A Review: Can Cytokines Induce Vascular Inflammation as a Sequela of Viral Infections?
ABSTRACT
Background and Aim
There are many unknown consequences of viral infections. In this piece, we looked at one of these effects that influence the venous system of the body, vasculitis, an inflammation of the blood vessels.
Discussion
The study illustrates that viral infections attack host cells through viral proteins and surface receptors, activate NF-kappaβ via various receptors and signaling pathways, and subsequently induce a cytokine storm through the release of various pro-inflammatory cytokines, including IL-6, TNF-α, and CCL2, likely due to endothelial dysfunction caused by reactive oxygen species generation. Generally, overproduction of these mediators has been identified as a contributor to vascular inflammation and the subsequent development of atherosclerotic plaque, which may facilitate the initiation of vascular inflammation. This article also discusses potentially effective inhibitors for particular cytokines that contribute to vascular inflammation. Inhibiting the expression of these cytokines can diminish atherosclerotic lesions.
Conclusion
This article addresses the need for further investigation into the link between post-viral infection effects and vascular inflammation by discussing the potential mechanism by which the immune system acts upon pathogen entry, the factors responsible for influencing the immune system, and the prevention of infectious disease transmission.
1 Background
Infectious diseases are characterized by a condition brought on by a pathogen or its toxic byproduct and contracted by a susceptible host from an infected person, animal, inanimate substance, or all of the above [1]. During an infectious state, the body develops inflammation as a defense mechanism. This biological response to a pathogen is mediated through a number of proteins named cytokines, which are secreted at the time of infection and regulate the kind of response to be generated by the body. Depending on the severity of the disease caused by the pathogen, different types of cytokines are released at differing concentrations. The abundance of cytokines produced in response to an infection to mitigate the pathogen is termed “cytokine storm” [2]. The term “storm” refers to the pathophysiology of cytokine storm, which is characterized by an inflammatory reaction triggered by an uncontrollably active immune system. Furthermore, a situation known as a “cytokine storm” is defined by an overstimulated pro-inflammatory response and a deficient anti-inflammatory response [3]. There is recent evidence that inflammation and infection can independently initiate the development of atherosclerosis [4]. Studies have shown that certain bacteria, such as Chlamydia pneumoniae and Helicobacter pylori, as well as various viral agents, including SARS-CoV-2, influenza viruses, hepatitis viruses, herpes simplex viruses (HSV), human papillomavirus (HPV), human cytomegalovirus (HCMV), and human immunodeficiency virus (HIV), can contribute to the development of vascular inflammation. This occurs through mechanisms such as the excessive production of cytokines, molecules that attract immune cells, molecules that promote cell adhesion, and growth factors following infection. Additionally, these infections lead to increased oxidation and uptake of low-density lipoprotein (LDL), as well as enhanced resistance against cell death (apoptosis) [5, 6]. The cause of vascular inflammation is not fully clear, but it may be brought on by food, illness, medication, or an infection. Generally, cytokines are one of the major factors in developing vascular inflammation. This article aims to propose a hypothesis concerning the development of vascular inflammation resulting from cytokine storms in infectious diseases, and there is a possibility that these cytokines may initiate or make the existing inflammatory situation worse and suggests that inhibiting the expression of these cytokines may alleviate chronic vascular inflammation, such as atherosclerosis.
