Evaluating the knowledge, attitudes, and practices of healthcare workers regarding high-risk nosocomial infections: A global cross-sectional study
Abstract
Background
Healthcare workers (HCWs) play a vital role in delivering care and are frequently exposed to the risk of acquiring infections within the hospital setting. Around 15% of hospitalized patients suffer from these infections globally. However, the role and awareness of HCWs in the transmission of hospital-acquired infections (HAIs) or nosocomial infections is still unclear. This study aimed to evaluate the knowledge, attitude, and practices (KAP) toward high-risk microbial infections among HCWs on a global scale to identify measures to address this problem.
Method
A cross-sectional descriptive study was conducted between 2022 and 2023, with HCWs selected as the study population. Data concerning KAP were collected through a self-administered online survey questionnaire, using a nonprobability convenience sampling method. Descriptive statistics and regression analysis were used to analyze the data.
Results
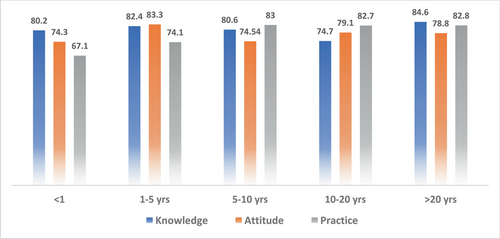
A total of 743 HCWs from various countries participated in the study, with the majority of respondents being doctors (64.9%). Data were mainly obtained from Saudi Arabia (26.78%), Iraq (25.84%), India (15.7%), the United States of America (15.2%), and Africa (Sudan, Nigeria) (13.98%). The frequency of good KAP scores among physicians (KAP: 82.5, 80.66, and 70.5), nurses (KAP: 74.1, 73.07, and 88.7), medical practitioners (KAP: 87.2, 77.58, and 75.1), and technicians (KAP: 76.1, 74.38, and 89.6) were obtained as mentioned. With respect to experience, HCWs showed good KAP scores in 1–5 years (KAP: 82.4, 83.3, and 74.1), 5–10 years (KAP: 80.6, 74.54, 83), 10–20 years (KAP: 74.7, 79.1, and 82.7), and >20 years (KAP: 84.6, 78.8, and 82.8) categories.
Conclusion
This study suggests that HCWs have good KAP regarding infection prevention, but there is still room for improvement. Educational seminars and awareness programs can provide better adherence to barrier protection measures such as hand washing, use of gloves, and hand disinfection.
1 INTRODUCTION
Hospital-acquired infections (HAIs) or nosocomial infections are localized or systemic. A healthcare worker (HCW) usually acquires these infections during their interaction with the patients during the examination and other day-to-day activities, including the collection of specimens, their processing and discarding, and handling of medical equipment. However, patients acquire these infections during their stay at the hospital.1, 2 The symptomatology of these infections appears 48–72 h after the patient's admission or within 10 days after the discharge from the healthcare facility.2-4 People of all age groups are susceptible to these infections, whereas those with weak immune systems, the elderly, and children are the most vulnerable. These infections have been known to severely affect the quality of healthcare services.2, 3 According to 2011 reports from the US Centers for Disease Control and Prevention (CDC), there were 37,000 HAI-associated deaths in the US compared to 111,000 in Europe. These infections result in approximately 16 million hospital admissions every year, imposing significant costs and burdens on the healthcare system.5 Bacteria such as Escherichia coli, Staphylococcus aureus, Streptococcus, and Clostridium sp., along with other nonbacterial microorganisms such as viruses, parasites, and fungi, can cause HAIs due to their presence in the air or on various surfaces in healthcare settings. The worldwide estimated rate of these infections is estimated at 12.9% for urinary tract infection (UTI); 21.8% for surgical site infections; 4.0% for lower respiratory tract infections; 21.8% for pneumonia; 9.9% for bloodstream infections; and 5.6% for ENT and mouth infections. Other infections include skin and soft tissues infections accounting for 3.2%, cardiovascular infections 1.2%; bone and joint infections 1.0%; central nervous system (CNS) 0.8%; reproductive tract infections 0.6%; and systemic infections 0.2%.2, 3, 6 The global HCWs' knowledge scores for nosocomial infections remain unknown, despite being the most prevalent causative factor of poor healthcare quality. HAI infections mainly affect low- and middle-income countries (LMICs). Although the overall global incidence of nosocomial infections has reportedly declined over time, the pooled prevalence remains significantly higher in resource-constrained settings: 15.5% in LMICs compared to 7.6% in high-income countries. However, this picture of the endemic burden of infections in developing countries is extremely doubtful owing to the scarcity of reliable data.7
In recent studies in Pakistan8 and Iran,9 nurses scored only 43% on knowledge and practice regarding the prevention of HAIs. The lack of knowledge and practice in this area may be a key factor in the spread of these infections.10 India reported a 57.5% score,11 while two Nigerian tertiary care hospitals orchestrated a 50.8% score for HCWs' practice in the prevention of healthcare-associated infections.12 Two studies from Ethiopia, one conducted in Bahir Dar, revealed a 54.2% practice score of HCWs in the prevention of HAI.13 Another study from Amhara regional state referral hospitals in Ethiopia reported 40.7% knowledge scores and 48.7% practice scores for the prevention of nosocomial infections.14 Knowledge regarding infectious agents, their mode, and transmission route plays an important role in planning and executing infection control. While inadequate knowledge and incorrect attitudes among HCWs can directly influence their practices and lead to delayed diagnosis, poor infection control practices, and the spread of the disease.15 The risk factors for the increased rate of inadequate practice and knowledge toward preventing HAIs include the years of experience, gender distribution among HCWs, their understanding and educational status, and lack of training and adherence to guidelines and workload.10
