Characteristics and In-Hospital Outcomes of Patients With Septic Shock in the Adult Medical Intensive Care Unit of a Tertiary Hospital in Ethiopia: A Prospective Observational Study
ABSTRACT
Background and Aims
Sepsis and septic shock are major healthcare problems, affecting millions of people around the world each year and claiming the lives of one-sixth to one-third of those affected. However, there is insufficient data on the characteristics and outcomes of septic shock patients in resource-limited settings. This study aimed to assess the characteristics and in-hospital outcomes of septic shock among patients admitted to the adult medical intensive care unit (ICU) of a tertiary hospital in Ethiopia.
Methods
A prospective observational study was conducted among patients diagnosed with septic shock according to consensus criteria who were admitted to the adult medical ICU of a tertiary hospital in Ethiopia from January 1, 2023, to September 30, 2023.
Results
A total of 144 patients were included in this study, with a mean age of 46 ± 19 years. The respiratory system was the most common source of infection, affecting 70.2% of the patients. The blood culture positivity rate was 8.4%, and antibiotics were revised for only 2.8% of the patients tailored to culture results. Adrenaline was the most frequently used vasopressor (73.6%), followed by noradrenaline (27.8%). The main complications were acute kidney injury (51.4%) and acute lung injury (50.7%). The in-hospital mortality rate was 66.7%, with a median hospital stay of 6 days (interquartile range: 2–11 days). Multivariable Cox regression analysis showed that a Glasgow Coma Scale score below 15 (AHR: 2.23; 95% CI, 1.35–3.69) and peripheral oxygen saturation under 90% (AHR: 8.74; 95% CI, 1.18–14.84) were independently associated with increased in-hospital mortality risk.
Conclusions
This study demonstrated a high mortality rate among septic shock patients with more than two-thirds of patients dying during their hospital stay. The significant prognostic indicators of in-hospital mortality were impaired mental status and peripheral oxygen desaturation.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- ALI
-
- acute lung injury
-
- CNS
-
- central nervous system
-
- CONS
-
- coagulase-negative staphylococcus
-
- DM
-
- diabetes mellitus
-
- EGDT
-
- early goal-directed therapy
-
- GCS
-
- Glasgow coma scale
-
- HIV
-
- human immunodeficiency virus
-
- ICU
-
- intensive care unit
-
- SIRS
-
- systemic inflammatory response syndrome
-
- SOFA
-
- sepsis-related organ failure assessment
-
- SpO2
-
- peripheral oxygen saturation
-
- SSA
-
- Sub-Saharan Africa
1 Background
The 1991 American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference defined sepsis as a systemic inflammatory response to infection meeting at least two systemic inflammatory response syndrome (SIRS) criteria, severe sepsis as sepsis with organ dysfunction, and septic shock as sepsis-induced hypotension resistant to fluid resuscitation [1]. The 2016 Sepsis-3 definitions redefined sepsis as life-threatening organ dysfunction due to a dysregulated response to infection, identified by a sepsis-related organ failure assessment (SOFA) score > 1 or a quick SOFA (qSOFA) score ≥ 2 (Glasgow Coma Scale (GCS) < 15, systolic blood pressure (SBP) ≤ 100 mmHg, respiratory rate (RR) ≥ 22 breaths per minute). Septic shock was defined as a subset of sepsis with the requirement of vasopressor(s) to maintain mean arterial pressure (MAP) ≥ 65 mmHg and lactate level ≥ 2 mmol/L [2]. However, the 2021 Surviving Sepsis Campaign advised against qSOFA as a sole screening tool for sepsis or septic shock due to its lower sensitivity compared to SIRS [3, 4].
Sepsis and septic shock are major healthcare problems, affecting millions of people around the world each year and claiming the lives of one-sixth to one-third of those affected [5-7]. The 2017 Global Burden of Disease (GBD) study estimated 48.9 million sepsis cases and 11 million sepsis-related deaths, accounting for one-fifth of global deaths [8]. This report indicated that the highest rates of sepsis incidence and mortality were seen in low- and middle-income countries, particularly sub-Saharan Africa (SSA). A large retrospective U.S. database analysis found mortality rates of 6% for sepsis, 15% for severe sepsis, and 34% for septic shock, with associated costs of about $16,000, $25,000, and $38,000, respectively [9]. A systematic review and meta-analysis of 170 studies from Europe, North America, and Australia (2009–2019) found a 30-day septic shock mortality rate of 34.7% [10].
