Clinical Indication-Based Pediatric Diagnostic Reference Level Values for Abdominal Computed Tomography: A Descriptive Cross-Sectional Study
ABSTRACT
Background and Aims
Computed tomography (CT) poses a concern in pediatric patients because of their higher sensitivity to radiation. At the time of the study, nephroblastoma was the most prevalent clinical indication for abdominal CT examinations in pediatric patients. Therefore, the aim of this study was to establish pediatric typical diagnostic reference levels (DRLs) for contrast-enhanced abdominal CT examinations of pediatric patients presenting with nephroblastoma.
Methods
The volume CT dose index (CTDIvol), dose-length product (DLP) and patient weight of 121 patients with nephroblastoma were collected retrospectively. Size-specific dose estimates (SSDE), CTDIvol and DLP were used to calculate DRL values. The SSDE was added as an additional parameter because dose estimates based on the patient's size are considered more precise. Patients were categorized into five weight groups for which DRL values were established per group. The pediatric DRL values in this study were set at the median of the data distribution.
Results
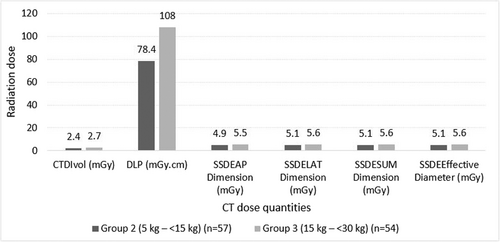
Diagnostic reference level values were only established for two weight groups that adhered to the International Commission on Radiological Protection (ICRP) guidelines. The DRL values for CTDIvol ranged from 2.4 to 2.7 mGy, while the DLP ranged from 78.4 to 108 mGy.cm, and SSDEsum ranged from 4.9 to 5.6 mGy. The DRL values of this study were lower than the European Commission (EC) DRL values and higher than those of other international studies. The lower DRL values of this study will be used to further tailor the radiation dose to be lower than usual for optimization of the radiation dose received by pediatric patients for CT abdomen examinations.
Conclusion
For efficient dose optimization, clinical indication, body weight, and SSDE should be considered when developing pediatric DRL values. Future optimization strategies will benefit from adapting patient size and clinical indication as suggested and supported by the findings of this study.
Abbreviations
-
- AAPM
-
- American Association of Physicists in Medicine
-
- AP
-
- anterior-posterior
-
- ASIR
-
- adaptive statistical iterative reconstruction
-
- ATCM
-
- automated tube current modulation
-
- cm
-
- centimeter
-
- CT
-
- computed tomography
-
- CTDIvol
-
- volume computed tomography dose index
-
- DLP
-
- dose-length product
-
- EC
-
- European Commission
-
- ICRP
-
- International Commission on Radiological Protection
-
- IR
-
- iterative reconstruction
-
- kg
-
- kilogram
-
- kVp
-
- kilovoltage peak
-
- LAT
-
- lateral
-
- LDRL
-
- local diagnostic reference level
-
- mAs
-
- milliampere-seconds
-
- mGy
-
- milligray
-
- mGy.cm
-
- milligray per centimeter
-
- mm
-
- millimeter
-
- NDRL
-
- national diagnostic reference level
-
- N/I
-
- not indicated
-
- PACS
-
- Picture Archiving and Communication System
-
- RDRL
-
- regional diagnostic reference level
-
- SSDE
-
- size-specific dose estimate
1 Introduction
With ongoing technological developments, computed tomography (CT) has become essential to pediatric radiology and has been established as an important and increasingly common part of the diagnostic workup of patients [1, 2]. As the gold standard for detecting and managing multiple diseases, CT is fundamental in pediatric medicine [3]. However, pediatric CT imaging poses a particular concern since pediatric patients are highly sensitive to radiation [4]. Computed tomography uses more radiation than conventional X-ray imaging methods [5]. Furthermore, CT use increases cancer risk over a child's lifetime. Compared to adults, children's organs and tissues are inherently more prone to cellular damage [6, 7]. The radiation dose received by children during routine CT scans, in some instances, is higher than what is required, which can lead to cancer in children [8]. Therefore, the justification and optimization of pediatric CT examinations are important [4].
