II atomic force microscopy (AFM) system (JPK Instruments AG, Berlin, Germany) installed on top of an inverted light microscope (Zeiss Axio Observer, Oberkochen, Baden-Württemberg, Germany). Contact mode was operating by a T1L450B cantilever (NANOSENSORSTM, Neuchatel™, Switzerland) with nominal spring constant 0.02 N/m to identify and localize the scanning region and a 0.2 Hz scanning rate was used.
Results
Two Novel Missense Mutations in the SEPT12 Gene
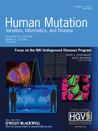
We screened 160 infertile men and 200 fertile men to assess whether SEPT12 mutation was responsible for male infertility. We sequenced 10 exons of SEPT12 and detected 2 novel heterozygous mutations in exon 3 (c.266C>T) and exon 6 (c.589G>A) in 2 infertile men. Both mutations were located in the GTPase domain, replacing threonine with methionine (T89M) and aspartate with asparagine (D197N), respectively (Fig. 1A–C). They were confirmed by TaqMan assays (Fig. 1D and E). These mutations were not identified in 200 fertile men with normal semen parameters. Semen analyses showed that the man carrying T89M had asthenoteratozoospermia, whereas the patient carrying D197N had oligoasthenozoospermia (Table 1). The GTPase functional domain of SEPT12 consists of 6 conserved motifs, including P-loop, switch I, switch II, guanine-recognition site, trans loop I, and trans loop II [Weirich et al., 2008]. T89M and D197N are located in the switch I motif and guanine-recognition site, respectively. Both threonine and aspartate are highly conserved in other species (Supp. Fig. S1).
Two missense mutations of SEPT12 identified in infertile men. A: Schematic representations of the SEPT12 protein (top) and the SEPT12 locus (bottom), showing two novel nonsynonymous nucleotide changes (c.266C>T and c.589G>A located in exon 3 and exon 6, respectively) found in two infertile men. The exons are numbered, and the coding regions are indicated with black boxes. B and C: The upper panels show the corresponding sequences from control subjects, the lower panels show nucleotide changes (c.266C>T and c.589G>A; asterisks) of the patients. D and E: The TaqMan assay used to confirm the mutations. The green and red circles pinpoint the heterozygous nucleotide alterations in the two patients and the homozygous wide-type alleles of control subjects, respectively. The black dots indicate negative control. F and G: Three-dimensional cartoon representations of SEPT12 structures. The SEPT12T89M structure (F; white) or SEPT12D197N structure (G; blue) is superimposed with the SEPT12WT structure (F and G; green). The six functional motifs of the GTP-binding domain are shown separately in Fii and Gii. The switch I motif or guanine-recognition motif of SEPT12WT (Fii and Gii; magenta), SEPT12T89M (Fii; blue), and SEPT12D197N (Gii; white) are presented in different colors. Inset: magnified images of the Thr89/Met89 (Fi) and Asp197/Asn197 (Gi) residues denoted by the arrows. All images were generated by the PyMOL software [DeLano, 2002].
| Motility typesd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence variationa | Amino acid variationb | Age (years) | Karyotype | Clinical feature | Ratioc | Sperm count | Morphology | Total motility | a | b | c |
| c.266C>T | p.Thr89Met (T89M) | 36 | 46, XY | Asthenoteratozoospermia | Patient: 1/160, Control: 0/200 | 20.5 × 106/ml | 8% normal, 92% abnormal | 48% | 42% | 6% | 52% |
| c.589G>A | p.Asp197Asn (D197N) | 33 | 46, XY | Oligoasthenozoospermia | Patient: 1/160, Control: 0/200 | 0.9 × 106/ml | 20% normal, 80% abnormal | 22% | 0% | 22% | 78% |
- aNucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence (SEPT12, NM_144605.3).
- bThe initiation codon is codon 1.
- cThe ratio of case that harbors mutant allele.