Despite extensive research on sepsis and septic shock globally, there is a lack of population-based data from low-income countries, especially in sub-Saharan Africa [11]. A systematic review of five studies from SSA on septic patients admitted to the adult medical intensive care units (ICU) found a pooled prevalence of 31% and a mortality rate of 46% [12]. Studies conducted in Ethiopia showed a high level of septic shock mortality rate, with 58.3% at Tikur Anbessa Specialized Hospital [13] and 42% at the University of Gondar Hospital [14]. Various studies have identified key predictors of in-hospital mortality from septic shock, including delayed sepsis recognition, late ICU admission, older age, comorbidities, low MAP, high SOFA score, respiratory failure, lack of pathogen identification, and inappropriate antibiotic treatment [13-18].
While the healthcare impact of septic shock in resource-limited settings is presumably much higher than that of the developed nations, there have been insufficient data on the characteristics and outcomes of septic shock patients in such settings. Therefore, this study aimed to assess the characteristics and in-hospital outcomes of septic shock among patients admitted to the ICU of a tertiary hospital in Ethiopia.
2 Methods
2.1 Study Design, Period, and Area
A prospective observational study was conducted from January 1, 2023, to September 30, 2023, among septic shock patients admitted to the adult medical ICU of Yekatit 12 Hospital Medical College (Y12HMC), a teaching hospital in Addis Ababa, Ethiopia. It is one of the largest hospitals in the country, with over 500 beds, treating approximately 310,000 patients each year, and has 21 departments. The hospital has a 10-bed adult medical ICU that serves about 350–400 patients per year. Septic shock patients who were admitted to the adult medical ICU were managed by a team of nurses, residents, anesthesiologists, emergency medicine and critical care physicians, and internists.
2.2 Source and Study Population
The source population was all septic shock patients who were admitted to the adult medical ICU in public hospitals in Addis Ababa. The study population was all septic shock patients aged ≥ 15 years who were admitted to the adult medical ICU at Y12HMC.
2.3 Eligibility Criteria
Eligible patients were those aged 15 years or older admitted to the adult medical ICU at Y12HMC with septic shock, defined as a subset of sepsis with a suspected or confirmed focus of infection, requiring a vasopressor to maintain a MAP ≥ 65 mmHg. Patients with septic and cardiogenic shock were excluded to reduce the potential for misclassification between the two conditions and to ensure that our analysis was focused specifically on septic shock cases. Additionally, patients who had incomplete documentation of essential clinical or laboratory data and those who were unable to provide consent were excluded from the study.
2.4 Study Variables
The dependent variable was the incidence of in-hospital mortality. Independent variables were demographic data and medical history (age, sex, prior hospitalization, and comorbidities), clinical characteristics (duration of illness, vital signs, GCS, and source of infection), microbiological evidence, treatment-related factors (timing of antimicrobial initiation, type of antimicrobial agent, type of vasopressor, and steroid use), length of hospital stay, and systemic complications.
2.5 Operational Definitions
SIRS was defined by the presence of at least two of the following four clinical characteristics: axillary body temperature < 36°C or > 38°C; heart rate (HR) > 90 beats/min; hyperventilation, evidenced by a RR > 20 breaths/minute or partial arterial pressure of carbon dioxide < 32 mmHg; and white blood cell (WBC) count > 12,000 cells/μL or < 4000 cells/μL or with > 10% immature forms [1].
The SOFA score was a 24-point measure of organ dysfunction that used six organ systems (cardiovascular, pulmonary, renal, hepatic, neurologic, and hematologic), where 0–4 points were assigned per organ system [2].
Septic shock was defined as a subset of sepsis with a suspected or confirmed focus of infection, in which a vasopressor was required to maintain a patient's MAP ≥ 65 mmHg [2].
Low GCS was defined by a GCS score less than or equal to 14, indicating mild to severe impairment of consciousness or neurological function [2].
Low SpO2 was defined by peripheral oxygen saturation levels below 90%, indicating hypoxemia [19].
Acute lung injury (ALI) was defined by acute respiratory insufficiency with tachypnea, peripheral oxygen saturation < 90%, and diffuse pulmonary infiltrates on chest X-ray [20].
The timing of antimicrobial initiation was defined by the time between septic shock diagnosis documented by a physician and the first dose of antimicrobial administration.
Outcomes included systemic complications, in-hospital mortality, and the length of hospital stay.
2.6 Sample Size Determination and Sampling Technique
The sample size was calculated as 374 using the single population proportion formula: , using a 42% in-hospital mortality rate (p = 0.42) derived from a study conducted at the University of Gondar Hospital in Ethiopia [18], a 95% confidence level (Zα/2 = 1.96), and a 5% margin of error (d = 0.05). After adjusting for an estimated population of 225 patients over 9 months, the sample size was reduced to 140 using the correction formula . Including a 15% nonresponse rate, the final sample size was 161. A convenient sampling technique was used for data collection and all patients with septic shock who fulfilled the inclusion criteria were included.