2 Infectious Diseases and Inflammatory Mediators
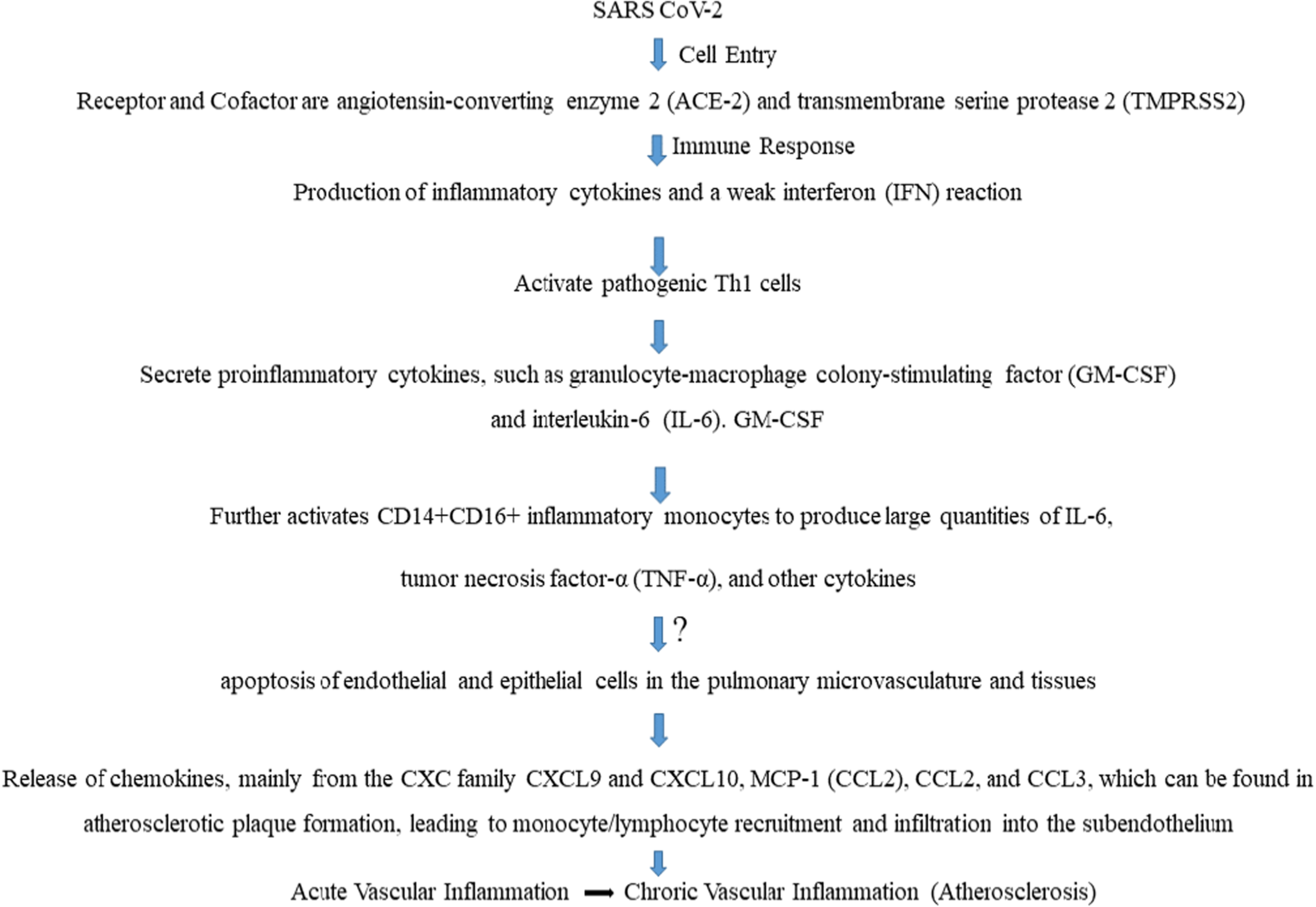
Various kinds of inflammatory mediators are expressed and released in the event of an infectious disease (Table 1). These include cytokines such as chemokines, interleukins, interferons, etc. Multiple lines of evidence establish a causal relationship between inflammatory mediator release and vascular inflammation [14-16]. As an example, here, we show the recent worldwide epidemic of COVID-19, which has been linked to significant vascular inflammation in the pulmonary and extrapulmonary vasculature on both the macro- and microvascular levels. The ACE-2 receptor and the transmembrane protease serine 2 are ubiquitously expressed, allowing SARS-CoV-2 to infiltrate host endothelium cells via endocytosis [2]. Regarding the immune response, micro- and macrovasculature (Leukocytoclastic vasculitis) inflammation has been linked to a type-3 hypersensitivity reaction in COVID-19. Another immune response seen in almost every case, mild or very severe, is cytokine storm developed by activation of NF-κB by neutralizing its inhibitory component IκB [2]. Immune cell activation and the release of pro-inflammatory cytokines like IL-6, TNF-α, and CCL2 result from this mechanism [14]. In COVID-19, there is an increase in the relative frequencies of plasmablasts and circulating activated CD4+ and CD8+ T lymphocytes. TNF-α and IL-1β seem to have prominent effects on the endothelium function. Post-COVID severity has also become a matter of concern as it seems to develop multiorgan diseases, such as heart conditions, neurological conditions, or blood clots, compared with people who have not had COVID-19 [17]. Figure 1 is intended to hypothesize the mechanism by which viral infections, such as SARS-CoV-2, stimulate the production of cytokines and subsequently cause vascular inflammation [18-20].