- dGrade of sperm motility according to WHO criteria: a, rapid progressive motility; b, slow progressive motility; c, nonprogressive.
Structural Alterations of SEPT12T89M and SEPT12D197N
To gain further structural insight into the impact of the mutations, we modeled the structures of SEPT12WT, SEPT12T89M, and SEPT12D197N using the (PS)2 program [Chen et al., 2006]. Structural comparison of SEPT12WT and SEPT12T89M revealed that the major α-helices and β-sheets were well superimposed, except for a difference in the switch I motif conformation (Fig. 1Fi, inset; magenta and blue), whereas comparison of SEPT12WT and SEPT12D197N, including the guanine-recognition motif, showed good superimposition (Fig. 1Gi, inset; magenta and white). As both mutated residues (T89M and D197N) are located within the GTP-binding pocket of the GTPase domain, we inspected the conformational change only at the GTP-binding pocket. Although the overall structures of the GTP-binding pockets of SEPT12T89M and SEPT12D197N were similar to that of SEPT12WT, the side-chain orientations of the residue Thr89 and its substitution Met89 were deviated from each other (Fig. 1Fii). Furthermore, the substitution of Asp197 with Asn197 changed a charged residue to a noncharged residue, but did not seem to significantly affect the conformation of the guanine-recognition motif (Fig. 1Gii). In addition, the surface model revealed that residues at the switch I motif in SEPT12T89M were exposed to the solvent-accessible surface in comparison to SEPT12WT (Supp. Fig. S2A). In the guanine-recognition motif of SEPT12D197N, the surface area was only slightly changed (Supp. Fig. S2B). These results suggest that a mutation of the highly conserved residue Thr89 in SEPT12 might have a significant effect on the protein conformation. Both mutations therefore may interfere with intrinsic GTP binding and hydrolysis capability by substitutions of these critical residues.
The GTP-Binding and Hydrolysis Functions Were Perturbed by SEPT12 Mutations
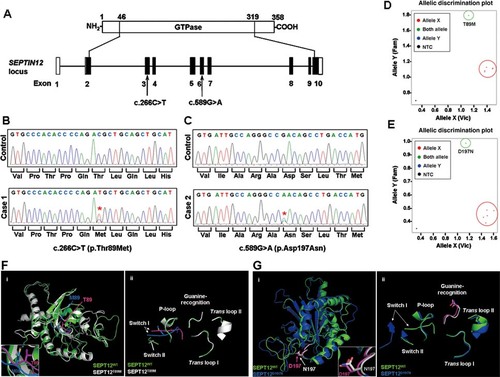
Incorporation of GTP and GDP into septins is critical for septin filament assembly [Kinoshita et al., 2002; Mendoza et al., 2002]. In order to assess how protein conformational changes might affect the intrinsic GTPase function of SEPT12T89M and SEPT12D197N, we measured GTP binding and hydrolysis abilities of the mutant proteins in vitro. We purified His–SEPT12WT, His–SEPT12T89M, and His–SEPT12D197N that were expressed in bacteria (Fig. 2A), and tested their abilities to bind GTP–agarose. His–SEPT12WT and His–SEPT12T89M were readily pulled down by the GTP–agarose beads, but His–SEPT12D197N and His-only were not (Fig. 2B). Quantification of the protein signals showed significant differences in the levels of GTP-bound His–SEPT12D197N and GTP-bound His–SEPT12WT (Fig. 2C). It has been demonstrated previously that the side chain of Asp197 of SEPT12 forms two hydrogen bonds with the guanine base of GTP [Sirajuddin et al., 2009]. Considering that the conformation of SEPT12D197N was not dramatically changed (Fig. 1G), His–SEPT12D197N may have reduced GTP-binding capability due to the noncharged asparagine substitution, similar to the effect of the guanylate-binding protein 1 (GBP1) D184N mutation, which substituted a residue equivalent to Asp197 in SEPT12 [Praefcke et al., 2004].