2.7 Data Collection Procedure and Data Quality Control
Two internal medicine residents, who were trained on how to interview patients and extract data from electronic health records, filled out a prestructured questionnaire, which was prepared by reviewing different literature. Data regarding age, medical history, and duration of illness were collected on the day of admission to the adult medical ICU by interviewing patients and/or their legal guardians, and these were cross-checked for consistency with the patients' electronic health records. Additional clinical characteristics, source of infection, laboratory findings, management-related factors, and outcomes were extracted from the EMR system on the day of admission to the adult medical ICU and during the subsequent in-hospital stay. The data collection process was supervised and monitored by the principal investigators. The completeness and consistency of the collected data were ensured on a daily basis.
2.8 Statistical Analysis
Epi Info, version 7, and SPSS, version 25 (developed by IBM's team based in Chicago, Illinois, USA) were utilized for data entry and statistical analysis, respectively. Data cleaning and consistency checks were performed to ensure the accuracy and reliability of the data set. The characteristics and outcomes were summarized using descriptive statistics. Categorical variables were reported as frequencies and percentages, while continuous variables were presented as means with standard deviations for normally distributed data, and as medians with interquartile ranges for non-normally distributed data. Crude hazard ratios (CHR) and adjusted hazard ratios (AHR) with a 95% confidence interval (CI) were calculated using Cox proportional hazards regression models to identify the predictors of in-hospital mortality associated with septic shock. Variables with a p value < 0.25 in the crude Cox regression analysis were included in the multivariable Cox regression analysis, and a p value < 0.05 in the final model was considered statistically significant. The model fit was assessed using the omnibus test of model coefficients. The −2 Log Likelihood value (754.5) indicates a good fit, and the significant χ2 test (p < 0.01) suggests that at least one coefficient in the model is non-zero. The incidence of in-hospital mortality was illustrated using the hazard function curve.
2.9 Ethical Approval and Consent to Participate
Ethical approval was obtained from the Institutional Review Board of Y12HMC (protocol number: 314/22). For adult patients (≥ 18 years), written informed consent was taken from themselves and/or their caregivers. For those patients under the age of 18 years, written informed consent was obtained from their parents or legal guardians.
3 Results
3.1 Demographic Data and Medical History
A total of 156 patients were assessed for eligibility, of which 12 were excluded for not meeting the inclusion criteria. Finally, 144 patients were included for analysis (Figure 1).

The mean age (standard deviation) was 46 ± 19 years with a range of 15–95 years. The majority of patients (55.6%) were male. Approximately one-third of the patients had a history of hospitalization 3 months before the current study. The most common comorbidity was human immunodeficiency virus (HIV) (18.8%), followed by diabetes mellitus (DM) (17.4%) and chronic lung disease (14.6%). Furthermore, the majority (68%) had one or more comorbidities (Table 1).
| Characteristics | Overall, n (%) | Survivors, n (%) | Non-survivors, n (%) |
|---|---|---|---|
| Sex | |||
| Male | 80 (55.6) | 29 (20.1) | 51 (35.4) |
| Female | 64 (44.4) | 19 (13.2) | 45 (31.3) |
| Agea | |||
| 15–24 | 22 (15.3) | 9 (6.3) | 13 (9.0) |
| 25–34 | 22 (15.3) | 11(7.6) | 11 (7.6) |
| 35–44 | 28 (19.4) | 9 (6.3) | 19 (13.2) |
| 45–54 | 28 (19.4) | 7 (4.9) | 21 (14.6) |
| 55–64 | 18 (12.5) | 7 (4.9) | 11 (7.6) |
| ≥ 65 | 26 (18.1) | 5 (3.5) | 21 (14.6) |
| Comorbidites | |||
| HIV | 27 (18.8) | 6 (4.2) | 21 (14.6) |
| Diabetes mellitus | 25 (17.4) | 7 (4.9) | 18 (12.5) |
| Chronic lung disease | 21 (14.6) | 7 (4.9) | 14 (9.7) |
| Cancer | 19 (13.2) | 5 (3.5) | 14 (9.7) |
| Hypertension | 16 (11.1) | 9 (6.3) | 7 (4.9) |
| Stroke | 9 (6.3) | 4 (2.8) | 5 (3.5) |
| Chronic liver disease | 8 (5.6) | 1 (0.7) | 7 (4.9) |
| Chronic kidney disease | 7 (4.9) | 2 (1.4) | 5 (3.5) |
| Othersb | 14 (9.7) | 3 (2.1) | 11 (7.6) |
| Number of comorbidities | |||
| No comorbidity | 46 (31.9) | 20 (13.9) | 26 (18.0) |
| One comorbidity | 67 (46.5) | 17 (11.8) | 50 (34.7) |
| Multiple comorbidities | 31 (21.6) | 11 (7.6) | 20 (13.9) |
- a Ethiopian demographic and health survey (EDHS) age category.