An essential part of optimization is developing diagnostic reference level (DRL) values [9]. The International Commission on Radiological Protection (ICRP) described DRL values as a form of investigation levels applied to an easily measured quantity [10]. In practice, DRL values are recommended levels that an imaging facility should use to examine its methods and determine if acceptable image quality can be attained at lower doses [11]. The ICRP [10] stated that DRL values are not dose limits based on individual patients but on a specific group of patients. Furthermore, the ICRP [10] recommends that the DRL values should be based on patient weight since weight is the most reliable factor associated with the DRL quantity. Therefore, weight should be used for categorizing, and age can be used if the age of the patient is the only available measure, as the ICRP [10] proposed.
Most CT consoles display the volume CT dose index (CTDIvol) and dose-length product (DLP) for a particular patient's CT scan, based on the reference phantom selected by the scanner [12]. The reference phantom size does not take into account the size differences between children and adults [10]. The American Association of Physicists in Medicine (AAPM) [13] developed the size-specific dose estimates (SSDE) taking into consideration the patient's body size [14]. Dose estimates based on the patient's size are considered more precise and should be used when the size parameters are available [10]. Additionally, different clinical indications for an examination may need different image qualities and, consequently, different amounts of radiation. Therefore, setting DRL values without considering the clinical indication has little significance [10].
Nephroblastoma is a dense malignant tumor of the kidney originating from kidney cells and different developmental stages of various tissue types, such as muscle, cartilage, and epithelium [15]. Nephroblastoma is prevalent in children between 1 and 5 years of age and represents 5.9% of all childhood malignancies [16]. Local diagnostic reference level (LDRL) values for CT brain, temporal bones, cervical spine, trunk, chest and abdomen have been established at two university hospitals in South Africa, using the CTDIvol and DLP for different age groups [17]. Other pediatric LDRL values for emergency pediatric head CT scans based on the patient's age have been developed at a South African tertiary-level hospital, using the CTDIvol and DLP [18]. At the time of this study, no pediatric CT DRL values based on nephroblastoma have been established in South Africa. Internationally, DRL values for pediatric patients were compiled by several authors using the DLP and CTDIvol based on the patient's weight [19, 20]. Hwang et al. [21] developed pediatric DRL values using the DLP, CTDIvol, and SSDE based on weight. Clinical indication-based DRL values for tumor and infection in pediatric CT abdomen were developed by Almén et al. [20] using the DLP and CTDIvol for weight groups.
A literature evaluation revealed that no documented pediatric DRL values were available for CT examinations to investigate and stage nephroblastoma in South Africa and internationally. Nephroblastoma was the most common clinical indication for pediatric patients presenting for abdominal CT examinations at the participating hospital [22]. The absence of established clinical indication-based pediatric DRL values in South Africa limits the ability to identify unusually high dose levels and ensure optimal radiation protection during contrast-enhanced abdominal CT examinations of pediatric patients with nephroblastoma. The aim of the study was to establish pediatric DRL values for contrast-enhanced abdominal CT examinations to diagnose and stage nephroblastoma cases based on patient weight group.
2 Materials and Methods
2.1 Ethical Considerations
Ethical approval to conduct the study was obtained from the Health Sciences Research Ethics Committee (HSREC) of the University of the Free State (approval number: UFS-HSD2022/0341/3008-0001). Permission to use patient information from the participating hospital was obtained from the Free State Province Department of Health (DoH). Due to the retrospective nature of this study and using anonymised CT dose data, informed consent was not required [23].