The incidence rate of nosocomial infections in LMIC has been estimated to be very high due to the limited knowledge of professional risks, adherence to universal precautions, and limited availability of personal protective equipment (PPE).16 Several epidemiological studies have reported that physicians, nurses, dentists, and laboratory personnel are the main sources of transmission of nosocomial infections due to aseptic precautions during medical procedures. Based on the guidelines of CDC, nosocomial transmission can be efficiently prevented by personal hygiene, use of PPE and proper handling of contaminated beddings and clothing during direct contact with the patients. Another preventive measure could be environmental decontamination which includes utilizing superheated water and sterile patient equipment. Proper training in the prevention of healthcare-associated infections, incorporated into both preservice and in-service medical education for healthcare professionals, along with effective monitoring and evaluation methods, can play a crucial role in improving their understanding, awareness, attitude, and practice of universal precautions and infection control when handling patients.3 While guidelines are available for HCWs to reduce HAIs,5, 17 factors such as poor awareness, noncompliance, and logistical and organizational barriers have hindered their effective implementation.6 This can be compounded by inadequate knowledge and inappropriate attitudes of HCWs, leading to improper practices that can impact the quality of diagnosis, treatment, and infection control, as well as increase the potential for microbial transmission. Analyzing the knowledge, attitudes, and practices (KAP) of HCWs will assess the transmission risk of nosocomial infections. Additionally, it may help to estimate and compare the quality of treatments in remedial settings. In this regard, the current study envisioned evaluating the attitude, awareness, and practices toward high-risk microbial infections among HCWs in a global setting to indicate measures to be taken to address the problem.
2 METHODOLOGY
A cross-sectional-descriptive research study was conducted globally between 2022 and 2023, with HCWs selected as the study population. The study utilized nonprobability sampling to collect responses from a global audience over a specific time interval using a web-based, and self-administered survey questionnaire. We distributed the questionnaire through various methods to reach a diverse group of healthcare professionals globally. These methods included online surveys sent to hospitals and healthcare facilities, social media platforms, and personal messaging to individual healthcare professionals. By utilizing these multiple channels, we aimed to capture a wide range of participants from different regions and healthcare settings. Sample size was calculated based on the following factors: Z = standard normal distribution value at 95% confidence level = 1.96, margin of error (d) = 5%, and good knowledge = 50%.
2.1 Selection criteria
It included the participation of HCWs actively working in hospitals and medical outreach. All HCWs, including doctors, nurses, medical practitioners, technicians (e.g., laboratory technicians, radiologic technologists), pharmacists, midwives, allied health professionals (e.g., physical therapists, occupational therapists, and dieticians), and other hospital staff (e.g., cleaning personnel) were invited to participate in the study. All professionals other than HCWs were excluded from the study.
2.2 Definition and classification of HCWs
HCWs and professionals are individuals who work to improve the health of others. They diagnose, treat, and manage diseases, injuries, and health-related issues based on the needs of the population. The international classification of HCWs includes medical practitioners (generalized and specialists), nursing professionals, midwifery professionals, traditional and complementary medicine professionals, paramedical practitioners, dentists, pharmacists, environmental and occupational health and hygiene professionals, physiotherapists, dieticians and nutritionists, audiologists and speech therapists, optometrists and ophthalmic opticians, medical and pathology laboratory technicians, paramedical technicians and assistants, and medical and dental prosthetic technicians and their assistants.18
2.3 Design of questionnaire
A comprehensive questionnaire consisting of 27 questions was developed based on guidelines and points from other validated published questionnaires2, 19 to assess socio-demographic and sample characteristics, such as age group, profession, years of experience, knowledge, practice, and attitude of HCWs. Names and genders were excluded to maintain participant anonymity. The questionnaire included 12 questions to evaluate knowledge scores, six questions for attitude scores, and five questions to analyze the infection control practices of HCWs. Knowledge was assessed based on the understanding of transmission and sources of infection, hygiene, handling of disposable syringes, those at risk of infection, PPE, and disinfectants that should be used. Situation-based questions were provided to analyze the attitude of HCWs in several instances. The practice of HCWs was assessed by determining the habits of handwashing, wearing masks, using needles and syringes, and dealing with blood exposure accidents. The level of knowledge, attitudes, and practices was considered appropriate when participants answered 80%–100% of the questions correctly. The questionnaire is available in the Supporting Information: Appendix.