- b Alcohol use disorder, Alzheimer's disease, anemia, pancreatitis, benign prostatic hyperplasia, malnutrition, Parkinson's disease, rheumatoid arthritis, systemic lupus erythematous (each accounted one case), venous thromboembolism (five cases). HIV, human immunodeficiency virus.
3.2 Clinical and Laboratory Profiles
The most frequently identified SIRS variables were RR > 20 breaths per minute and HR > 90 beats per minute, observed in 97.9% and 97.2% of the patients, respectively. Among the SOFA variables, SBP ≤ 100 mmHg was present in all patients, while a SpO2 < 90% and a GCS score < 15 were recorded in 93.1% and 56.9% of the patients, respectively. Additionally, the majority of the patients (63.9%) experienced electrolyte imbalances (Table 2).
| Overall, n (%) | Survivors, n (%) | Non-survivors, n (%) | |
|---|---|---|---|
| SIRS variables at presentation | |||
| RR > 20 breaths per minute | 141 (97.9) | 45 (31.3) | 96 (66.7) |
| HR > 90 beats per minute | 140 (97.2) | 46 (31.9) | 94 (65.3) |
| Temperature < 36°C or > 38°C | 135 (93.7) | 45 (31.3) | 90 (62.5) |
| WBC count < 4 × 103 or > 12 × 103 cells/µL | 135 (93.7) | 46 (31.9) | 89 (61.8) |
| SOFA variables at presentation | |||
| SBP ≤ 100 mmHg | 144 (100) | 48 (33.3) | 96 (66.7) |
| SpO2 < 90% | 134 (93.1) | 39 (27.1) | 95 (65.9) |
| GCS < 15 | 82 (56.9) | 15 (10.4) | 67 (46.5) |
| Creatinine ≥ 1.2 mg/dL | 74 (51.4) | 27 (18.8) | 47 (32.6) |
| Platelets ≤ 150 × 103 cells/µL | 69 (47.9) | 22 (15.3) | 47 (32.6) |
| Total bilirubin ≥ 1.2 mg/dL | 21 (14.6) | 10 (6.9) | 11 (7.6) |
| Electrolyte imbalancesa | 92 (63.9) | 25 (17.4) | 67 (46.5) |
- Abbreviations: GCS, Glasgow coma scale; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; SIRS, systemic inflammatory response syndrome; SOFA, sepsis-related organ failure assessment; SpO2, peripheral oxygen saturation; WBC, white blood cell count.
- a Electrolyte imbalances included sodium, chloride, potassium, calcium, and/or magnesium disorders.
3.3 Sources of Infection
The respiratory system was the most common source of infection, identified in 70.2% of septic shock patients, followed by the gastrointestinal system in 34.7% and the central nervous system (CNS) in 10.4% (Table 3). Of note, more than a quarter of the patients (27.8%) had more than one source of infection.
| Variables | Overall, n (%) | Survivors, n (%) | Non-survivors, n (%) |
|---|---|---|---|
| Source of infection | |||
| Respiratory system | 101 (70.2) | 27 (18.8) | 74 (51.4) |
| Gastrointestinal system | 50 (34.7) | 18 (12.5) | 32 (22.2) |
| Central nervous system | 15 (10.4) | 1 (0.7) | 14 (9.7) |
| Skin/soft tissuea | 14 (9.7) | 3 (2.1) | 11 (7.6) |
| Genitourinary system | 6 (4.2) | 3 (2.1) | 3 (2.1) |
- a 13 patients had sepsis of wound focus.
3.4 Microbiologic Evidence
Blood cultures were performed on a total of 83 patients (57.6%). Samples for blood cultures were obtained before the administration of antimicrobials in 78 patients (94%). The available antimicrobials for culture and sensitivity testing were amoxicillin-clavulanic acid, ceftriaxone, ceftazidime, cefepime, ciprofloxacin, clindamycin, cotrimoxazole, erythromycin, gentamycin, meropenem, and vancomycin.