| Infectious diseases | Mediators expressed | References |
|---|---|---|
| Influenza A | TNF-α, IL-6, IL-1β, IL-18, IL-17 | [7] |
| Dengue | VEGF-A, IL-10, IL-6, IL-8 | [8] |
| AIDS | IL-1, IL-6, IL-8, TNF-α | [9] |
| Chickenpox | IL-6, IL-8, IL-10 | [10] |
| Infectious mononucleosis | IL-6, TNF-α | [11] |
| Tuberculosis | TNF-α, IL-12 | [12] |
| COVID-19 | IL-2, IL-7, IL-10, TNF-α, | [2] |
| Swine flu | IL-8, IL-9, IL-17, IL-6, TNF-α, IL-15, IL-12p70 | [13] |
| Avian flu | IL-8 | |
| SARS | IL-1β, IL-6, IL-12, IFN-γ | |
| MERS | IFN-γ, TNF-α, IL-15, IL-17 |
The three primary PRR families consist of Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [21]. The abnormal signaling pathways via these receptors excessively stimulate inflammatory cytokines and chemokines. SARS-CoV-2 antigens are detected by PRRs in the cytosol, on the cell surface, and on endosomal membranes. This triggers innate immune signaling pathways. Pro-inflammatory cytokines can be produced by TLR2 and TLR4, activating the NF-κB and MAPK signaling pathways via MyD88 (Myeloid differentiation primary response 88) [2]. IRF3 can be activated, and type I interferon (IFN) production can be induced by TLR4 and TLR3 signaling TRIF. Through MyD88, TLR7/8 and TLR9 can send signals that activate NF-κB and IRF, causing the creation of IFN-I and inflammasomes. Pathogenesis is a result of hyperinflammation. To generate IFNs, RLRs detect viral RNA and activate IRF3. After sensing viral RNA, spike (S) and nucleocapsid (N) proteins, and open reading frame 3A (ORF3A), the NLRP3 inflammasome is formed. The result of this assembly is interleukin (IL)−1β production. In general, COVID-19 disease may be caused by these three signaling pathways being delayed or overactivated [2, 22].
Primorac and the peers discussed bout the adaptive immunological response to the first and second interactions with the SARS-CoV-2 antigen (spike protein) [23]. Several antigens undergo phagocytosis and degradation within antigen-presenting cells (APCs) after the initial interaction. Major histocompatibility complex class II (MHC-II) molecules on the surface of APCs mediate the process by which they present the antigen in smaller peptide form. After that, the antigens are transmitted to several cell types within the host, with a focus on CD4 + T helper cells (along with B and CD8+ cells). Antibodies are secreted by B cells, which eventually become plasma cells; these antibodies block the viral particle from entering healthy cells. Different subtypes of T helper cells are activated by APC and go on to perform distinct roles through cell-to-cell interaction and cytokine production. By secreting IL-4 and interacting with CD40/CD40L, Th2-differentiated T helper cells aid in the maturation of humoral responses by sending a second signal to B cells [23]. Memory B cells and long-lived high-affinity antibody-producing plasma cells are regulated by the interactions that take place in the germinal centers, which are governed by a subset of CD4+ cells called T follicular helper cells (Tfh). Memory T helper cells are produced by a separate subgroup of CD4+ T cells. When it comes to building cellular responses, Th1 T helper cells are vital. Through simultaneous interaction with APCs, they pivot the MHC-I activation of CD8+ cells (cytotoxic T lymphocytes, or CTLs). After that, activated CTLs take action by triggering cell death in SARS-CoV-2-infected host cells through FasL and ligand-FasL interaction [23]. In response to further antigen encounters, a subset of CTLs undergoes differentiation into memory cytotoxic T cells, whose role is to rapidly restore the CTL response. Interaction between NK cells and the virally infected cell triggers a comparable process of cellular death. Programmed cell death is caused by the secretion of granules containing IFNγ and TNF-α found in their cytoplasm [23].