2.2 Data Collection

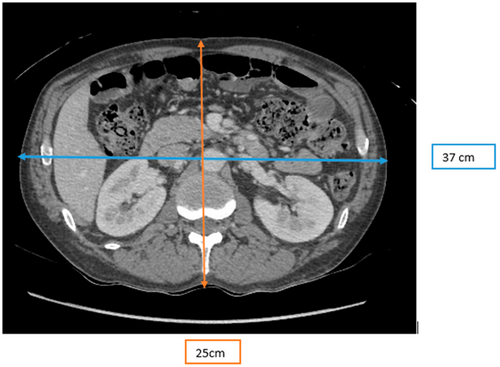
Figure 2 illustrates the measurement of the AP and LAT dimensions on a patient's CT axial image. The measurements were obtained on the widest part of the patient on a CT axial image. The AP measurements were made on the level of the vertebral bodies between thoracic vertebra 12 and lumbar vertebra 3, and the LAT measurements were made on the level of the center of both kidneys. Electronic callipers were used to measure the AP and LAT dimensions in millimeters (mm), which were converted into centimeters (cm).
The contrast-enhanced abdominal examinations of the pediatric patients were performed with the General Electric (GE) HealthCare CT unit Discovery model CT750HD (GE HealthCare Technologies Inc.; Chicago, IL, USA) at the participating hospital. The annual quality control (QC) tests were performed on the CT unit, and the QC test results adhered to the manufacturer's recommended guidelines. The results of the QC tests were satisfactory, and the same CT unit was used throughout the study period. The GE HealthCare CT unit used for the CT scanning of participants involved in this study used the automatic tube current modulation (ATCM) (SmartmA and AutomA) and the iterative reconstruction (IR) algorithm, namely adaptive statistical iterative reconstruction (ASIR).
The target population consisted of pediatric patients who presented with nephroblastoma and underwent contrast-enhanced abdominal CT examinations at the participating hospital. Out of 265 patients that formed part of the study population, only 121 patients adhered to the inclusion criteria of this study to form the sample size. Therefore, the sample size of pediatric patients forming part of this study was 121 patients. Stratified sampling was used to randomly select pediatric patients based on age, weight, and clinical indication [27]. Patients aged younger than 18 years who weighed between 0 and less than 80 kg were considered pediatric patients. Only patients who presented with nephroblastoma and underwent contrast-enhanced chest-abdomen-pelvis CT examinations between January 1, 2018 and October 9, 2022 were included in the study. The exclusion criteria were patients who were 18 years and older; patients not presenting with nephroblastoma for contrast-enhanced chest-abdomen-pelvis CT examinations during the study period; and patients booked for CT examinations of the brain-chest-abdomen, neck-chest-abdomen, neck-chest-abdomen-pelvis, pulmonary and/or abdominal angiogram.
2.3 Grouping of Patients
The patients were categorized into five weight categories, as proposed by the ICRP [10]. The grouping of patients according to their weight was as follows: group 1 (0 to < 5 kg), group 2 (5 to < 15 kg), group 3 (15 to < 30 kg), group 4 (30 to < 50 kg) and group 5 (50 to < 80 kg). The age of the patients has been used in the past to define groups for pediatric DRL values. An analysis of age alone has shown that it is not a reliable indicator. The European Commission (EC) [28] recommended the weight groups in Table 1, indicating the age groups to which they correspond. However, this correspondence will vary significantly in different parts of the world.
| Description | Weight group (kg) | Age group based on weight-for-age charts | Most common age groups used for previous national DRLs (years) |
|---|---|---|---|
| Neonate | < 5 | < 1 month | 0 |
| Infant, toddler, and early childhood | 5 to < 15 | 1 month to < 4 years | 1 |
| Middle childhood | 15 to < 30 | 4 to < 10 years | 5 |
| Early adolescence | 30 to < 50 | 10 to < 14 years | 10 |
| Late adolescence | 50 to < 80 | 14 to < 18 years | 15 |
- Abbreviations: DRL = diagnostic reference level, kg = kilogram.