Positive blood cultures were identified in only seven patients (8.4%), with four showing gram-negative isolates and three showing gram-positive isolates. Two species of Klebsiella, one species of Escherichia coli, and one species of Streptococcus pneumoniae were isolated, all sensitive to the tested antimicrobials. A species of Acinetobacter baumannii was also isolated, exhibiting resistance to all tested antimicrobials. Additionally, two species of coagulase-negative staphylococci were identified, although drug susceptibility results were indeterminate. Sputum GeneXpert testing was conducted on 14 patients, with three testing positive for Mycobacterium tuberculosis. Importantly, none of the positive cases demonstrated resistance to rifampicin.
3.5 Treatment Modalities
All patients received crystalloid fluid, antimicrobials, and vasopressors. In 95% of the cases, antimicrobial therapy was initiated within the first 3 h of diagnosis. The three commonly utilized antimicrobials were vancomycin (97.9%), ceftazidime (51.4%), and cefepime (29.9%). Only four patients had their antimicrobial regimens revised based on bacterial isolates that showed sensitivity to specific tested antibiotics, while the vast majority (97.2%) continued to receive empiric antibiotics. The most widely used vasopressor was adrenaline (73.6%), followed by noradrenaline (27.8%). Add-on vasopressor was required for 12.5% of the patients, while hydrocortisone was needed for 64.6% of the patients due to refractory septic shock, as shown in (Table 4). All of these therapies were administered via peripheral intravenous access.
| Frequency | Percent | |
|---|---|---|
| Antimicrobials utilized | ||
| Vancomycin | 141 | 97.9 |
| Ceftazidime | 74 | 51.4 |
| Cefepime | 43 | 29.9 |
| Meropenem | 28 | 19.4 |
| Metronidazole | 23 | 16.0 |
| Ceftriaxone | 15 | 10.4 |
| Ciprofloxacin | 9 | 6.3 |
| Ampicillin | 6 | 4.2 |
| Antimicrobial initiation | ||
| < 1 h | 32 | 22.2 |
| 1–3 h | 105 | 72.9 |
| 3–6 h | 7 | 4.9 |
| Initial vasopressor | ||
| Adrenaline | 101 | 70.1 |
| Noradrenaline | 40 | 27.8 |
| Dopamine | 3 | 2.1 |
| Add-on vasopressor | 18 | 12.5 |
| Dopamine | 13 | 9.0 |
| Adrenaline | 5 | 3.5 |
3.6 Outcomes of Septic Shock Patients