Another significant concern that remains to be uncovered is the occurrence of post-COVID-19 sequelae resulting in multiorgan failure. The symptoms of long COVID, sometimes referred to as post-acute sequelae of COVID-19, have been well recorded [24]. However, there is a lack of comprehensive understanding of the natural progression of the condition, including its impact on symptoms, organ damage, and overall functioning [25]. According to a Han et al. comprehensive analysis, 8591 COVID-19 survivors had a pooled prevalence of fatigue/weakness (28%) and dyspnea/breathlessness (18%) at a 1-year follow-up, which raises additional concerns about long-term COVID care [26]. The features of extended COVID were examined in a systematic review conducted by Michelin et al. There were 60 signs and symptoms—psychological and physical—that were widely prevalent. Weakness, overall malaise, exhaustion, a lower quality of life, difficulty concentrating, poor lung function, and dyspnea were the most frequent [27]. It is crucial to confirm the occurrence of multiorgan dysfunction in 29% of individuals with prolonged COVID at both the 6 and 12-month intervals. This dysfunction is marked by enduring symptoms and a deterioration in overall physiological functioning [28]. Post-COVID-19 syndrome is still a mystery, and its exact pathophysiological mechanism is not fully understood. Long-lasting COVID-19 symptoms need to be documented through vigorous studies to increase awareness and raise concerns.
An infection with the human monkeypox virus (MPXV) induces a diverse array of cardiac symptoms. Research suggests that the virus can penetrate endothelial cells, disrupting their normal physiological functions, which leads to endothelial dysfunction and subsequently atherosclerosis [29, 30]. Multiple signal transduction pathways, such as TLR-4, NF-κB, and MAPK, can activate various pro-inflammatory cytokines and macrophages during MPXV infection. Activated macrophages can release pro-inflammatory cytokines, including TNF-α. These signal transduction pathways also induce endothelial dysfunction and consequent cardiovascular inflammation [31]. In endothelial cells, the specified signal transduction pathways may lead to an elevation in reactive oxygen species (ROS) and oxidative stress. Consequently, similar to other viral infections, MPXV may induce an increase in reactive oxygen species (ROS) generation and damage to antioxidant mechanisms, thereby compromising the immune system [32, 33]. The cytokine storm caused by MPXV infection is associated with a robust Th2 immune response, characterized by elevated serum concentrations of IL-4, IL-6, IL-5, and IL-10, alongside a reduction in Th1-related cytokines such as TNF-α, IL-2, IL-12, IFN-α, and IFN-γ [34]. For example, they can activate leukocytic pro-inflammatory signaling pathways, including NF-κB, MAP kinases, and STATs, which regulate the expression of numerous pro-inflammatory genes. Inflammatory cytokines, adhesion molecules, and chemokines are generated as a consequence of those signaling activations [29].
The Hepatitis B virus is a hepatotropic virus that replicates in the liver; this viral infection can induce various extrahepatic diseases, impacting multiple organs and systems inside the human body. The extrahepatic manifestations of HBV infection encompass immune-mediated syndromes and direct viral effects, leading to various clinical presentations, including renal disorders (glomerulonephritis, membranous nephropathy, membranoproliferative glomerulonephritis, IgA nephropathy), systemic manifestations (cryoglobulinemia, polyarteritis nodosa, serum sickness-like syndrome), as well as rheumatologic, hematologic, neurologic, ophthalmologic, and dermatologic disorders. These extrahepatic consequences may arise in both acute and chronic HBV infections [35, 36]. The liver, with its unique anatomical and immunological structure, is also considered an immunological organ that produces large amounts of cytokines and chemokines (such as IL-1, IL-2, IL-6, IL-10, IL-21, etc.) under pathological conditions, playing a significant role in the progression of HBV infection [37].