2.4 Statistical analysis
Data were statistically analyzed using SPSS (version 23). The normality of the data was determined using the Shapiro-Wilk test. Descriptive analysis was carried out for qualitative and categorical variables at a nominal scale, which included age, experience, profession, country, KAP variables. The demographic data were presented in frequencies and percentages. The chi-square test was independently applied to profession and experience to determine the association with KAP variables. Cramer's v was used to assess the correlation between profession and experience with knowledge, practice, and attitudes. A range of 0–1 was used to depict the strength of the correlation between the two variables, where 0 indicated a weak correlation while 1 indicated a strong correlation. Binary logistic regression with Hosmer Lemeshow goodness of fit was applied to determine the relationship between the understanding of HCWs with the route of transmission of infection and their practice. The results were depicted as odds ratio (OR), where odds of >1 indicated a high rate of practice while odds <1 indicated decreased practice, with 95% confidence intervals (CIs). A p-value of <0.05 was considered an indicator of statistical significance. The statistical tests were two-sided.
2.5 Consent for publication
Before data collection, all study participants signed an informed consent form that addressed the purpose, significance, and privacy concerns of the study.
2.6 Ethical approval
The study obtained ethical approval from the Taibah University College of Medicine Research Ethics Committee (Study ID/Ref no.: TU-019-22) and Ajman University Research Ethics Committee (Ref no.: MFS6Feb) on February 15th and February 6th respectively. Before data collection, all study participants signed an informed consent form that addressed the purpose, significance, and privacy concerns of the study. Confidentiality and anonymity of the participants' data were assured, and they had the right to refuse or withdraw from the study at any point in time. No incentives or rewards were offered to the participants.
3 RESULTS
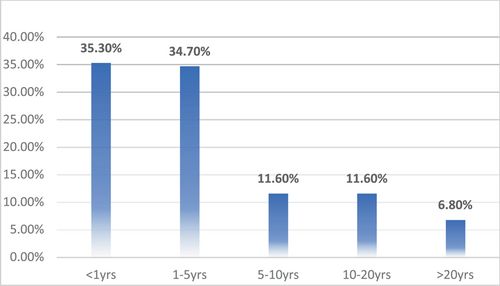
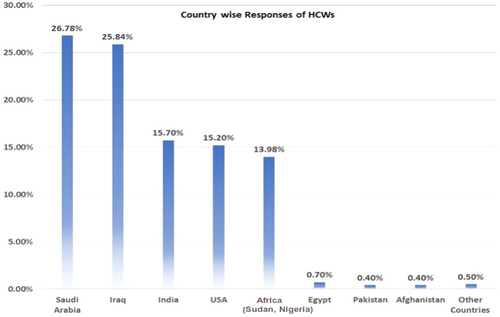
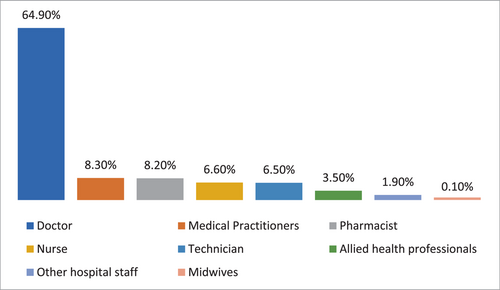
A total of 743 respondents participated in the study. Among them, 404 (4.4%) respondents were in 17–25 age bracket, 210 (28.3%) in 25–35, 73 (9.8%) in 35–45, and 56 (7.5%) in the 45–60 age groups. Other sociodemographic characteristics that were taken into consideration included years of experience (Figure 1), healthcare profession (Figure 2), and location/country (Figure 3). About 35.3% of the study participants had less than 1 year of experience, while 34.7% had between 1 and 5 years of experience (Figure 1). The majority of respondents (64.9%) were doctors, as shown in (Figure 3). We primarily obtained data from Saudi Arabia (26.78%), Iraq (25.84%), India (15.7%), the United States (15.2%), Africa (13.98%), Egypt, (0.7%), UAE (0.7%), Pakistan (0.4%), and Afghanistan (0.4%). Other countries accounted for 0.5% including Bangladesh (0.1%), Oman (0.1%), Qatar (0.1%), Russia (0.1%), and Canada (0.1%) (Figure 2). Figure 4 depicts the frequency of good KAP scores among physicians (KAP: 82.5, 80.66, and 70.5), nurses (KAP: 74.1, 73.07, and 88.7), medical practitioners (KAP: 87.2, 77.58, and 75.1), and technicians (KAP: 76.1, 74.38, and 89.6) (Table 1). With regard to experience (Figure 5), HCWs highlighted good KAP scores in 1–5 years (KAP: 82.4, 83.3, and 74.1), 5–10 years (KAP: 80.6, 74.54, and 83), 10–20 years (KAP: 74.7, 79.1, and 82.7), and >20 years (KAP: 84.6, 78.8, and 82.8) categories (Table 1, Figure 5).
