Investigating the causal relationship between human blood/urine metabolites and periodontal disease using two-sample Mendelian randomization
Abstract
Background and Aims
The aim is to investigate the cause-and-effect connection between metabolites found in blood/urine and the likelihood of developing periodontal disease (PD) through the utilization of a two-sample Mendelian randomization (MR) method.
Methods
Using an inverse variance weighted (IVW) method and two additional two-sample MR models, we examined the relationship between blood/urine metabolites and PD by analyzing data from a comprehensive metabolome-based genome-wide association study and the Genome-Wide Association Studies (GWAS) of PD. To assess the consistency and dependability of the findings, diversity, cross-effects, and sensitivity analyses were conducted.
Results
Out of the 35 metabolites found in blood and urine, a total of eight metabolites (C-reactive protein, Potassium in urine, Urea, Cystatin C, Non-albumin protein, Creatinine, estimated Glomerular Filtration Rate, and Phosphate) displayed a possible causal connection with the risk of dental caries/PD using the inverse variance weighted (IVW) method (p < 0.05). This includes five metabolites in the blood and three in the urine. No metabolites were statistically significant in IVW MR models (p < 3.68 × 10−4). Even after conducting sensitivity analysis with the leave-one-out method and removing the confounding instrumental variables, the impact of these factors on dental caries/PD remained significant.
Conclusion
Based on the available evidence, it is not possible to establish a significant causal link between the 35 blood metabolites and the likelihood of developing dental caries and PD.
1 INTRODUCTION
Dental hygiene plays a crucial role in global physical well-being. Dental caries and periodontal diseases, like gingivitis and periodontitis, are the prevalent ailments that impact oral well-being. According to the 2016 study on the Global Burden of Disease, Injury, and Risk Factors, dental caries in permanent teeth and periodontitis were identified as the primary and eleventh most widespread sources of illness globally in 2016.1 It is crucial to identify the cause of these illnesses as they impose substantial health and financial consequences, with dental disease worldwide costing over $540 billion in 2015.2, 3 Dental caries is a disease process that can lead to irreversible damage to dental tissue.4, 5 The Decayed, Missing, and Filled teeth index (DMF index), which includes decayed, missing, and filled teeth for both primary and permanent dentition, is a widely employed approach for measuring the prevalence of dental caries.6 Meanwhile, Periodontal disease, a condition characterized by inflammation in the supporting tissues of the teeth caused by microorganisms, impacts around half of the adult population, with 10% experiencing the severe form of the disease.7 According to recent studies on the global burden of disease (1990–2017), it has been estimated that around 796 million individuals experience severe periodontitis, with a reported age-standardized prevalence of 9.8%.8 Prior research has indicated that numerous changeable risk factors are linked to dental cavities and gum disease, including inadequate eating patterns (for instance, regular intake of processed sugars), substandard dental care, tobacco use, and alcohol consumption.9-11 Nevertheless, there are still unidentified modifiable factors linked to dental caries and periodontitis.
To routinely assess and track chronic illnesses, measuring biomarkers in serum and urine at regular intervals is a standard practice.12 Knowing the genetic predisposition to particular biomarker conditions and the variables that complicate them could potentially impact the management of diseases. While lipids,13, 14 glycemic traits,15, 16 and measures of renal function17, 18 have been thoroughly investigated, the genetic aspects of certain biomarkers have also been extensively examined. Large population-scale datasets have not been used to investigate the genetic foundation of most biomarkers.19 The most extensive Genome-Wide Association Studies (GWAS) up to now has mapped the genetic blueprint of human blood metabolites,19 offering a significant benchmark for the genetic foundation of blood and urine metabolomics. We explored the cause-and-effect connection between these blood metabolites and the susceptibility to periodontal disease and the formation of cavities using this approach. Although it has not been reported yet, gaining more understanding of the pathogenesis of periodontal disease and caries can offer fresh perspectives on the clinical treatment of patients with these conditions.