GTP binding and hydrolysis of wild-type and mutants SEPT12. A: Silver staining of purified SEPT12 recombinant proteins on a gel. Open arrowhead points to the location of these proteins. B: The GTP-binding assay. (Top panel) Recombinant proteins bound to GTP–agarose beads were immunoblotted with an anti-His antibody. His-only served as a negative control. (Bottom panel) Immunoblot of 1% of the proteins used in the GTP-binding assay. Arrowheads and arrows indicate the positions of the various His–SEPT12 proteins and His-only, respectively. C: Quantification of the GTP–agarose-bound proteins was performed and normalized to the corresponding proteins in the 1% input. *P < 0.05; **P < 0.01, one-way analysis of variance (ANOVA) (Tukey's multiple comparison test for posterior comparison). Data are represented as means ± SE (n = 3). D: The GTP hydrolysis activities of wild-type and mutants SEPT12. Various amounts of purified proteins were incubated with 0.5 mM GTP, followed by quantification of released phosphate at optical density at 635 nm. His-only served as negative control (P < 0.001, one-way ANOVA, Tukey's multiple comparison test for posterior comparison). Data are represented as mean ± SE (n = 5). E: Immunoblots of 0.15 µmol loading control of the various His–SEPT12 and His-only proteins used in the GTP hydrolysis assay. The arrowhead and the arrow indicate the protein positions as indicated.
Septins possess GTPase activity [Field et al., 1996; Huang et al., 2006; Sheffield et al., 2003]. Even though the GTP-binding capability of His–SEPT12T89M was not significantly different from that of His–SEPT12WT, whether His–SEPT12T89M maintained its GTP hydrolytic activity remained unknown. To answer the question, colorimetric GTPase assay was performed. Compared with the modest GTPase activity for His–SEPT12WT, the activities for both His–SEPT12T89M and His–SEPT12D197N were significantly reduced (Fig. 2D). Western blotting showed comparable levels of each protein in the assays (Fig. 2E). Taken together, these results indicate that His–SEPT12WT hydrolyzes GTP at a modest rate. Although the T89M mutation does not affect GTP binding, it compromises the GTPase enzymatic activity of the protein. Because His–SEPT12D197N cannot bind GTP, its hydrolysis is similar to that of the His-only control.
The Formation of SEPT12 Filamentous Structure is Inhibited by Mutant SEPT12 Proteins in a Dose-Dependent Manner
At least four types of septin subcellular staining patterns have been observed: periphery, punctate, filaments, and whole cytoplasm distribution [Lindsey and Momany, 2006]. Indeed, SEPT12 showed various patterns in NT2D1 cells. Of the three enhanced green fluorescent protein (EGFP)-tagged SEPT12 proteins, only SEPT12WT presented filament fibers in 27.7% of cells transfected with the expression vector, but the other two did not form filament fibers (Supp. Fig. S3).
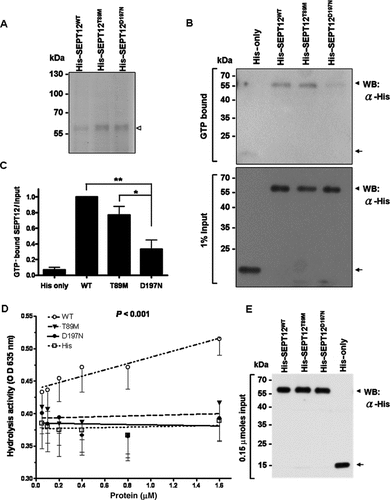
In light of the heterozygosity of the mutations in the patients, we wondered whether the mutant SEPT12 proteins interfered with the filaments formation of the wild-type SEPT12. We therefore transfected a constant amount of the EGFP–SEPT12WT construct together with various amounts of FLAG-tagged wild-type or mutant SEPT12 constructs into NT2D1 cells. The ratio of the EGFP–SEPT12WT plasmid related to the SEPT12T89M or SEPT12D197N plasmid ranged between 4:1 and 1:4 (Fig. 3). By counting cells with EGFP-labeled filament fibers, we found that with increasing amounts of FLAG–SEPT12T89M and FLAG–SEPT12D197N, the fraction of cells containing EGFP–SEPT12WT filaments significantly diminished (Fig. 3, lanes 2–9; middle and bottom panels), compared to the FLAG–SEPT12WT control (Fig. 3, lane 1; middle and bottom panels). Importantly, when SEPT12WT was present in the same amount as the mutant proteins, a situation in the heterozygous patients, its ability to form filament was severely blocked. Further increase in the mutant SEPT12 completely blocked filament formation (∼0%), a finding similar to the lack of filament in cells expressing the mutant SEPT12 only (Supp. Fig. S3C). These observations suggest that SEPT12T89M and SEPT12D197N adversely affect filament formation of SEPT12WT in a dose-dependent manner.