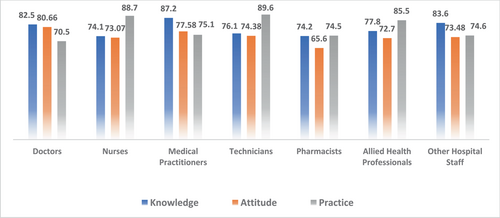
| Knowledge | Attitude | Practice | Total | |
|---|---|---|---|---|
| Profession | ||||
| Doctors | 82.5 | 80.66 | 70.5 | 77.9 |
| Nurses | 74.1 | 73.07 | 88.7 | 78.6 |
| Medical practitioners | 87.2 | 77.58 | 75.1 | 80.0 |
| Technicians | 76.1 | 74.38 | 89.6 | 80.0 |
| Pharmacists | 74.2 | 65.6 | 74.5 | 71.4 |
| Allied health professionals | 77.8 | 72.7 | 85.5 | 78.7 |
| Other hospital staff | 83.6 | 73.48 | 74.6 | 77.2 |
| Experience (years) | ||||
| <1 | 80.2 | 74.3 | 67.1 | 73.9 |
| 1–5 | 82.4 | 83.3 | 74.1 | 79.9 |
| 5–10 | 80.6 | 74.54 | 83 | 79.4 |
| 10–20 | 74.7 | 79.1 | 82.7 | 78.8 |
| >20 | 84.6 | 78.8 | 82.8 | 82.1 |
There was a significantly favorable professional attitude of HCWs toward HAI prevention. They strongly rejected the reuse of catheters and surgical forceps, stressed the importance of glove use during patient contact, and acknowledged the high HAI risk (Table 2). The study also demonstrated a strong correlation between HCWs' experience levels and their attitudes (Table 3).
| Attitude | Profession (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Doctors | Nurses | Medical professional | Technicians | Pharmacists | Midwives | Allied health professionals | Other hospital staff | p-Value | Cramer's v | |
| List of reusable equipment | ||||||||||
| Syringes | ||||||||||
| Yes | 45 (9.3) | 6 (12.2) | 10 (16.1) | 6 (12.5) | 11 (18.0) | 0 (0) | 4 (15.4) | 1 (7.1) | 0.426 | 0.097 |
| No | 437 (90.7) | 43 (87.8) | 52 (83.9) | 42 (87.5) | 50 (82.0) | 1 (100) | 22 (84.6) | 13 (92.9) | ||
| Stethoscope | ||||||||||
| Yes | 424 (88.0) | 44 (89.8) | 53 (85.5) | 44 (91.7) | 45 (73.8) | 1 (100) | 22 (84.6) | 12 (84.7) | 0.124 | 0.124 |
| No | 58 (12.0) | 5 (10.2) | 9 (14.5) | 4 (8.3) | 16 (26.2) | 0 (0) | 4 (15.4) | 2 (14.3) | ||
| Catheters | ||||||||||
| Yes | 81 (16.8) | 29 (59.2) | 14 (22.6) | 24 (50.0) | 19 (31.1) | 0 (0) | 13 (50.0) | 3 (21.4) | <0.001* | 0.318 |
| No | 401 (83.2) | 20 (40.8) | 48 (77.4) | 24 (50.0) | 42 (68.9) | 1(100) | 13 (50.0) | 11 (78.6) | ||
| Endoscope | ||||||||||
| Yes | 346 (71.8) | 34 (69.4) | 44 (71.0) | 42 (87.5) | 42 (68.9) | 0(0) | 15 (57.7) | 12 (85.7) | 0.078 | 0.131 |
| No | 136 (28.2) | 15 (30.6) | 18 (29.0) | 6 (12.5) | 19 (31.1) | 1 (100) | 11 (42.3) | 2 (14.3) | ||
| Surgical sponges | ||||||||||
| Yes | 50 (10.4) | 5 (10.2) | 7 (11.3) | 2 (4.2) | 5 (8.2) | 0 (0) | 3 (11.5) | 3 (21.4) | 0.733 | 0.077 |
| No | 432 (89.6) | 44 (89.8) | 55 (88.7) | 46 (95.8) | 56 (91.8) | 1 (100) | 23 (88.5) | 11 (78.6) | ||