Mendelian randomization (MR) offers an alternative approach to address the issue of observational bias.20, 21 In MR, genetic information is utilized as an arbitrary origin of exposed diversity, ensuring that the origin of diversity remains unaffected by confounding factors.22, 23 By harnessing the inherent variability in an individual's genetic composition, similar to the approach of a randomized controlled trial, this method utilizes genetic variation in instrumental variable analysis to deduce the impact of a modifiable exposure on an outcome. Therefore, MR offers a dependable comprehension of the impacts of alterable exposures on characteristics of concern in contrast to conventional observational studies that are vulnerable to confounding or reverse causality.23
Hence, in this study, a two-sample MR analysis was conducted to explore the causal association between 35 blood/urine metabolites and the progression of dental caries and periodontal disease. The analysis was performed from a molecular mechanism viewpoint, utilizing the aforementioned extensive GWAS data as the exposure data and an additional vast GWAS data on periodontal disease and dental caries as the outcome data. Additionally, this research possesses a specific foundation in theory and holds significance in clinical application. The findings can serve as a guide for the advancement of tools used in predicting and treating dental caries and periodontal disease.
2 MATERIALS AND METHODS
2.1 Design of the study
To explore the potential causal connection between exposure and the outcome of interest, genetic variations closely linked to exposure will serve as instrumental variables in MR analyses.24 To enhance inferences about the potential causal impact on outcomes, MR utilizes genetic variants associated with exposure. The method utilizes Mendel's principles of separation and autonomous categorization, where genetic variations are assigned autonomously without considering environmental and other genetic factors (except for variations nearby due to linkage disequilibrium [LD]).23, 25 For each IV to be considered valid, three key conditions must be met: (1) a strong connection between the instrument and the exposure; (2) the instrument affecting the outcome solely through the exposure; and (3) genetic variation being unrelated to factors that may confound the association between exposure and outcome.26 The findings of this research are presented following the guidelines provided by STROBE-MR and the Mendelian Randomization Survey Guidelines.27 The study procedure and specifics were not previously registered.
2.2 Data sources
The exposure data for this study was obtained from the largest GWAS data published in Nature Genetics in 2021, conducted by Armstrong et al.19 The meta-analysis includes 363,228 Europeans who underwent rigorous quality control. It encompasses a comprehensive set of 2.1 million SNP loci and 35 blood and urine metabolites for conducting genome-wide association analysis. These metabolites can be divided into several metabolite categories: lipids, glycemic traits, and vitamins, energy products, heterologous biological metabolites. The database website 10.35092/yhjc.12355382 provides public access to summary data for all association analyses. Table 1 contains comprehensive details.
| Phenotype | Abbreviation | Units of measurement | Trait category |
|---|---|---|---|
| Alanine aminotransferase | ALT | U/L | Liver |
| Albumin | ALB | g/L | Liver |
| Alkaline phosphatase | ALP | U/L | Bone and Joint |
| Apolipoprotein A | APOA | g/L | Cardiovascular |
| Apolipoprotein B | APOB | g/L | Cardiovascular |
| Aspartate aminotransferase | AST | U/L | Liver |
| AST to ALT ratio | AST2ALT | N/A | Liver |
| C-reactive protein | CRP | mg/L | Cardiovascular |
| Calcium | CA | mmol/L | Bone and Joint |
| Cholesterol | CHOL | mmol/L | Cardiovascular |
| Creatinine | CRE | µmol/L | Renal |
| Creatinine in urine | UCR | µmol/L | Renal |
| Cystatin C | CYS | mg/L | Renal |
| Direct bilirubin | BILD | µmol/L | Liver |
| eGFR | EGFR | mL/min/1.73 m2 | Renal |
| Gamma glutamyltransferase | GGT | U/L | Liver |
| Glucose | GLU | mmol/L | Diabetes |
| HbA1c | HBA1C | mmol/mol | Diabetes |
| HDL cholesterol | HDL | mmol/L | Cardiovascular |
| IGF-1 | IGF1 | nmol/L | Hormone |
| LDL cholesterol | LDLD | mmol/L | Cardiovascular |
| Lipoprotein A | LPA | nmol/L | Cardiovascular |
| Microalbumin in urine | URMA | mg/L | Renal |
| Non-albumin protein | NAP | g/L | Renal |
| Phosphate | PHOS | mmol/L | Renal |
| Potassium in urine | URK | mmol/L | Renal |
| SHBG | SHBG | nmol/L | Hormone |
| Sodium in urine | URNA | mmol/L | Renal |
| Testosterone | TES | nmol/L | Hormone |
| Total bilirubin | TBIL | µmol/L | Liver |
| Total protein | TP | g/L | Renal |
| Triglycerides | TRIG | mmol/L | Cardiovascular |
| Urate | UA | µmol/L | Renal |
| Urea | BUN | mmol/L | Renal |
| Vitamin D | VITD | nmol/L | Bone and Joint |
- Abbreviation: eGFR, estimated glomerular filtration rate.