2.4 Setting of DRL Values
Computed tomography chest-abdomen-pelvis protocol was performed in the portal venous phase, with the injection of contrast media sufficient to characterize nephroblastoma [29]. The chest-abdomen-pelvis imaging protocol was used for patients of different sizes at the participating hospital. The imaging parameters for the protocol, as displayed in the CT unit at the participating hospital, are shown in Table 2. It is important to note that the specific parameters used for each patient were not documented since this study was performed retrospectively. Moreover, only the dose report of each patient was available to the authors to establish DRL values.
| Body part | kV | mA | Pitch | Recon destination | Rotation time (s) |
|---|---|---|---|---|---|
| Chest-abdomen-pelvis | 100–120 kV | Smart mA and automatic mA | 0.53– 0.98 | PACS | 0.5 s |
- Abbreviations: kV = kilovoltage, mA = milliampere, PACS = picture archiving and communication system, s = seconds.
The pediatric DRL values developed in this study were based on the median of data distribution, as recommended by the ICRP [10] and the EC [28]. A minimum of 20 patients for each group was required to develop typical DRL values based on the median. The ICRP further stated that typical values might be derived from the median of the distribution if an institution lacked sufficient data to use the third quartile [10]. Therefore, the typical DRL values in this study are presented as the median of the various dose quantities, namely, CTDIvol, DLP, SSDEAP Dimension, SSDEEffective Diameter, SSDELAT Dimension, and SSDESUM Dimension.
2.5 Statistical Analysis
The data were captured electronically from the structured data recording document and entered into a spreadsheet compiled in Microsoft Excel, version 3.2.7 (Microsoft Corporation; Redmond, WA, USA). Further analysis was performed using SAS Version 9.4 software (SAS Institute Inc.; Cary, NC, USA). Descriptive statistics, namely frequencies and percentages, were calculated for categorical data. Medians, interquartile range (IQR), and percentiles were calculated for numerical data. A Spearman's correlation test was used to calculate the Spearman's coefficient (r) and p values to assess the relationship between the dose quantities for each weight group. The Spearman's correlation tests were two-tailed to observe and determine whether a significant difference exists between variables in each weight group. In addition, Kruskal-Wallis tests were performed to observe the overall effect of the weight groups on all dose quantities (CTDIvol, DLP, SSDEAP Dimension, SSDEEffective Diameter, SSDELAT Dimension, and SSDESUM Dimension) and if any significant differences occurred. A p value of less than 0.05 indicated a significant difference between the proportions in weight groups.
3 Results
The data of 121 patients who adhered to the study's inclusion criteria were analyzed, where 66 patients (54.6%) were male. The patients were initially categorized into five weight groups. However, when developing pediatric DRL values, the ICRP [10] recommended collecting data from at least 20–30 patients per category with agreed weights for CT examinations. Therefore, three weight groups, group 1 (0 to < 5 kg) with one (0.83%) patient, 4 (30 to < 50 kg) with seven (5.76%) patients, and 5 (50 to < 80 kg) with two (1.65%) patients, did not have the required number of patients. Consequently, DRL values were established only for weight groups 2 (5 to < 15 kg) with 57 (47.11%) patients and 3 (15 to < 30 kg) with 54 (44.63%) patients.
The pediatric typical DRL values developed in this study were based on the median of the data distribution. The pediatric DRL values were different for various weight groups for all dose quantities, as depicted in Figure 3. The pediatric DRL values based on the CTDIvol ranged from 2.4 to 2.7 mGy for weight groups. The DLP, pediatric DRL values ranged from 78.4 to 108 mGy.cm for weight groups. The DRL values based on the SSDE parameters (AP, LAT, SUM, and effective diameter) ranged from 4.9 to 5.6 mGy weight groups.
A weak positive correlation was observed between patient weight and dose quantities (CTDIvol, DLP, and SSDE parameters) for weight group 2 with r = 0.04 for CTDIvol, r = 0.01 for DLP, and r = 0.01–0.05 for SSDE parameters. All these dose quantities had a nonsignificant correlation (Spearman correlation test; p > 0.001) with weight for weight group 2. Weight group 3 showed a positive significant correlation (Spearman correlation test; p < 0.001) between the patient weight and all dose quantities with r = 0.54–0.62 for CTDIvol, DLP, and SSDE parameters. The p values determined by the Kruskal-Wallis test indicated a significant (Kruskal-Wallis; p < 0.001) difference between both weight groups and among dose quantities. To investigate whether there was a significant difference between CTDIvol and SSDEsum, the median of the paired differences was calculated. The median difference between CTDIvol and SSDEsum was 2.87, with 95% confidence limits of 2.73–2.96. Since the confidence interval does not include zero, this indicates a statistically significant difference between the two measures.