| Surgical forceps | ||||||||||
| Yes | 333 (69.1) | 17 (34.7) | 40 (64.5) | 17 (35.4) | 20 (32.8) | 1 (100) | 13 (50.0) | 5 (35.7) | <0.001* | 0.299 |
| No | 149 (30.9) | 32 (65.3) | 22 (35.5) | 31 (64.6) | 41 (67.2) | 0 (0) | 13 (50.0) | 9 (64.3) | ||
| Average percentage of HCWs risk of developing hospital-acquired infections (HAIs) (between 0% and 100%) | ||||||||||
| 0%–25% | 167 (34.6) | 7 (14.3) | 6 (9.7) | 5 (10.4) | 9 (14.8) | 0 (0) | 2 (7.7) | 1 (7.1) | <0.001* | 0.185 |
| 25%–50% | 98 (20.3) | 3 (6.1) | 14 (22.6) | 5 (10.4) | 9 (14.8) | 1 (100) | 3 (11.5) | 3 (21.4) | ||
| 50%–75% | 75 (15.6) | 8 (16.3) | 13 (21.0) | 9 (18.8) | 10 (16.4) | 0 (0) | 7 (26.9) | 3 (21.4) | ||
| 75%–100% | 37 (7.7) | 11 (22.4) | 8 (12.9) | 14 (29.2) | 8 (13.1) | 0 (0) | 8 (30.8) | 0 (0) | ||
| I do not know | 105 (21.8) | 20 (40.8) | 21 (33.9) | 15 (31.3) | 25 (41.0) | 0 (0) | 6 (23.1) | 7 (50.0) | ||
| Wearing similar gloves while contacting different patients | ||||||||||
| True | 72 (14.9) | 1 (2.0) | 14 (22.6) | 7 (14.6) | 13 (21.3) | 0 (0) | 5 (19.2) | 2 (14.3) | 0.121 | 0.124 |
| False | 410 (85.1) | 48 (98.0) | 48 (77.4) | 41 (85.4) | 48 (78.7) | 1 (100) | 21 (80.8) | 12 (85.7) | ||
| Wearing gloves while having a direct contact when a patient seems healthy | ||||||||||
| Yes | 404 (83.8) | 44 (89.8) | 46 (74.2) | 38 (79.2) | 37 (60.7) | 0 (0) | 19 (73.1) | 10 (71.4) | <0.001* | 0.197 |
| No | 78 (16.2) | 5 (10.2) | 16 (25.8) | 10 (20.8) | 24 (39.3) | 1 (100) | 7 (26.9) | 4 (28.6) | ||
| Best way of dealing with a hazard to ensure others are not put at risk | ||||||||||
| Remove it immediately | 312 (64.7) | 27 (55.1) | 40 (64.5) | 26 (54.2) | 256 (42.6) | 1 (00) | 19 (73.1) | 6 (42.9) | <0.001* | 0.18 |
| Leave it for others to sort out | 27 (5.6) | 9 (18.4) | 3 (4.8) | 12 (25.0) | 2 (3.3) | 0 (0) | 3 (11.5) | 2 (14.3) | ||
| Place a barrier tape around it | 54 (11.2) | 7 (14.3) | 11 (17.7) | 7 (14.6) | 21 (34.4) | 0 (0) | 1 (3.8) | 1 (7.1) | ||
| Display a notice or warning sign | 89 (18.5) | 6 (12.2) | 8 (12.9) | 3 (6.3) | 12 (19.7) | 0 (0) | 3 (11.5) | 5 (35.7) | ||
| Using goggles while cutting catheter bags, cleaning bedpans, and emptying suction cups | ||||||||||
| Yes | 429 (89.0) | 37 (75.5) | 55 (88.7) | 37 (77.1) | 52 (85.2) | 1 (100) | 22 (84.6) | 11 (78.6) | 0.078 | 0.131 |
| No | 53 (11.0) | 12 (24.5) | 7 (11.3) | 11 (22.9) | 9 (14.8) | 0 (0) | 4 (15.4) | 3 (21.4) | ||
- * Statistically significant at p < 0.05.