The results of this magnetic resonance study included dental decay, number of teeth, and gum disease. For each tooth surface that was available, we assessed caries indicators using two metrics: the total of tooth surfaces with decay, missing, and fillings (DMFS) and the total of tooth surfaces with decay and fillings (DFSS). In individuals of European descent, a recent meta-analysis of GWAS was conducted to acquire summary data on DMFS, DFSS, the number of teeth, and periodontitis.28 The Genetic Lifestyle Interactions in Dentistry Endpoints (GLIDE) consortium performed a GWAS meta-analysis, which involved 9 studies for the initial examination of DMFS (n = 26,792), 8 for DFSS (n = 26,533), 9 for N teeth (n = 27,949), and 7 for periodontitis (17,353 cases and 28,210 controls). Clinical dental records provided the data for DMFS and DFSS. Clinical dental records were used to collect data for N teeth in all studies, except for one study where self-reporting was used. Periodontitis was diagnosed using the Centers for Disease Control and Prevention/American Academy of Periodontology definition or similar criteria.29 Table 2 displays comprehensive details for every study.
| Study | Full name | No with GWAS in paper | Age |
|---|---|---|---|
| ARIC | Atherosclerosis Risk in Communities | DMFS (4409) DFSS (4409) N teeth (4409) Periodontitis (4655) |
45–64 years |
| COHRA1 | The Center for Oral Health in Appalachia cohort 1 (COHRA1) part of GENEVA caries | DMFS (887) DFSS (887) N teeth (887) Periodontitis (711) |
18+ years |
| DRDR | Dental Registry and DNA Repository (DRDR) of the University of Pittsburgh School of Dental Medicine | DMFS (229) DFSS (229) N teeth (229) Periodontitis (0) |
17–84 years |
| MDC | Malmö Diet and Cancer Study | DMFS (842) DFSS (842) N teeth (842) Periodontitis (0) |
45–64 years |
| NFBC 1966 | Northern Finland Birth Cohort 1966 | DMFS (1483) DFSS (1483) N teeth (1483) Periodontitis (0) |
46–47 years |
| SHIP | Study of Health in Pomerania | DMFS (3362) DFSS (3362) N teeth (3362) Periodontitis (3065) |
20–81 years |
| SHIP-TREND | Study of Health in Pomerania Trend | DMFS (944) DFSS (944) N teeth (944) Periodontitis (879) |
20–83 years |
| TWINGENE | Swedish Twin Biobank | DMFS (2820) DFSS (2820) N teeth (2820) Periodontitis (1944) |
46–93 years |
| WGHS | Women's Genome Health Study | DMFS (0) DFSS (0) N teeth (1353) Periodontitis (22,290) |
45+ years |
- Abbreviation: GWAS, Genome-Wide Association Studies.
2.3 Statistical analysis
The relationship between the concentrations of blood/urine and outcomes was primarily assessed using two-sample MR analysis with the IVW method, which relies on inverse variance weighting. The IVW approach is a perfect estimation and an efficient analysis assuming that all genetic variants act as effective instrumental variables, demonstrating a robust capability to identify causality.24 However, the IVW method specifically mandates that genetic variations solely impact the desired result via the studied exposure. Despite the exclusion of identified confounding SNPs to the best of our ability, numerous undisclosed confounding variables remain, which impact gene pleiotropy and introduce bias in the estimation of effect values. To ensure the reliability and stability of the findings, two other techniques, specifically MR-Egger regression30 and the weighted median method (WME),31 were employed for testing. We conducted MR analysis for each metabolite individually. If the three MR models yielded comparable estimates of the causal effect, we deemed the metabolite's causal relationship with dental caries/periodontal disease to be consistent and trustworthy. The findings were analyzed as beta coefficients and 95% confidence intervals (CIs) for DMFS, DFSS, and N teeth per 1 standard deviation (SD) rise in blood/urine levels. To test for significant causality, the IVW analysis was employed, utilizing a stringent multiple hypothesis test threshold of p < 3.68 × 10−4. We also focused on metabolites with p values greater than or equal to 1.03 × 10−4 but less than 0.05 as potential risk predictors for caries/periodontal disease. The associations with p values below 0.05 underwent the subsequent examinations for heterogeneity and genetic pleiotropy.