Central nervous system (CNS) vasculitis primarily impacts cerebral vessels and, to a lesser extent, those in the spinal cord, leading to various consequences including ischemic stroke, intracerebral or subarachnoid hemorrhage, mycotic aneurysms, venous thrombosis, and transient ischemic attacks (TIAs). Numerous microbiological agents have been linked to secondary CNS vasculitides, typically occurring during or following a clinically apparent infection. Bacterial infections, including Pneumococcal meningitis, are particularly perilous, exhibiting elevated fatality rates and significant neurological sequelae in survivors. Viral infections such as human immunodeficiency virus (HIV) may result in various neurological consequences, including vasculitis, particularly in advanced stages. CNS vasculitis is infrequent (under 1% of occurrences), although HIV-associated vasculopathy constitutes a considerable proportion of strokes in these individuals. Fungal infections, particularly Aspergillosis, are the most common invasive mold infections globally, mostly affecting immunocompromised individuals. Nonetheless, CNS vasculitis and strokes may also occur in immunocompetent patients [38-40].
3 Infectious Diseases and Vascular Inflammation
Higher organisms developed the inflammatory response as a protection mechanism against infection and damage. The initial response of the organism to harmful stimuli is acute inflammation and is characterized by increasing blood flow, enabling plasma and leukocytes to reach extravascular sites of injury, elevating local temperatures, and causing pain. When a microorganism invades into the cells, a strong host response gets initiated. The simple interaction of viral surface proteins with cellular surface proteins causes the first wave of cytokine production [41].
Endothelial cells (ECs), vascular smooth muscle cells (VSMCs), inflammatory cells such as neutrophils, lymphocytes, monocytes, macrophages, and the extracellular matrix (ECM) all interact intricately throughout the vascular inflammatory response [42]. Increased production of adhesion molecules by ECs, recruitment of inflammatory cells, growth factors, and cytokines, and subsequent effects on ECs, VSMCs, and ECM are related to vessel damage. Interleukins, lymphokines, monokines, interferons, colony-stimulating factors, and transforming growth factors (TGFs) are all examples of cytokines [2, 14]. Platelets, ECs, and VSMCs, as well as macrophages, T cells, and monocytes, generate cytokines. When circulating cytokines bind to their receptors on distinct cell types, they trigger an inflammatory response that includes cell adhesion, permeability, and apoptosis by activating the JAK-STAT, NF-κB, and Smad signaling pathways [2].
Interferons are generally known as the killers of viral infection by interfering with viral replication. As the cell dies, nearby macrophages and monocytes pick up dead cells as well as virions and get infected. Naturally, macrophages will start producing cytokines like IL-12, IL-6, TNF-α. These cytokines are super potent and start creating leaky endothelium. Concurrently, the Th1 cells produce IFNγ, IL-2, and TNF-β, which again activate macrophages, thus the cycle of cytokine production and cell recruitment continues [43]. In response to cytokine and from microbial invasion, reactive oxygen species (ROS) are produced by host defense cells like neutrophils. The development of many inflammatory disorders depends heavily on the production of ROS. The widely studied free radicals are superoxide anion, hydroxyl radical, and hydrogen peroxide. These free radicals can harm healthy cells and cause inflammation [44]. Thus, we are concerned to propose a hypothesis that through endothelial dysfunction, pro-inflammatory cytokines overexpression and cellular apoptosis in response to viral infections, may lead to the initiation or progression of vascular inflammation and possibly atherosclerosis at a later stage (Figure 2).

Atherosclerosis begins with endothelial dysfunction and structural changes. These alterations result in the buildup of circulating low-density lipoprotein (LDL) within the subendothelial layer of arteries. Reactive oxygen enzymes released by inflammatory cells cause oxidative changes in LDL, leading to the production of oxidised LDL. Oxidised low-density lipoproteins (oxLDLs) induce the production of adhesion molecules and pro-inflammatory cytokines from endothelial cells, leading to the recruitment of immune cells into the intima. The process of phagocytosis of oxLDL by immune cells, particularly macrophages, results in the production of foam cells and induces apoptosis in cells. The presence of apoptotic cells and cholesterol crystals leads to the production of necrotic shapes, ultimately causing the development of plaque [45].
Various viruses have been discovered to induce damage to the endothelium, impair vascular function, and produce extensive effects on many organ systems by disturbing the usual balance of the endothelial cells. This endothelial damage by the viral infection may trigger an inflammatory response, the production of cytokines, and the leaking of blood vessels, which can affect several organs to different extents [46, 47]. Tumour necrosis factor (TNF) is a cytokine that has been demonstrated to cause dysfunction in the endothelium barrier and is one of several cytokines involved in the pathology of influenza. Elevated levels of TNF, IL-6, and IL-1β after influenza virus infection have been demonstrated to stimulate the production of trypsin, leading to the degradation of the endothelium tight junction protein, zonula occludens-1 (ZO-1), and a consequent increase in vascular permeability. Increased chemokine expression during influenza virus infection may also contribute to endothelial barrier dysfunction [48].