| Attitude | Years of experience (%) | ||||||
|---|---|---|---|---|---|---|---|
| <1 | 01-May | 05-October | 20-October | >20 | p-Value | Cramer's v | |
| List of reusable equipment | |||||||
| Syringes | |||||||
| Yes | 39 (14.9) | 22 (8.5) | 11 (12.8) | 9 (10.5) | 2 (3.9) | 0.077 | 0.107 |
| No | 223 (85.1) | 236 (91.5) | 75 (87.2) | 77 (89.5) | 49 (96.1) | ||
| Stethoscope | |||||||
| Yes | 223 (85.1) | 225 (87.2) | 73 (84.9) | 77 (84.9) | 47 (92.2) | 0.591 | 0.061 |
| No | 39 (4.9) | 33 (12.8) | 13 (15.1) | 13 (15.1) | 4 (7.8) | ||
| Catheters | |||||||
| Yes | 48 (18.3) | 48 (18.3) | 34 (39.5) | 33 (38.4) | 20 (39.2) | <0.001* | 0.219 |
| No | 214 (81.7) | 210 (81.4) | 52 (60.5) | 53 (61.6) | 31 (60.8) | ||
| Endoscope | |||||||
| Yes | 153 (58.4) | 202 (78.3) | 65 (75.6) | 75 (87.2) | 40 (78.4) | <0.001* | 0.234 |
| No | 109 (41.6) | 56 (21.7) | 21 (24.4) | 11 (12.8) | 11 (21.6) | ||
| Surgical sponges | |||||||
| Yes | 38 (14.5) | 25 (9.7) | 5 (5.8) | 5 (5.8) | 2 (3.9) | 0.024* | 0.123 |
| No | 224 (85.5) | 233 (90.3) | 81 (94.2) | 81 (94.2) | 49 (96.1) | ||
| Surgical forceps | |||||||
| Yes | 157 (59.9) | 171 (66.3) | 36 (41.9) | 51 (59.3) | 31 (60.8) | 0.003* | 0.147 |
| No | 105 (40.1) | 87 (33.7) | 50 (58.1) | 35 (40.7) | 20 (39.2) | ||
| Average percentage of HCWs at risk of developing healthcare-associated infection (between 0% and 100%) | |||||||
| 0%–25% | 61 (23.3) | 103 (39.9) | 7 (8.1) | 14 (16.3) | 12 (23.5) | <0.001* | 0.192 |
| 25%–50% | 65 (24.8) | 46 (17.8) | 14 (16.3) | 7 (8.1) | 4 (7.8) | ||
| 50%–75% | 53 (20.2) | 38 (14.7) | 13 (15.1) | 15 (7.4) | 6 (11.8) | ||
| 75%–100% | 16 (6.1) | 14 (5.4) | 19 (22.1) | 24 (27.9) | 13 (25.5) | ||
| I do not know | 25.6 | 57 (22.1) | 33 (38.4) | 26 (30.2) | 16 (31.4) | ||
| Wearing similar gloves while contacting different patients | |||||||
| True | 48 (18.3) | 39 (15.1) | 12 (14.0) | 10 (11.6) | 5 (9.8) | 0.4 | 0.074 |
| False | 214 (81.7) | 219 (84.9) | 74 (86.0) | 76 (88.4) | 46 (90.2) | ||
| Wearing gloves while having a direct contact when a patient seems healthy | |||||||
| Yes | 207 (79.0) | 217 (84.1) | 67 (77.9) | 71 (82.6) | 36 (70.6) | 0.178 | 0.092 |
| No | 55 (21.0) | 41 (15.9) | 19 (22.1) | 15 (17.4) | 15 (29.4) | ||
| Best way of dealing with a hazard to ensure others are not put at risk | |||||||
| Remove it immediately | 137 (52.3) | 189 (73.3) | 45 (52.3) | 50 (58.1) | 36 (70.6) | <0.001* | 0.149 |
| Leave it for others to sort out | 19 (7.3) | 12 (4.7) | 10 (11.6) | 15 (17.4) | 2 (3.9) | ||
| Place a barrier tape around it | 40 (15.3) | 30 (11.6) | 17 (19.8) | 9 (10.5) | 6 (11.8) | ||
| Display a notice or warning sign | 66 (25.2) | 27 (10.5) | 14 (16.3) | 12 (14.0) | 7 (13.7) | ||
| Using goggles while cutting catheter bags, cleaning bedpans, and emptying suction cups | |||||||
| Yes | 221 (84.4) | 240 (93.0) | 73 (84.9) | 73 (84.9) | 37 (72.5) | 0.001* | 0.162 |
| No | 41 (15.6) | 18 (7.0) | 13 (15.1) | 13 (15.1) | 14 (27.5) | ||
- * Statistically significant at p < 0.05.
3.1 Knowledge of HCWs about HAI prevention
3.1.1 Correlation of HCWs' professional nature and knowledge scores
The results of the study showed significant variations in the knowledge scores of HCWs based on their profession, particularly regarding the transmission of infections through blood-borne viruses like Hepatitis C and D (p < 0.002), chickenpox/shingles (p < 0.001), tuberculosis (p < 0.01), and HIV (p < 0.001). We additionally observed significant differences in responses related to the transmissibility of skin and soft tissue infections through routes such as kissing (p < 0.001), sexual intercourse (p < 0.003), and respiratory transmission (p < 0.001). The study further identified significant variations (p < 0.001) in the knowledge of HCWs through respiratory transmission. The response on transmission modes of diarrheal illnesses showed fluctuations considering respiratory (p < 0.007) and fecal-oral routes (p < 0.001). The analysis of HCWs' technical knowledge revealed an uneven distribution with respect to hygienic practices, such as the reuse of syringes (p < 0.015) and preferences for cleaning methods (p < 0.001) (Table 4).