The estimation of causal effects may be biased in the two-sample MR analysis method due to heterogeneity arising from variations in the analysis platform, experimental conditions, enrollment population, and SNP. Thus, in this research, the heterogeneity examination was conducted on the primary IVW analysis technique and MR-Egger regression. If the p value exceeded 0.05 in the test, it indicated the absence of heterogeneity in the incorporated instrumental variables, allowing the disregard of heterogeneity's impact on estimating causal effects. The MR-Egger regression analysis is applicable for assessing the bias of genetic pleiotropy. The magnitude of pleiotropy can be evaluated by the regression intercept, and the likelihood of pleiotropy decreases as the intercept approaches zero. The study utilized the p value from the genetic pleiotropy test to assess the existence of genetic pleiotropy in the analysis. If the p value exceeded 0.05, the presence of genetic pleiotropy in the causal analysis was deemed insignificant, and its impact could be disregarded.24
To test the reliability and stability of the results, the MR-Egger regression method, the WME, the simple estimation method based on the plural, and the plural-based weighted estimation method are utilized, in addition to the four methods stated earlier. In addition, sensitivity analysis was conducted using the leave-one-out method in the study. In other words, the metabolites’ sensitivity analysis successfully passed both the heterogeneity test and gene multiplicity test, and their p values in the IVW method used for sensitivity analysis were less than 0.05. After eliminating metabolites that had a p value below 0.05 in the IVW technique and successfully passing the heterogeneity and pleiotropy assessments, we excluded each associated SNP and computed the collective impact of the remaining SNPs. To evaluate the impact of individual SNPs on the metabolites, the cumulative influence of the remaining SNPs was computed. The impact of every single SNP on metabolites was assessed by eliminating each pertinent SNP and computing the collective impact of the remaining SNPs.32
3 RESULTS
3.1 Characteristics of the selected SNPs
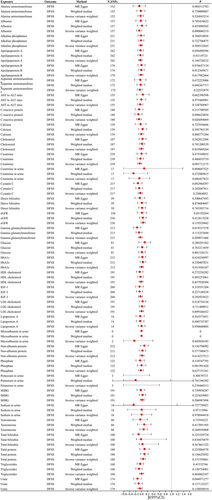
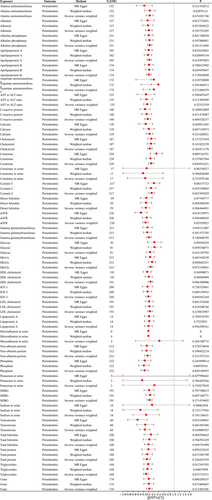
Out of the 35 metabolites, there were a total of 34,211 SNPs that showed association with p values less than 5 × 10−5. After LD analysis, 3567 independent SNPs were obtained, of which 477 SNPs were associated with at least two metabolites. Out of the 477 SNPs, there was a single SNP (rs174547) in the PhenoScanner database that showed an association with glycemia traits. In the subsequent analysis, 3566 SNPs were included after excluding confounding SNPs. The median number of instrumental variables for each metabolite was 79, and two metabolites (Microalbumin in urine and Potassium in urine) with instrumental variables less than or equal to 3 were excluded from the subsequent analysis.
3.2 Correlation between levels of blood/urine concentrations and the likelihood of developing dental caries and periodontitis
The primary approach employed in this study was the IVW technique to evaluate the causal association between metabolites and dental caries. Three metabolites (C-reactive protein, urine Potassium, and Urea) exhibited potential significant (p < 0.05) causal effects on dental caries, while none of the metabolites remained significant following multiple hypothesis testing (p < 3.68 × 10−4).