Nearly two-thirds of the patients suffered from multiple organ dysfunctions due to sepsis. The most prevalent complications were acute kidney injury (AKI) in 51.4% and acute lung injury (ALI) in 50.7% of the cases (Table 5). In addition, five patients developed venous thromboembolism during their hospital stay.
| Overall, n (%) | Survivors, n (%) | Non-survivors, n (%) | |
|---|---|---|---|
| Systemic complications | |||
| Acute kidney injury | 74 (51.4) | 25 (17.4) | 49 (34.0) |
| Acute lung injury | 73 (50.7) | 11 (7.6) | 62 (43.1) |
| Thrombocytopenia | 42 (29.2) | 14 (9.7) | 28 (19.4) |
| Sepsis-associated encephalopathy | 27 (18.8) | 2 (1.4) | 25 (17.4) |
| Acute liver injury | 17 (11.8) | 5 (3.5) | 12 (8.3) |
| Arrhythmias | 16 (11.1) | 2 (1.4) | 14 (9.7) |
| Number of organ failures | |||
| One | 14 (9.7) | 6 (4.2) | 8 (5.6) |
| Two | 35 (24.3) | 15 (10.4) | 20 (13.9) |
| More than two | 95 (66.0) | 27 (18.8) | 68 (47.2) |
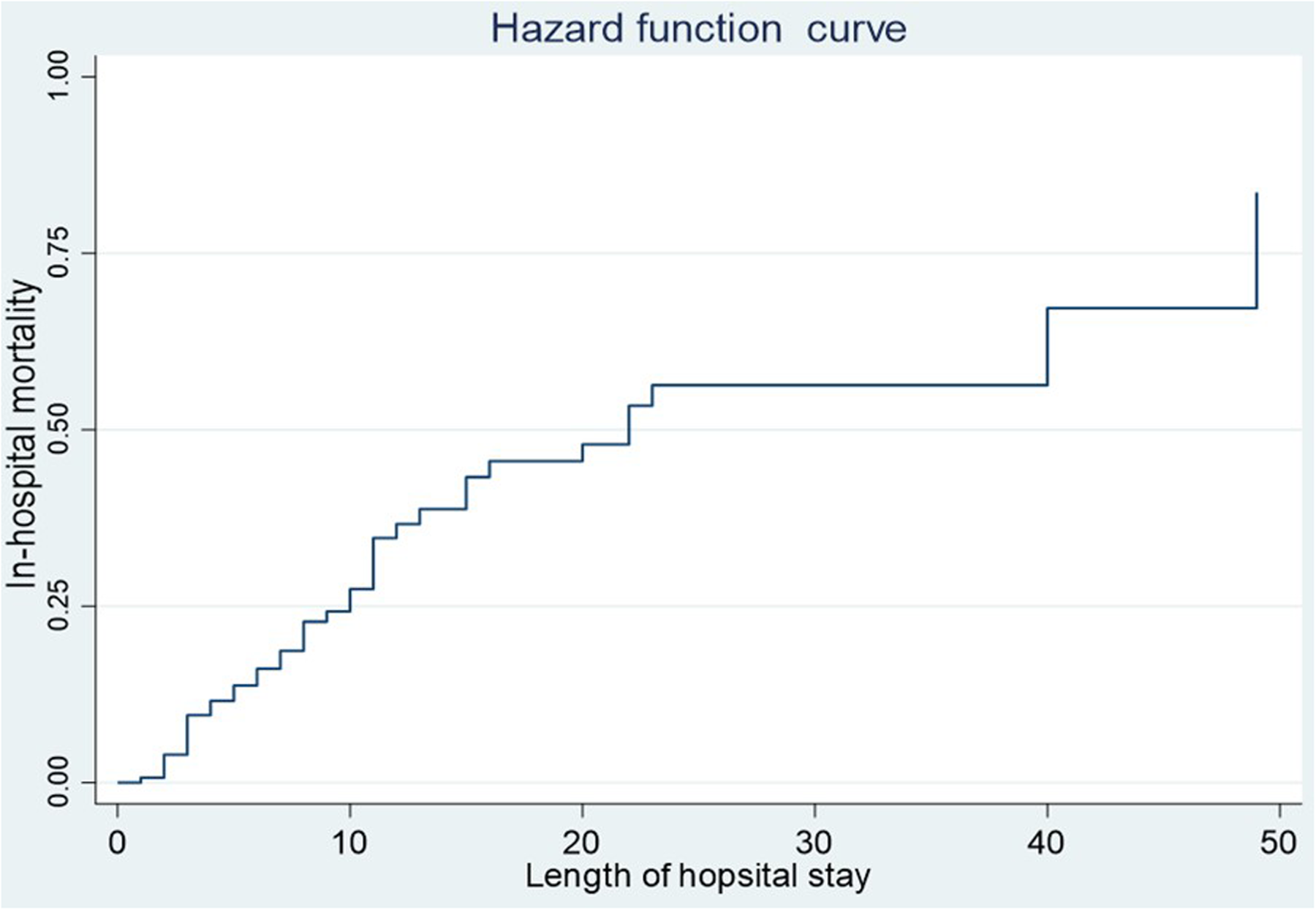
The in-hospital mortality rate of septic shock patients was 66.7%. The median length of hospital stay was 6 days (interquartile range: 2–11 days). The minimum and maximum lengths of hospital stay ranged from 1 to 49 days. Nearly a quarter of the cases died in the first 10 days of admission. Half of the total cases died within the first 3 weeks of admission, with a median survival time of 22 days (Figure 2).
3.7 Predictors of Septic Shock In-Hospital Mortality
The bivariable Cox regression analysis identified that a low GCS score (CHR: 1.84, 95% CI, 1.19–2.86, p = 0.01) and ALI (CHR: 1.52, 95% CI, 1.22–2.32, p = 0.04) were significantly linked to an increased risk of in-hospital mortality in septic shock patients. Additionally, low SpO2 showed a borderline significant association with mortality (CHR: 5.24, 95% CI, 4.71–7.73, p = 0.05) (Table 6).
| Bivariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Predictors | CHR | 95% CI for CHR | AHR | 95% CI for AHR |
| Low GCS | 1.84 | 1.19–2.86 | 2.23 | 1.35–3.69* |
| Low SpO2 | 5.24 | 4.71–7.73 | 8.74 | 1.18–14.84* |
| Acute lung injury | 1.52 | 1.22–2.32 | 1.44 | 0.88–2.37 |
- Abbreviations: AHR, adjusted hazard ratio; CHR, crude hazard ratio; CI, confidence interval; GCS, Glasgow Coma Scale; SpO2, peripheral oxygen saturation.
- * p values < 0.05.