| Knowledge | Profession (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Doctors | Nurses | Medical professional | Technicians | Pharmacists | Midwives | Allied health professionals | Other hospital staff | p-Value | Cramer's v | |
| Microbes that are mainly transmitted through blood | ||||||||||
| Influenza | ||||||||||
| Yes | 34 (7.1) | 3 (6.1) | 6 (9.7) | 0 (0) | 6 (9.8) | 0 (0) | 2 (7.7) | 3 (21.4) | 0.237 | 0.112 |
| No | 448 (92.9) | 46 (93.9) | 56 (90.3) | 48 (100) | 55 (90.2) | 1 (100) | 24 (92.3) | 11 (78.6) | ||
| Hepatitis C and D virus | ||||||||||
| Yes | 285 (59.1) | 45 (91.8) | 44 (71.0) | 47 (98.9) | 46 (75.4) | 1 (100) | 23 (88.5) | 11 | <0.001* | 0.278 |
| −78.6 | ||||||||||
| No | 197 (40.9) | 4 (8.2) | 18 (29.0) | 1 (2.1) | 15 (24.6) | 0 (0) | 3 (11.5) | 3 (21.4) | ||
| Tuberculosis | ||||||||||
| Yes | 50 (10.4) | 5 (10.2) | 8 (12.9) | 4 (8.3) | 18 (29.5) | 0 (0) | 4 (51.4) | 5 (35.7) | <0.01* | 0.187 |
| No | 432 (89.6) | 44 (89.8) | 54 (87.1) | 44 (91.7) | 43 (70.5) | 1 (100) | 22 (84.6) | 9 (64.3) | ||
| Chicken pox and Shingles (caused by herpes virus) | ||||||||||
| Yes | 34 (7.1) | 6 (12.2) | 5 (8.1) | 5 (8.1) | 15 (24.6) | 0 (0) | 8 (30.8) | 4 (28.6) | <0.001* | 0.221 |
| No | 448 (92.9) | 43 (87.8) | 57 (91.9) | 43 (89.6) | 46 (75.4) | 1 (100) | 18 (69.2) | 10 (71.4) | ||
| HIV | ||||||||||
| Yes | 165 (34.2) | 0 (0) | 12 (19.4) | 0 (0) | 7 (11.5) | 0 (0) | 1 (3.8) | 1 (7.1) | <0.001* | 0.307 |
| No | 317 (65.8) | 49 (100) | 50 (80.6) | 48 (100) | 54 (88.5) | 1 (100) | 25 (96.2) | 13 (92.9) | ||
| 70% alcohol kills germs | ||||||||||
| Yes | 401 (83.2) | 43 (87.8) | 51 (82.3) | 47 (97.9) | 54 (88.5) | 1 (100) | 25 (96.2) | 10 (71.4) | 0.06 | 0.135 |
| No | 81 (16.8) | 6 (12.2) | 11 (17.7) | 1 (2.1) | 7 (11.5) | 0 (0) | 1 (3.8) | 4 (28.6) | ||
| Healthy person is capable of transmitting infections | ||||||||||
| Yes | 458 (95) | 43 (87.8) | 58 (93.5) | 41 (85.4) | 52 (85.2) | 1 (100) | 20 (76.9) | 11 (78.6) | 0.001* | 0.186 |
| No | 24 (5.0) | 6 (12.2) | 4 (6.5) | 7 (14.6) | 9 (14.8) | 0 (0) | 6 (23.1) | 3 (21.4) | ||
| Sharing of food with patients spreads viral or bacterial infections | ||||||||||
| Yes | 394 (81.7) | 38 (77.6) | 53 (85.5) | 41 (85.4) | 44 (72.1) | 1 (100) | 18 (69.2) | 12 (85.7) | 0.348 | 0.103 |
| No | 88 (18.3) | 11 (22.4) | 9 (14.5) | 7 (14.6) | 17 (27.9) | 0 (0) | 8 (30.8) | 2 (14.3) | ||
| Cleaning hands while caring for patients with vomiting or diarrheal illnesses | ||||||||||
| Alcohol | 89 (18.5) | 15 (30.6) | 19 (30.6) | 14 (29.2) | 21 (34.4) | 0 (0) | 8 (30.8) | 4 (28.6) | <0.001* | 0.221 |
| Soap | 177 (36.7) | 5 (10.2) | 9 (14.5) | 1 (2.1) | 7 (11.5) | 0 (0) | 0 (0) | 2 (14.3) | ||
| Any of them | 216 (44.8) | 29 (59.2) | 34 (54.8) | 33 (68.8) | 33 (54.1) | 1 (100) | 18 (69.2) | 8 (57.1) | ||
| Bending, shearing, breaking, or re-capping of disposable needles or removal from disposable syringes | ||||||||||
| Yes | 116 (24.1) | 5 (10.2) | 18 (29.0) | 2 (4.2) | 12 (19.7) | 0 (0) | 4 (15.4) | 4 (28.6) | 0.015* | 0.153 |
| No | 366 (75.9) | 44 (89.8) | 44 (71.0) | 46 (95.8) | 49 (80.3) | 1 (100) | 22 (84.6) | 10 (71.4) | ||