The MR study using the IVW method discovered a potential correlation between genetic susceptibility to C-reactive protein and DFFS (β: −0.083; 95% CI: −0.157 to −0.009; p = 0.027). There was a potential correlation between urinary potassium and DMFS, with a β value of −0.980 (95% CI: −1.831 to −0.128; p = 0.024). The concentration of urea showed a correlation with DMFS (β: −0.180; 95% CI: −0.283 to −0.077; p = 0.001) as depicted in Figures 1, 2, and Table 3. In the MR Egger model, there was a positive correlation between Gamma glutamyltransferase, Urate, Urea, and DFSS (Figure 3). The MR Egger model showed a nominal association between sodium levels in urine, C-reactive protein, and DMFS. Cholesterol was positively associated with DMFS in the Weighted median model (Table S1). Both the approach of calculating the median with weights and the MR-Egger estimation yielded inconclusive findings and demonstrated an absence of a causal relationship. Although all three MR models did not reach statistical significance, they all had similar effect values in most blood/urine metabolites, probably because the IVW method had higher test power than the other two MR models. The intercept p values for the MR-Egger test were all greater than 0.05. No substantial heterogeneity was observed (all p values for Cochran Q > 0.05). The sensitivity analysis, which involved leaving out one SNP at a time, revealed that none of the individual SNPs had a significant impact on the overall outcome (Table S2).
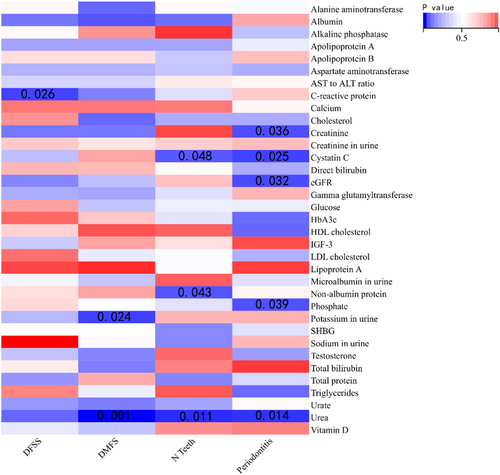
| Exposure | Outcome | IVW-derived p value | β | Upper 95% CIs | Lower 95% CIs | Cochran’ s Q-derived p value | MR-Egger intercept-derived p value |
|---|---|---|---|---|---|---|---|
| C-reactive protein | DFSS | 0.026 | −0.083 | −0.156 | −0.009 | 0.444 | 0.946 |
| Potassium in urine | DMFS | 0.024 | −0.98 | −1.831 | −0.128 | 0.464 | 0.532 |
| Urea | DMFS | 0.001 | −0.180 | −0.283 | −0.077 | 0.082 | 0.735 |
| Cystatin C | N teeth | 0.048 | −0.071 | −0.141 | −0.001 | 0.075 | 0.664 |
| Non-albumin protein | N teeth | 0.043 | −0.068 | −0.134 | −0.002 | 0.23 | 0.825 |
| Urea | N teeth | 0.010 | 0.12 | 0.028 | 0.212 | 0.468 | 0.093 |
| Creatinine | Periodontitis | 0.036 | 0.107 | 0.006 | 0.208 | 0.701 | 0.057 |
| Cystatin C | Periodontitis | 0.025 | 0.126 | 0.015 | 0.237 | 0.159 | 0.377 |
| eGFR | Periodontitis | 0.032 | −0.112 | −0.215 | −0.009 | 0.857 | 0.108 |
| Phosphate | Periodontitis | 0.039 | −0.147 | −0.287 | −0.007 | 0.022 | 0.981 |
| Urea | Periodontitis | 0.014 | 0.1847 | 0.035 | 0.333 | 0.369 | 0.676 |
- Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; MR, Mendelian randomization.

3.3 Blood/urine metabolites and risk of periodontitis
The primary approach used in this study was the IVW method to evaluate the causal connection between metabolites and the number of teeth affected by periodontitis. Nominal significant causal effect values (p < 0.05) were observed for six metabolites (Cystatin C, Non-albumin protein [NAP], Urea, Creatinine, estimated glomerular filtration rate [eGFR], and Phosphate) concerning N teeth/periodontitis. However, none of the metabolites remained significant after multiple hypothesis testing (p < 3.68 × 10−4).
According to the IVW model, the MR study discovered that an increase of 1 SD in Cystatin C levels potentially showed a positive correlation with the number of teeth (β: −0.070; 95% CI: −0.141 to −0.001; p = 0.048). There was a potential positive correlation between NAP and the number of teeth (β: −0.068; 95% CI: −0.134 to −0.002; p = 0.043). Urea showed a potential positive correlation with the number of teeth (β: 0.120; 95% CI: 0.028 to 0.212; p = 0.010) as depicted in Figure 4 and Table 3. In the IVM method, there was a potential positive correlation between Creatinine, Cystatin C, eGFR, Phosphate, and Urea with periodontitis. On the other hand, in the MR Egger model, Creatinine, eGFR, and Testosterone showed a positive association with periodontitis (Figure 5 and Table 3). The additional results are shown in Tables S1 and S2. The intercept of the MR-Egger test had a p value greater than 0.05. Based on leave-one-out analyses, it was indicated that no single SNP had a significant impact on the overall outcome of N teeth/periodontal disease.