Endothelial cells (ECs) exhibit inflammatory activity, which leads to an upregulation of selections, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1). This upregulation facilitates the recruitment of monocytes. Pro-inflammatory cytokines, such as IL-1β and TNF-α, induce the expression of adhesion molecules. Additionally, the acute-phase protein C-reactive protein (CRP), which is produced by the liver in response to IL-6, also induces adhesion molecule expression [16, 45]. Adhesion molecule expression can also be triggered by protease-activated receptor signalling, ox-LDL uptake via ox-LDL receptor-1 (LOX-1), and interactions between CD40/CD40 ligands (CD40L and CD154). Endothelial dysfunction can lead to the development of vascular disease through a series of pathological events [49].
Atherosclerosis, a primary consequence of diabetes mellitus (DM), is characterized by various pathogenic causes, including endothelial dysfunction and oxidative stress [50]. Inflammasomes are heterogeneous assemblies generated by pattern recognition receptors that are triggered by diverse physiological or pathogenic stimuli. These complexes generate an innate immune response, enabling the elimination of pathogens and injured cells. NLRP3, or “pyrin domain-containing protein 3”, is the most well-known inflammasome. It is a member of the family of nucleotide-binding and oligomerization domain-like receptors (NLRs) [51]. Two signaling stages must be fulfilled for the NLRP3 inflammasome to become active. NLRP3 and IL-1β expression can be upregulated by the first signal, which can include lipopolysaccharide or cytokines that activate the NF-κB pathway. The second signal can facilitate the assembly of the NLRP3 inflammasome complex by containing a range of damage- and pathogen-associated molecular patterns (DAMPs), including lysosomal disruption, ROS increase, endoplasmic reticulum stress, mitochondrial dysfunction, and Ca2+ signaling. Pro-caspase-1 is cleaved into active caspase-1, which subsequently cleaves to the mature form and causes pyroptosis. A major part of inflammation and a number of illnesses are mediated by NLRP3. The NLRP3 inflammasome may play a role in the onset of diabetes mellitus and atherosclerosis, according to mounting data [52].
The exact mechanism via which numerous viruses, such as the influenza virus, generate atherosclerosis is not fully understood; nevertheless, inflammation and coagulopathy seem to be key factors. The mechanisms associated may include the following: (a) antigenic cross-reactivity; (b) elevated levels of pro-inflammatory and prothrombotic cytokines, including IL-2, IL-6, IL-10, and IL-18; (c) expression of pro-inflammatory cytokines by infected monocytes and reduced clotting time; (d) enhanced macrophage infiltration into the arterial wall; and (e) induction of procoagulant activity in infected endothelial cells, diminished clotting time, and heightened expression of tissue factor [53]. Recurrent influenza virus infection can harm vascular endothelial cells and initiate the inflammatory response that accelerates and worsens atherosclerosis, resulting in vascular inflammation. The incidence of atherosclerosis may also potentially increase due to Herpes Simplex Virus (HSV) [54]. The principal receptor protein for oxidized low-density lipoprotein (ox-LDL), lectin-like oxidized LDL receptor-1 (LOX-1), is increased by HSV [55]. Consequently, endothelial cells demonstrate elevated ox-LDL uptake, lipid accumulation, and metabolism, driven by the augmented acquisition of saturated cholesteryl esters and triacylglycerols. This leads to coronary artery calcium accumulation and thrombosis, all of which are linked to the advancement of vascular inflammation and subsequent atherosclerosis [56]. Moreover, a significant correlation exists between atherosclerosis and human immunodeficiency virus (HIV) infection, with individuals who are HIV-positive exhibiting a greater prevalence of atherosclerosis than their HIV-negative counterparts [57]. The specific effects of viral infections on vascular inflammation may vary depending on the type of virus, the host's immune response, and pre-existing medical conditions.