Effects of the SEPT12 mutants on the filament formation of SEPT12WT. The expression vector for EGFP-tagged SEPT12WT was mixed with various amounts of the expression vector for FLAG-tagged SEPT12WT (lane 1), SEPT12T89M (lanes 2–5), or SEPT12D197N (lanes 6–9), and transfected into NT2D1 cells. More than 100 cells were scored for each experiment and cells with EGFP filament fibers were counted. The value was normalized to that of the wild-type control (lane 1) (P < 0.01, one-way ANOVA, Dunnett's multiple comparison test for posterior comparison). Data are represented as mean ± SE (n = 3). (Middle panels) Low magnification (400×) images of cells are presented in lanes 1, 2, and 6; the dotted line indicates expression vectors-transfected cells. The boxed areas show filament fibers, the images of which are enhanced in the bottom panels (a–c 1,000×).
SEPT12 Was Missing from the Annulus in the Infertile Man Carrying SEPT12D197N
To further study the effects of SEPT12 mutations on spermatogenesis, we examined the sperm characteristic of the patients. Sperm was available for re-examination only from the infertile man carrying SEPT12D197N. We enrolled 5 fertile control subjects to gain further insight into the mRNA level and localization of wild-type and mutant SEPT12 in sperm. The abundance of SEPT12 transcript was comparable between the SEPT12D197N patient and control subjects (Supp. Fig. S4). Immunofluorescence staining showed strong SEPT12 or SEPT4 signals at sperm annulus in the control subjects (Fig. 4A–C, insets). Conversely, SEPT12 was absent from the annulus in the SEPT12D197N patient. Loss of annulus SEPT12 or SEPT4 signals were observed in sperm with abnormal morphology (Fig. 4D–G, and Supp. Fig. S5), indicating that SEPT12 interacts with SEPT4 to form a septin protein complex at the annulus. Some sperm of the SEPT12D197N patient contained a deformed tail with reduced diameter at the midpiece–principal piece junction referred to as the annulus site (Fig. 4D, E, and G, insets). Defective annulus was further confirmed by AFM, which detected loss of the annulus structure, bent tail, and the axoneme of the flagellar were exposed outward in the SEPT12D197N patient (Fig. 5A, A′, C, and C′). The height of the annulus site in SEPT12D197N sperm was about 20–40 nm, significantly lower than that in the control sample (about 160 nm) (Fig. 5A′′ and C′′; arrowhead). In three-dimensional overview of sperm, the annulus defect was clearly visible in SEPT12D197N sperm (Fig. 5B and D). This finding is in line with previous observations in Sept4 and Tat1 knockout mice where the annulus was not found or malformed [Ihara et al., 2005; Kissel et al., 2005; Lhuillier et al., 2009; Toure et al., 2007]. Under bright-field and fluorescence microscope, we also found that the SEPT12D197N sperm had multiple defects. In addition, transmission electron microscopy detected noncondensed chromatin material and cytoplasmic droplet in SEPT12D197N sperm. The joining points of connecting piece had shrunken with minimal connection, and the tail also bent (Supp. Fig. S6). In fertile men, SEPT12 signals were not detectable at the annulus of only a minority (14.96 ± 3.89%) of abnormal-looking sperm, as compared with the SEPT12D197N patient, in whom a significant fraction (55.95%) of abnormal sperm lost their SEPT12 signals (Supp. Table S1). Considering abundance of SEPT12 transcript amount in patients and detectable SEPT12 proteins on the neck and cytoplasmic droplets of abnormal sperm (Fig. 4D and Supp. Figs. S5C–E), we hypothesize that SEPT12 protein is dislocated from annulus of SEPT12D197N patient. It is tempting to speculate that SEPT12 is responsible for maintaining annulus integrity and the mutant protein disrupts annulus integrity and causes characteristic sperm pathology in a dominant negative fashion.