The pediatric DRL values developed in this study were also compared with DRL values developed in international studies [19-21], with the comparison summarized in Table 3. For all indicated groups, the DRL values of this study were lower than the EC [28] DRL values and higher than those of other international studies.
| Median and/or (75th percentile) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Clinical indication | DRLs (local, national, regional, typical) | Weight groups (kg) | n | CTDIvol (mGy) | DLP (mGy.cm) | SSDESUM dimension (mGy) |
| This study (2024) | Nephroblastoma | Typical DRLs | 5 to < 15 | 57 | 2.4 (2.6) | 78.4 (94.5) | 5.1 (5.5) |
| 15 to < 30 | 54 | 2.7 (3.2) | 108 (132.3) | 5.6 (6.5) | |||
| European Commission [28] | N/I | NDRLs | 5 to < 15 | N/I | (3.5) | (120.0) | — |
| 15 to < 30 | N/I | (5.4) | (150.0) | — | |||
| Almén et al. [20] | Tumor and infection | RDRLs | 15 to < 30 | 60 | 1.8 (2.6) | 70.5 (91.7) | — |
| Hwang et al. [21],a | N/I | LDRLs | 5 to < 15 | 259 | 1.9 (2.2) | 59.9 (73.8) | 4.1 (4.8) |
| 15 to < 30 | 520 | 2.3 (2.7) | 94.1 (101.2) | 4.2 (5.5) | |||
- Abbreviations: AP = anterior-posterior, cm = centimeter, CTDIvol = volume computed tomography dose index, DLP = dose-length product, DRLs = diagnostic reference levels, kg = kilogram, LAT = lateral, LDRLs = local diagnostic reference levels, mGy = milligray, mGy.cm = milligray per centimeter, NDRLs = national diagnostic reference levels, N/I = not indicated, RDRLs = regional diagnostic reference levels, SSDE = size-specific dose estimate.
- a Hwang et al. [20] did not report DRL values for age and weight separately. The specific SSDE parameter for their study was not indicated [20].
4 Discussion
This study aimed to establish pediatric DRL values for contrast-enhanced chest-abdomen-pelvis CT examinations in patients with nephroblastoma. To our knowledge, there is a paucity of literature specifically addressing DRL development for CT contrast studies in this patient population. Most existing DRL values are based on anatomical regions rather than clinical indications. However, this approach (anatomical DRL values) presents limitations, as different clinical indications for the same anatomical region may require varying imaging qualities, scan phases, and, ultimately, different radiation doses [10]. Dalah and Bradley [30] observed that radiation doses can vary among studies involving patients with identical clinical indications. This variation may be attributed to several factors, including patient size and scan parameters. According to Dalah and Bradley [30], developing scan-acquisition-based DRLs per protocol involves comparing patients subjected to the same protocol, clinical indication, and scan acquisition series. Our study's findings support Dalah and Bradley's [30] suggestions, as all participants with a clinical diagnosis of nephroblastoma underwent a contrast-enhanced CT chest-abdomen-pelvis protocol performed with one scan phase, namely the portal venous phase.
Various scanning parameters were considered because of their known impact on radiation dose, including kilovoltage (kV), milliampere-seconds (mAs), gantry rotation time, section thickness, pitch, and scan length. The ATCM was used in all scans, allowing most parameters to remain consistent except for mAs, which varied according to tissue attenuation. While dose reports were retrieved from the PACS, the GE CT unit used, did not provide detailed imaging parameter documentation, limiting retrospective analysis. The kVp was set at 100–120, and the pitch ranged between 0.53 and 0.98. As reported by Raman et al. [31], ATCM increases mAs in areas with higher attenuation and decreases it in lower-density regions. Although this study did not investigate the specific impact of each parameter on dose, prior literature confirms that radiation dose generally increases with higher mAs, kVp, thinner slices, and extended scan ranges, while decreasing with higher pitch, faster rotation, and proper patient centering [8].