4 Discussion
Pathogens may induce vasculitis in several ways. One way is the cytokine overproduction and adhesion of molecules participating in the complex interactions between leukocytes and endothelial cells, that results in vascular inflammatory response [58]. But it is likely that direct endothelial invasion and damage are the primary mechanism. Leukocytoclastic vasculitis is also believed to have a primary humoral immune response with immune complex production and deposition in and around vessel walls. Immune dysregulation brought on by infection is a less frequently proposed cause of vasculitis [59].
A 19-year national survey indicated an overall increase in the incidence of severe illnesses from 1998 to 2016. They noted that sepsis surpassed pneumonia as the primary serious infection in 2011–2012. In 2015–2016, sepsis manifested 2.3 times more frequently than pneumonia in persons with vasculitis [60].
Cytokine storm can participate in endothelial activation. Endothelium is a tissue that controls the exchange of nutrients, fluids, and metabolites and is regarded as a key mediator of processes such as vasoconstriction or relaxation, vascular remodeling, cellular adhesion, etc. When endothelial cells are exposed to inflammatory stimuli, they develop abnormalities in vascular tone and permeability. It undergoes morphological and functional changes in cases of infections or tissue destruction, which is known as endothelial dysfunction [61].
Infectious diseases may leave a marked effect, namely vasculitis, on the vascular system of the human body. To prevent further endothelial dysfunction and vasculitis, proper vaccination against said infectious diseases is necessary, as vaccines are considered as the only preventive measures against any viral infection [62]. If vaccination is not possible due to the fact that it is a fairly new disease and a reliable vaccine is yet to be made, the first treatment strategy against the disease should be followed according to the symptoms using various effective antiviral and antimicrobial drugs. To deter the spread of vasculitis, steroids and immunosuppressants are suggested. Multiple factors, including food consumption habits, lipids, infections, and early-life psychosocial factors, have been identified as significant contributors to the development of atherosclerosis (a chronic vascular inflammation) [4].
The primary systemic vasculitides (PSV) denote a series of autoimmune rheumatic disorders (AIRDs) distinguished by vascular inflammation, resulting in ischemia occurrences and end-organ impairment. Notwithstanding progress in the management of these disorders, infections continue to be a significant source of morbidity and mortality among individuals with vasculitis [63]. Screening for viral hepatitis should be conducted in all patients, preferably before initiating treatment. HBV vaccination must be administered before the commencement of treatment for patients with negative first HBV testing, and modified regimens have been proposed for individuals undergoing immunosuppressive therapy. Patients with autoimmune inflammatory rheumatic diseases (AIRDs) undergoing treatment with biologic therapies, other immunosuppressive agents, or medium to high doses of glucocorticoids ( > 7.5 mg/day prednisone equivalent) are at an elevated risk for reactivation of hepatitis B (HBV) or hepatitis C virus (HCV); therefore, screening for viral hepatitis is essential before initiating treatment. Vaccination should be advocated for all patients with autoimmune inflammatory rheumatic diseases (AIRD), including primary systemic vasculitis (PSV); but, in certain circumstances, such as life-threatening conditions, therapy for AIRDs should be initiated promptly, irrespective of vaccination status. Although instances of disease flares postvaccination have been documented, they are infrequently severe, and the advantages of immunization surpass the possible dangers, particularly in this high-risk demographic. Annual influenza vaccine, utilizing either high-dose or adjuvanted formulations, along with COVID-19 and pneumococcal vaccinations, is advised for all patients aged 65 and older with autoimmune inflammatory rheumatic diseases (AIRD) and for those aged 18 and older undergoing immunosuppressive therapy. Additionally, emphasis should be given to the utilization of prophylaxis in infectious diseases [64].
Therapies targeting cytokine storms can successfully mitigate tissue damage and enhance the elimination of invading microorganisms. The course of cytokine release syndrome (CRS) is marked by substantial and atypical elevations in the levels of different cytokines, several of which are crucial in inflammatory and pathogenic processes. Consequently, the neutralization of excessively high cytokines in the body via the delivery of targeted monoclonal antibodies may mitigate inflammation. IL-1 receptor inhibition has been linked to decreased mortality in sepsis patients, while substantial mitigation of cytokine release syndrome related to excessive IL-6 production has been accomplished with the humanized anti-IL-6 receptor antibody tocilizumab, which inhibits IL-6 synthesis [13]. For instance, individuals with COVID-19 may have higher levels of cytokines such as IL-6, IL-2, IL-7, and IL-10, all of which utilize a distinct JAK-mediated intracellular signaling mechanism [2]. Consequently, the inhibition of JAK may represent a viable approach for managing CRS linked to COVID-19 infection [65].