- a The model fit was assessed using the omnibus test of model coefficients.
The multivariable Cox regression analysis further demonstrated that both low GCS (AHR: 2.23, 95% CI: 1.35–3.69, p = 0.01) and low SpO2 (AHR: 8.74, 95% CI: 1.18–14.84, p = 0.03) at presentation were independent predictors of in-hospital mortality from septic shock (Table 6). Patients with low GCS and SpO2 had approximately 2 and 8 times higher risks of death, respectively, compared to those with normal values. Other variables such as age, sex, comorbidities, clinical findings (except low GCS and SpO2), laboratory results, infection source, treatment approaches (e.g., antimicrobials, vasopressors, steroids), timing of antibiotics, and systemic complications (except ALI) did not qualify for inclusion in the multivariable analysis.
4 Discussion
This study aimed to assess the characteristics and in-hospital outcomes of septic shock patients admitted to the adult medical ICU at a tertiary hospital in Addis Ababa, Ethiopia. It revealed that the overall in-hospital mortality of septic shock was high (66.7%). It also demonstrated that impaired mental status and oxygen desaturation at the time of presentation were independent predictors of septic shock in-hospital mortality.
This study found that septic shock primarily affected middle-aged patients, with a mean age of 46 years. Similarly, studies conducted in Tikur Anbessa Specialized Hospital [13], Northwest Amhara [21], and Cameroon [17] reported mean ages of 47.9 years, 45 years, and 52.9 years, respectively, for septic shock patients. In contrast, research from developed nations indicated that septic shock primarily affected elderly patients, with mean ages of 66 years in France [16] and 64 years in the United Kingdom [22]. Higher rates of septic shock in younger age groups may be due to Ethiopia's predominantly young population, increased exposure to infections, untreated chronic conditions like HIV and malnutrition, and delays in healthcare seeking.
Over half of the septic shock patients in our study had one or more comorbidities, consistent with existing literature [13-16, 18, 23]. In the current study, HIV infection was the leading comorbidity (18.8%), followed by DM (17.4%). Likewise, HIV infection was the commonest comorbidity (19.3%), followed by DM (15.6%) in a study done in four selected hospitals in Addis Ababa, Ethiopia [18]. Similarly, the commonest comorbidity in septic shock patients was HIV (31.6%) in a study from the University of Gondar Hospital [14]. On the other hand, malignancy (25%) and heart failure (23%) were the leading comorbidities in a study from France [16], while hypertension and cancer were the most frequent comorbidities in studies from Korea [23, 24]. Our findings indicated the double burden of communicable diseases like HIV and non-communicable diseases such as DM among septic shock patients in low-income countries like Ethiopia.
In the current study, the respiratory system was the main source of infection among septic shock patients, observed in 70% of the patients, consistent with studies from Ethiopia reporting 53.1% in four hospitals in Addis Ababa [18] and 54.8% at Tikur Anbessa Specialized Hospital [13]. A considerable portion of the patients in the current study (10.4%) had sepsis originating from the CNS. However, there has been a lack of substantial data, with only a few case reports identifying CNS infections, particularly bacterial meningitis, as a source of infection [25, 26]. The variation in the proportion of sources of infection across studies could be attributed to differences in patient characteristics, geographic disparities in disease prevalence, and the varying diagnostic methods used to identify the sources of infection.
In our study, blood cultures were done on 57.6% of septic shock patients, higher than the 32.4% reported in a study at four hospitals in Addis Ababa, Ethiopia [18]. However, our culture positivity rate was slightly lower at 8.4%, compared to 10.5% in the other study. In a study conducted in Cameroon [17], bacterial cultures were performed in 100% of septic shock patients; however, the positive culture result was much lower (2.8%) than that of our study. In contrast, a study from Korea found that blood cultures were performed in 99.3% of septic shock patients, with a positive rate of 47.1%, while a U.S. study reported that 37.7% of septic shock patients had at least one positive microbiologic culture [24, 27]. The low performance and yield of blood cultures in our setting might be related to various factors like underutilization of laboratory services, limited resources, improper sample handling, and prior antibiotic use. Our findings underscore the need for culture stewardship programs and further research to identify the reasons behind the low culture yield.