Pediatric DRL values in this study were calculated based on weight groups, as recommended by the ICRP [10], EC [28], and Hwang et al. [21], respectively. DRL values were reported using the median of dose quantities: CTDIvol, DLP, and SSDESUM. Additionally, the 75th percentile values, as shown in Table 3, were calculated to allow for more direct comparison with international reference levels, which often report DRL values based on the 75th percentile. Since this study was conducted at a single healthcare facility performing a high volume of specialized scans for nephroblastoma, we report typical DRL values, which are especially useful in settings where national DRLs are unavailable [10]. Using median values to derive these typical DRLs aligns with methodologies used by other studies, such as Tan et al. [32].
Our study's DRL values could not be directly compared to international DRL values for CT chest-abdomen-pelvis, as no published data exist for nephroblastoma-specific DRLs by weight group. Nonetheless, comparisons were drawn with studies employing anatomical regions and weight-based classifications that most closely aligned with those used the present study. The DRL values we reported were lower than those published by the EC [28] but higher than those reported by Almén et al. [20] and Hwang et al. [21]. The lower values in Hwang et al. [21] may be attributed to their use of a single scan phase, ATCM, and IR algorithms, all of which contribute to dose reduction. Our study also used ATCM (SmartmA and AutomA) and IR (ASIR), which can reduce dose by 30%–40% [10]. However, ATCM may increase pelvic radiation dose when the patient is improperly centered in the gantry, possibly contributing to higher DRL values in our study compared to Hwang et al. [21, 33]. Sebelego et al. [34] noted that variations in CT units and their age can impact DLP and CTDIvol values. The lower DRL values reported by Almén et al. [20] and Hwang et al. [21] may be attributed to differences in CT equipment, though they did not specify the units used. Almén et al. [20] and Hwang et al. [21] studies used CT abdomen-pelvis examinations, which have a shorter scan length, while our study included chest-abdomen-pelvis examinations, leading to longer scan lengths and higher doses. This explains their lower DRL values compared to ours. Almén et al. [20] did not specify the use of ATCM or IR and employed a different CT unit with a larger sample size and no contrast media administration, which could also explain their lower dose values. Importantly, Hwang et al. [21] is the only study besides ours to report SSDE values stratified by weight groups. This allowed for a more meaningful comparison between studies, as SSDE is a more patient-size–specific dose metric than CTDIvol or DLP alone. The similarity in methodology strengthens the relevance of the comparative analysis and highlights the value of including SSDE when developing pediatric DRL values. A significant difference between the CTDIvol and SSDE was observed, supported by a 95% confidence interval at 50% median, indicating that these quantities impact radiation exposure differently. Since the CTDIvol reflects only the scanner's output and not patient-specific doses [13], the AAPM [13] recommends using SSDE, which adjusts for patient size and provides a more accurate dose estimation.
Statistical analyses further validated the use of weight-based groupings. The Kruskal-Wallis test showed significant differences in dose quantities across weight groups, confirming that patient weight significantly influences radiation dose. Additionally, Spearman's correlation revealed a significant positive relationship between all dose quantities (CTDIvol, DLP, SSDESUM) in both weight groups. According to the Spearman's correlation analysis, the parameters DLP, CTDIvol, and SSDESUM did not exhibit a statistically significant correlation with weight in weight group 2. The lack of correlation in group 2 may be due to variations in ATCM responsiveness, mispositioning within the gantry, or patient anatomy, which affects surface-based dose modulation more than weight [8]. Despite this, patient weight strongly correlated with body measurements and age, as found in studies by Imai et al. [14] and Hwang et al. [21]. This supports ICRP [10] recommendations to categorize pediatric patients by size, particularly weight, when developing DRL values. Our study followed this approach, incorporating SSDE as a patient-size-specific dose quantity.