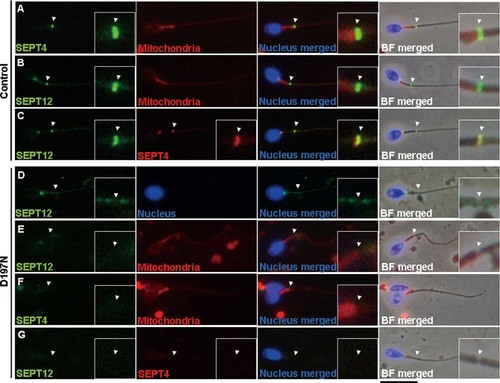
The endogenous SEPT12 protein in the spermatozoa of the D197N patient. Immunofluorescence staining showed SEPT4 or SEPT12 signal (A and B green), costaining of SEPT4/12 (C), and bright field (BF) merged with the fluorescence staining in the normal sperm of a fertile control. The four frames of each panel show the same sperm. D–G: In the D197N patient, SEPT12 or SEPT4 staining produced no signals in sperm with or without deformed tail. Insets, magnified images of the annulus denoted by the arrowheads. Scale bar, 10 µm.
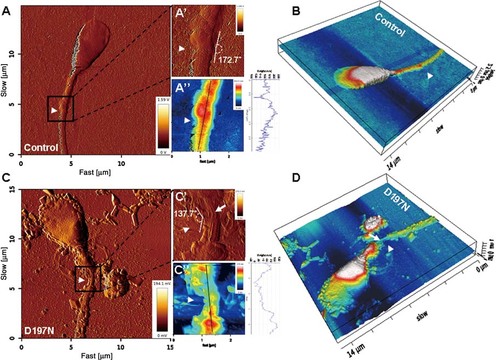
Absence of the annulus in the sperm of the SEPT12D197N patient. Height and error mode AFM images of sperm. Sperm of a control subject has an intact annulus structure (A); the magnification images of annulus are shown (A′ and A′′); the height profile is shown at right (blue line). Loss of annulus in the sperm of an infertile man with the SEPT12D197N mutation (C; C′ and C′′). The angles of tail bending at the annulus (A′ and C′); 3D images are displayed by the height mode (B and D). The arrowhead indicates the location of the annulus; the arrow indicates the axoneme.
Discussion
In this study, we identified two novel mutations of SEPT12 in infertile men. We found both mutant proteins (SEPT12T89M and SEPT12D197N) lost GTP hydrolysis ability and adversely affected septin filaments formation of the wild-type SEPT12. Importantly, sperm of the SEPT12D197N patient had multiple structural defects, notably a defective annulus with bent tail accompanied by loss of SEPT12 from annulus.