In the present study, 97.2% of septic shock patients were treated with empiric antibiotics, a trend also seen in 89.5% of patients in a study conducted at selected hospitals in Addis Ababa [18]. This could be explained by the low yield of the culture results. The corticosteroid utilization rate in this study (64.6%) was higher than that of the study from the University of Gondar Hospital (60.2%) [14], but lower than that of the study conducted in Northwest Amhara (86.6%) [21]. Conversely, the utilization rate of adrenaline (73.6%) was higher in the current study than that of the study from the University of Gondar Hospital (61.2%) [14] and lower than that of the study conducted in Northwest Amhara (85%) [21]. These findings indicated that the increased use of adrenaline corresponded with a higher proportion of corticosteroid use. Though the Surviving Sepsis Campaign guidelines recommend noradrenaline as the first-line vasopressor for septic shock patients [3], adrenaline was widely used in our study because of inaccessibility and high cost of noradrenaline.
The most common systemic complications among patients with septic shock in the current study were AKI (51.4%) and ALI (50.7%). Consistent with these findings, AKI and ALI were the most common complications of septic shock reported in other studies from Ethiopia, affecting up to 28.3% and 31.8% of patients, respectively [14, 18]. Similarly, in a study from Rwanda, AKI was the most common complication (19.3%), followed by acute respiratory distress syndrome (9.9%) [28]. The high prevalence of these complications emphasizes the need for early identification and intervention to improve patient outcomes and lessen the overall burden on healthcare systems.
The overall in-hospital mortality of septic shock patients in this study (66.7%) was found to be higher than that of the studies done in developed countries such as France (45%) [16], Germany (40.4%) [29], and Japan (34%) [30] as well as in upper middle-income countries such as China (37.3%) [31] and Brazil (65.3%) [32]. The mortality rate observed in our study was also higher than that of other studies done in Ethiopia, including Tikur Anbessa Specialized Hospital (58.3%) [13], University of Gondar Hospital (42%) [14], five hospitals in the Northwest Amhara (58.3% [21], and four hospitals in Addis Ababa (41.8%) [18]. The disproportionately higher in-hospital mortality observed in our study, compared to other studies, may be partly attributed to the lack of well-customized, hospital-based protocols for the timely identification, work-up, and management of septic shock.
There have been a number of studies that assessed predictors of mortality due to septic shock, albeit with a substantial disparity in their findings, with the most consistent predictors being the presence of comorbidities, organ failures, and initial severity of septic shock [14-16, 18, 27, 33, 34]. In contrast, our study revealed that impaired mental status (low GCS) and low SpO2 at presentation were predictors of in-hospital mortality among septic shock patients. There have been some studies that have revealed the impact of low GCS on the in-hospital mortality rate of septic patients [28, 35, 36]. However, there have been no adequate studies that assessed the prognostic role of low GCS on the mortality of septic shock patients. Consistent with the findings of our study, low GCS was a predictor of mortality among septic shock patients in studies conducted in Cameroon [17] and Haiti [37].
A pooled analysis of hospital-based cohort studies from six sub-Saharan African countries demonstrated an inverse relationship between in-hospital mortality and oxygen saturation [38]. However, there is still a lack of sufficient studies establishing oxygen saturation as a predictor of mortality in cases of sepsis or septic shock. One study showed that reduced oxygen saturation was found to be associated with poor prognosis in critically ill patients with sepsis [39]. In line with the findings of our study, low oxygen saturation was found to be associated with higher mortality rates according to a study that did a mortality prediction of septic shock based on routinely recorded data [40].
Our study was not without limitations. First, it was a single-center study with a relatively small sample size, which may restrict the generalizability of the results. Second, we did not measure serum lactate levels, a key diagnostic and prognostic marker in septic shock, limiting our ability to fully assess disease severity and patient outcomes.
5 Conclusions
This study demonstrated a high mortality rate among septic shock patients admitted to the adult medical ICU of a tertiary hospital, with more than two-thirds of patients dying during their hospital stay. The significant prognostic indicators of in-hospital mortality were impaired mental status and peripheral oxygen desaturation. Hence, it is imperative to develop targeted interventions to mitigate in-hospital mortality associated with septic shock.
Author Contributions
Amanuel Zeleke: conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing, validation. Gashaw Solela: conceptualization, validation, formal analysis, methodology, writing – original draft, writing – review and editing. Alazar Sitotaw: data curation, investigation. Balew Arega: writing – original draft, writing – review and editing. Elias Tewabe: formal analysis. Haileyesus Teshome: data curation, investigation. Hanna Alemu: data curation. Seble Legese: data curation. Sebrina Ahmed: formal analysis. Selam Gissa: writing – original draft, writing – review and editing. Simret Alemu: formal analysis. Anteneh Mitiku: conceptualization, methodology. Getabalew Endazenaw: conceptualization, methodology. Eyuel Tsegaye: conceptualization, methodology. Anteneh Eshetu: writing – original draft, writing – review and editing.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author, Gashaw Solela, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.