A major limitation in both our study and previous literature was the difficulty in collecting sufficient pediatric data. Almén et al. [20] and Hwang et al. [21] both reported challenges in establishing DRL values across all weight and age groups due to limited data. Similarly, our DRL values were only calculated for weight groups 2 (5 to < 15 kg) and weight group 3 (15 to < 30 kg). This limitation is reflective of the epidemiology of nephroblastoma, which is most prevalent in children aged 1–5 [16]. Additionally, during the time of data collection, the data available on the CT unit were of patients who underwent CT chest-abdomen-pelvis examinations to diagnose nephroblastoma during the period from January 1, 2018 to October 9, 2022. The COVID-19 pandemic may have further impacted patient attendance, as concerns over virus exposure likely caused delays or cancellations in scheduled imaging appointments. These pandemic-related disruptions may have contributed to the reduced sample size, particularly in older or heavier-weight groups. Moreover, the participating hospital does not have DRL values, and the current practice has not changed since; therefore, the authors are of the opinion that the DRL values can be used as a baseline.
Ultimately, patient weight remains the most dependable factor linked to a DRL quantity [10, 28] because the radiation dose used for pediatric examination varies greatly due to the great variation in patient weight [10]. However, further research should examine how ATCM adjusts exposures in pediatric populations across varying body sizes. To improve future DRL accuracy, we recommend that weight be measured directly rather than estimated from contrast dose. Additionally, SSDE should be prioritized when calculating DRL values due to its sensitivity to patient size [13]. Future studies should aim for a minimum of 20–30 patients per weight group and consider clinical indication in DRL development, as exposure levels may vary significantly for the same anatomical region depending on the diagnostic need.
4.1 Limitations of the Study
Firstly, the unavailability of the registers for patients who underwent abdominal CT before 2018 resulted in a small sample size. Thus, only data from January 1, 2018 to October 9, 2022 could be collected retrospectively. Secondly, the sample size of the study was relatively small and limited to one hospital. Consequently, it was not possible to achieve the minimum prescribed number of 20–30 patients, as recommended by the ICRP [10], for weight group 1 (0 to < 5 kg), group 4 (30 to < 50 kg) and group 5 (50 to < 80 kg) to develop DRL values for abdominal CT examinations, with nephroblastoma as the clinical indication. The third limitation was that the patient's weight was not always directly measured. Where a direct weight measurement was not available, the researcher had to convert the volume of contrast medium injected to determine the patient's weight. Therefore, a slight difference could exist between the patient's actual weight and the converted weight. Lastly, the dose reports were obtained from the PACS as the study was retrospective. The dose reports on the GE CT unit do not provide information relating to the imaging parameters. Consequently, the specific imaging parameters for each patient included in this study were not documented.
5 Conclusion
Clinical indication-based pediatric typical DRL values in chest-abdomen-pelvis CT examinations for body weight groups using SSDE were established. For efficient dose optimization, clinical indication, body weight, and SSDE should be considered when developing pediatric DRL values. The pediatric DRL values (based on the median) developed in this study were lower than the DRL values developed by EC [28] and higher than the DRL values developed in other studies [20, 21]. The pediatric DRL values developed in this study can be used as a baseline for future pediatric DRL values for the CT chest-abdomen-pelvis of nephroblastoma patients.
Author Contributions
Tebello Pitso: conceptualization, writing – original draft, investigation, data curation, methodology, formal analysis, and visualization. Ida-Keshia Sebelego: writing – review and editing, supervision, data curation, and validation. Henra Muller: writing – review and editing, supervision, and validation.
Acknowledgments
The authors thank the radiology practice for permitting the research study to be conducted; the Central University of Technology, Free State, for funding; Ms. Maryn Viljoen for the statistical analysis of data; and Dr. Daleen Struwig for the technical and editorial preparation of the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author, Ida-Keshia Sebelego, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
Data are available from the corresponding author upon reasonable request.