GTP Binding and Hydrolase Activity of SEPT12 Mutant Proteins
The Thr89 residue in SEPT12 is equivalent to Thr78 in SEPT2, Thr75 in guanylate-binding protein 1 (GBP1), and Thr65 in dynamin. These residues coordinate with the Mg2+ ion and form a hydrogen bond with the γ-phosphate of GTP. SEPT2T78G, GBP1T75A, and dynaminT65A mutations are all associated with decreased GTP binding as well as reduced GTP hydrolysis activity [Praefcke et al., 2004; Sirajuddin et al., 2009; Song et al., 2004]. It seems that SEPT12WT binds GDP through the conserved Thr89 in the switch I motif, which executes bona fide GTP hydrolysis activity. The ability of SEPT12T89M to bind GTP (Fig. 2) in contrast to the other mutants mentioned above may be explained by two possibilities. The first is our overnight incubation of SEPT12T89M with GTP beads instead of the few-minute incubation for fluorescence detection used in the previous studies [Praefcke et al., 2004; Sirajuddin et al., 2009]. The second possibility is that methionine provides a larger stereo pocket for GTP incorporation. This argument is supported both by our in silico modeling of GTP-binding pocket and by the data from other dynamin mutations (T65D and T65H) that do not affect GTP binding [Song et al., 2004]. However, in another study on dynaminT65A, substitution of threonine by a smaller alanine did not affect GTP binding but lost GTP hydrolysis ability [Marks et al., 2001]. It seems that functional significance of mutations within the switch I motif still remains difficult to predict. Nevertheless, loss of GTP hydrolysis is a common consequence observed in all studies [Marks et al., 2001; Praefcke et al., 2004; Sirajuddin et al., 2009; Song et al., 2004]. SEPT2 Thr78 is critical for GTP hydrolysis, a function required for the formation of the hexameric SEPT7/6/2/2/6/7 complex [Sirajuddin et al., 2007, 2009]. Hence, we also proved that loss of GTP hydrolytic activities in SEPT12T89M and SEPT12D197N perturbed filament formation (Fig. 3). In our study, SEPT12WT presented modest GTP hydrolysis ability, an observation consistent with the slow GTP hydrolysis during SEPT7/6/2/2/6/7 complex assembling [Sheffield et al., 2003]. Furthermore, some studies showed that EzrA, CRMP-2, and Orc6 facilitated tubulin or septin GTP hydrolysis [Chae et al., 2009; Chung et al., 2007; Huijbregts et al., 2009]. It may be inferred that other regulators are involved in modulating SEPT12 GTP hydrolysis and filament formation in mammalian cells.
Dominant-Negative Effect of the Mutant Proteins on Filament Formation
Septins have been shown to be critical for the compartmentalization of intracellular materials. They may also serve as scaffolds for various molecules (e.g., cell cycle regulators) or as barriers for proteins restriction at cilium, sperm annulus, and mother-bud neck in yeast [Hu et al., 2010; Kinoshita, 2006; Lindsey and Momany, 2006; Versele and Thorner, 2005]. Septins form homo- and hetero-oligomers with other septins through their GTPase domain interface as well as the N′-, C′-termini interface [Weirich et al., 2008]. However, the SEPT12 mutant proteins may not provide a functional GTPase domain interface for the interactions with other septins, resulting in the failure of oligomer assembly and filament formation. In this study, we included a 1:1 molar ratio of SEPT12WT/SEPT12T89M or SEPT12WT/SEPT12D197N to mimic the in vivo conditions (Figs. 1 and 3). With increasing amount of the mutant proteins, SEPT12WT lost its capability to form filaments in NT2D1 cells, conferring loss of filament formation and failure to form intracellular compartment in germ cells. Dominant-negative effect is usually observed with proteins that work as dimers or multimers. Drosophila miniature (M) encodes a membrane-anchored extracellular protein that is required for remodeling of cell shape and compartmentalization. Its Y117C mutant (Mtecta) has altered filament polymerization properties and exhibits a dominant-negative effect when expressed in wild-type Drosophila [Fernandes et al., 2010; Plaza et al., 2010]. In the septin family, SEPT9_v4 plays a dominant-negative role in cytokinesis, resulting in persistent midbodies and multinucleation [Estey et al., 2010]. SEPT9_v4 caused dislocalization of the endogenous SEPT9 and disrupted its polymerization. Expression of S148N, a SEPT9_v4 mutant, also acted in a dominant-negative fashion [Chacko et al., 2005]. Considering that SEPT1, SEPT4, SEPT6, SEPT7, and SEPT12 all localize in the sperm annulus [Ihara et al., 2005; Steels et al., 2007], it would be tempting to hypothesize that SEPT12T89M and SEPT12D197N prevent other septins from forming filaments through a dominant-negative effect.