Future cytokine-based therapies are presently being explored in preclinical trials of atherosclerosis. Inhibitory experimental compounds have been developed, including MCC950, arglabin, and VX765 [66]. MCC950 effectively mitigated myocardial ischemia/reperfusion injury in a porcine model of myocardial infarction by restricting neutrophil infiltration into the myocardium and reducing IL-1β levels [67]. Arglabin has been minimally examined in atherosclerosis research, demonstrating anti-inflammatory effects (transition to an anti-inflammatory macrophage phenotype) and hypolipidemic effects (down in total cholesterol and triglycerides) [68]. VX765, a pro-drug that inhibits caspase-1 and is activated by intracellular esterases, reduced pyroptosis in vascular smooth muscle cells and the progression of atherosclerosis in ApoE−/− animals fed a Western diet [69]. In anti-chemokine treatments, the inhibition of RANTES receptors reduced atherosclerosis, evidenced by less leukocyte migration and plaque stability. Modulating the CCL2/CCR2 axis may provide an intriguing strategy against atherogenesis [70, 71]. A recent meta-analysis of preclinical studies has been released, encapsulating the scientific information in this pathway. Inhibition of CCL2/CCR2 reduced atherosclerotic lesions in the aorta, carotid, and femoral arteries. Furthermore, it augmented collagen deposition and smooth muscle cell content while concurrently reducing macrophage accumulation [72].
Sedentary lifestyle of a person and their habits also dictate the overall physiological health of a person. Smoking, alcohol consumption, lack of exercise, and a healthy diet are crucial factors that affect the immune system, which ultimately renders the person susceptible to infectious diseases. An individual's food habit after the contraction of an infectious disease also affects vascular health. For instance, intake of highly fatty food results in lipid deposition on the vascular walls, which is instigated by the endothelial dysfunction caused by the initiation of the infectious disease. If the person didn't contract the disease, lipid deposition would have been much slower. So, food habits must be changed to healthier ones so that the person is able to receive adequate nutrition to boost the immune system and provide protection to the body. Some natural preventive measures can be taken, which include the reduction of caffeine intake, sweet beverages, and alcohol. Processed meat, raw seafood, or undercooked meat should also be avoided as they may contain microorganisms that are extremely dangerous to the body's immune system. Intake of fresh fruits, vegetables, low-fat dairy products, lean meat, and fish should be emphasized. Some supplements may be prescribed by the doctor as an extra measure when steroids are administered. Cleanliness and hygiene should be maintained thoroughly to prevent the spread of diseases. An individual who has recovered from an infectious disease should be checked at regular intervals for the markers indicating the presence of inflammatory mediators, which may cause further complications in the body. National and global surveillance is essential for combating infectious diseases. Poor and developing countries lack access to clean water, effective sewage treatment and disposal, and access to safer food and vaccination programs. The inability to discover, diagnose, and contain infectious diseases is hampered by a lack of healthcare professionals, disease monitoring programs, and modern laboratories.
5 Conclusion
Vasculitis is not a new concept, but it received the limelight during the recent COVID-19 pandemic because it has long-term consequences. Susceptibility of a person to an infectious disease differs from person to person based on their lifestyle, age, food habits, and various environmental factors, which was proven most recently during the COVID-19 pandemic. So, the general masses should be made aware of the infectious diseases and the effects they have on the human body. Especially in the developing and underdeveloped countries, special measures should be taken to provide basic and sufficient knowledge about the benefits of leading a healthy lifestyle, how to boost the immune system, and keep one healthy.
Author Contributions
Shahana Akhter Deena: conceptualization, writing – original draft. Samia Aziz Tonima: conceptualization, writing – original draft. Sakif Ahamed Khan: writing – review and editing. Mohammad Shahangir Biswas: writing – review and editing. Syed Masudur Rahman Dewan: conceptualization, writing – review and editing, supervision.
Acknowledgments
The authors received no specific funding for this work. All authors have read and approved the final version of the manuscript. S.M.R.D., the corresponding author, had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The corresponding author, Syed Masudur Rahman Dewan, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.