4 DISCUSSION
This study utilized extensive GWAS data available in public databases to investigate the causal links between 35 metabolites found in blood/urine and the likelihood of developing dental caries/periodontal disease. The analysis employed an impartial two-sample Mendelian randomization approach. Nevertheless, despite rigorous quality control measures, there is a lack of compelling evidence suggesting a direct causal link between these blood metabolites and the development of dental caries or periodontal disease.
Despite none of the 35 blood/urine metabolites examined in this research meeting the criteria for multiple hypothesis testing, they yielded 8 potential indicators of the risk of dental caries/periodontal disease. These include C-reactive protein, Potassium in urine, Urea, Cystatin C, NAP, Creatinine, eGFR, and Phosphate. Out of the eight metabolites, four metabolites (Urea, Creatinine, Cystatin C, and Urea) could potentially correlate with a higher susceptibility to periodontal disease. It was observed that the non-survivor group had elevated levels of high-sensitivity C-reactive protein compared to the survivor group. Additionally, during the 3-year follow-up period, the T3 group exhibited a significantly higher mean hs-CRP value than the T1-2 group.33 To examine the impact of treatment with silver diamine fluoride (SDF) and potassium iodide (KI) on secondary caries, the authors and others conducted an investigation. It was shown that the treatment with SDF + KI decreased the occurrence of secondary caries.34 In addition, urea was linked to the possibility of tooth decay35, 36 and gum disease.37, 38 The levels of the protein Cystatin C (CSTC), an inhibitor of cysteine protease, and the expression of the CST3 gene were notably elevated in individuals with periodontal disease compared to the healthy population.39 Additionally, a direct association was observed between the levels of the gene and protein. Urea, Creatinine, and eGFR are all important biomarkers of renal function. Several studies have reported that periodontitis is closely related to chronic kidney disease.40-43 Dysregulated Phosphate Metabolism was closed with Periodontal Disease.44 The urinary protein consists of both NAPs and albumin. NAPs consist of small proteins, such as mucoproteins (primarily Tamm-Horsfall protein), blood-group proteins, immunoglobulins, mucopolysaccharides, hormones, and enzymes.45 There are no reports on caries and periodontal disease for NAPs.
This research possesses several strengths. First, it investigates the causal connection between metabolites in blood/urine and the risk of dental caries/periodontal disease from a molecular mechanism perspective, which holds significant clinical research value and is supported by a strong theoretical foundation. Secondly, the study ensures reliability and stability by implementing rigorous quality control measures, analytical methods, and multiple models to evaluate causal effects. Last, unlike previous Mendelian randomization studies that focused on a single exposure factor, this research tackles numerous metabolites in blood, which presents a substantial workload and demands challenging analysis. This study has certain constraints. First, the GWAS data for periodontal disease, caries, and metabolites were collected from European populations. Therefore, it is necessary to conduct more extensive studies involving diverse ethnic groups. Additionally, despite utilizing the most extensive GWAS data available, future research should aim to include larger sample sizes to obtain a more precise evaluation of the genetic influence of metabolites. Despite utilizing the most extensive GWAS data available, additional research is required to offer a more precise evaluation of the genetic influence of metabolites.
To sum up, we employed a two-sample Mendelian randomization method to investigate the causal connections among 35 blood and urine metabolites and caries as well as periodontal disease. While no strong causal link was established between these blood/urine metabolitesand the risk of caries and periodontal disease, this study's findings on potential predictors of caries and periodontal disease risk offer fresh perspectives on the influence of genetic-exposure interactions in the development of these conditions.
AUTHOR CONTRIBUTIONS
Xinhai Yin: investigation; validation; writing—original draft. Yadong Wu: resources; software; visualization. Jukun Song: conceptualization; data curation; formal analysis; writing—review & editing. All authors have read and approved the final version of the manuscript
ACKNOWLEDGMENTS
We wish to acknowledge the participants and investigators of the UK Biobank and the GLIDE consortium.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Jukun Song affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Jukun Song had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.