Phenotype of the Patients
Of the two mutations, SEPT12T89M was presented with asthenoteratozoospermia (and borderline oligozoospermia), whereas SEPT12D197N was presented with oligoasthenozoospermia. Although the initial semen analysis of the SEPT12D197N patient did not fit a teratozoospermia diagnosis (20% of normal sperm), subsequent detailed analysis showed characteristic features in the majority of sperm (Supp. Fig. S6). Intriguingly, we found a correlation between the loss of SEPT12 signal and morphological defects, especially tail defect, in both SEPT12D197N patient and control cases (Supp. Table S1). The sperm without intact annulus are prone to bend while they move. The bent-tail defect was found in 17.36% of sperm in the SEPT12D197N patient, significantly higher than that observed in the fertile controls (4.14 ± 3.26%, data not shown). Taken together, the phenotypes of patients are in accordance with the Sept12 knockout mice, which presented with tail defect, maturation arrest, and increased apoptosis [Lin et al., 2009].
SEPT12 Mutations in Infertile Men Presenting with Distinctive Sperm Pathology
Annulus is a ring structure that demarcates the midpiece and the principal piece of the sperm tail. Its firm attachment to the flagellar membrane suggests that it may supply mechanical support and prevent displacement of the caudal mitochondrial helix [Fawcett, 1970]. Indeed, annulus-less sperm frequently have mitochondria defects, including abnormal arrangement, unequal size, irregular appearance, and reduced membranous material of mitochondria [Kissel et al., 2005; Lin et al., 2009; Toure et al., 2007]. In the mouse, Sept1/4/6/7 form the major septin complex in the sperm annulus. In Sept4 null mice, this complex failed to assemble due to the lack of Sept4, and Sept1/6/7 were dispersed throughout the cytoplasm. It was inferred that Sept4 is critical for septin complex assembly in the sperm annulus [Ihara et al., 2005]. The present study, together with a previous study, added SETP12 as one of the components of septin complex in the sperm annulus [Steels et al., 2007]. SEPT12 interacts with SEPT6 to form filamentous structure in HeLa cells [Ding et al., 2007]. It is possible that SEPT12 interacts with the SEPT1/4/6/7 complex to coordinate annulus formation. Considering heterozygous mutations of SEPT12 are sufficient to cause significant phenotypes, SEPT12 may be an important component of the septin complex in human sperm. Indeed, we observed defective annulus in the SEPT12D197N patient (Figs. 4 and 5; Supp. Table S1). We also found that SEPT12 transcript was equally abundant for D197N patient and control subjects (Supp. Fig. S4), suggesting that the mutant gene may produce fair amount of transcript and protein. We reason that SEPT12 mutants disrupt the septin complex through a dominant-negative effect, resulting in dislocalization or dispersal of SEPT12 (and other septin component) from the sperm annulus. For infertile men presenting with abnormal semen parameters, especially those with oligoasthenoteratozoospermia with defective annulus, attempts should be made to search for mutations of the SEPT12 gene.
Acknowledgements
We deeply appreciated the Transgenic Mouse Models Core of National Research Program for Genome Medicine for excellent technical assistance, and members of the Division of Reproductive Endocrinology (National Cheng Kung University Hospital) for their assistance in semen analysis, Dr. Pauline Yen (Institute of Biomedical Sciences, Academia Sinica, Taiwan) and Dr. Chun-Jung Chen (Life Science Group, Scientific Research Division, National Synchrotron Radiation Research Center, Taiwan) for critical reading of the manuscript.
Disclosure Statement: The authors declare no conflict of interest.