| Can we empty the contents of the sharp's disposal container into another container? | ||||||||||
| Yes | 80 (16.6) | 7 (14.3) | 16 (25.8) | 8 (16.7) | 16 (26.2) | 45 (73.8) | 9 (34.6) | 5 (35.7) | 0.06 | 0.135 |
| No | 402 (83.4) | 42 (85.7) | 46 (74.2) | 40 (83.3) | 0 (0) | 1 (100) | 17 (65.4) | 9 (64.3) | ||
| Only elderly, comorbid patients, and people with weak immune systems are affected by hospital-acquired infections (HAIs) | ||||||||||
| Yes | 112 (23.2) | 8 (16.3) | 17 (27.4) | 7 (14.6) | 18 (29.5) | 0 (0) | 4 (15.4) | 4 (28.6) | 0.458 | 0.095 |
| No | 370 (76.8) | 41 (83.7) | 45 (72.6) | 41 (85.4) | 43 (70.5) | 1 (100) | 22 (84.6) | 10 (71.4) | ||
| Wearing medical gowns during surgical procedures only | ||||||||||
| True | 123 (25.5) | 8 (16.3) | 12 (19.4) | 8 (16.7) | 14 (23.0) | 0 (0) | 8 (30.8) | 5 (35.7) | 0.493 | 0.093 |
| False | 359 (74.5) | 4 (83.7) | 50 (80.6) | 40 (83.3) | 47 (77.0) | 1 (100) | 18 (69.2) | 9 (64.3) | ||
| Transmission of skin and soft tissue infections | ||||||||||
| Skin contact | ||||||||||
| Yes | 450 (93.4) | 48 (98.0) | 56 (90.3) | 44 (91.7) | 57 (93.4) | 1 (100) | 23 (88.5) | 13 (92.9) | 0.811 | 0.071 |
| No | 32 (6.6) | 1 (2.0) | 6 (9.7) | 4 (8.3) | −6.6 | 0 (0) | 3 (1.0) | 1 (7.1) | ||
| Sexual intercourse | ||||||||||
| Yes | 241 (50) | 31 (63.3) | 43 (69.4) | 32 (66.7) | 32 (52.5) | 1 (100) | 11 (42.3) | 12 (85.7) | 0.003* | 0.171 |
| No | 241 (50) | 18 (36.7) | 19 (30.6) | 16 (33.3) | 29 (47.5) | 0 (0) | 15 (57.7) | 2 (14.) | ||
| Respiratory transmission | ||||||||||
| Yes | 95 (19.7) | 4 (8.2) | 17 (27.4) | 1 (2.1) | 10 (16.5) | 0 (0) | 1 (3.8) | 6 (42.9) | 0.001* | 0.186 |
| No | 387 (80.3) | 45 (91.8) | 45 (72.6) | 47 (97.9) | 51 (83.6) | 1 (100) | 25 (96.2) | 8 (57.1) | ||
| Fecal-oral route transmission | ||||||||||
| Yes | 50 (10.4) | 3 (6.1) | 4 (6.5) | 4 (8.3) | 8 (13.1) | 0 (0) | 3 (11.5) | 1 (7.1) | 0.895 | 0.062 |
| No | 432 (89.6) | 46 (93.9) | 58 (93.5) | 44 (91.7) | 53 (86.9) | 1 (100) | 23 (55.5) | 13 (92.9) | ||
| Kissing | ||||||||||
| Yes | 328 (68.0) | 23 (46.9) | 40 (64.5) | 21 (43.8) | 25 (41.0) | 1 (100) | 10 (38.5) | 8 (57.1) | <0.001* | 0.223 |
| No | 154 (32.0) | 26 (53.1) | 22 (35.5) | 27 (56.3) | 36 (59.0) | 0 (0) | 16 (61.5) | 6 (42.9) | ||
| Transmission of respiratory infections | ||||||||||
| Air droplets | ||||||||||
| Yes | 443 (91.9) | 47 (95.9) | 57 (91.9) | 44 (91.7) | 52 (85.2) | 1 (100) | 24 (92.3) | 13 (92.9) | 0.706 | 0.079 |
| No | 39 (8.1) | 2 (4.1) | 5 (8.1) | 4 (8.1) | 9 (14.8) | 0 (0) | 2 (7.7) | 1 (7.1) | ||
| Fecal oral route | ||||||||||
| Yes | 67 (13.9) | 4 (8.2) | 8 (12.9) | 1 (2.1) | 9 (14.8) | 0 (0) | 1 (3.8) | 1 (7.1) | 0.241 | 0.111 |
| No | 415 (86.1) | 45 (91.8) | 54 (87.1) | 47 (97.9) | 52 (85.2) | 1 (100) | 25 (96.2) | 13 (92.9) | ||
| Respiratory secretions | ||||||||||
| Yes | 421 (87.3) | 47 (95.9) | 52 (83.9) | 47 (97.9) | 52 (85.2) | 1 (100) | 24 (92.3) | 13 (92.9) | 0.183 | 0.117 |
| No | 61 (12.7) | 2 (4.1) | 10 (16.1) | 1 (2.1) | 9 (14.8) | 0 (0) | 2 (7.7) | 1 (7.1) | ||
| Sexual intercourse | ||||||||||
| Yes | 38 (7.9) | 3 (6.1) | 6 (9.7) | 5 (10.4) | 9 (14.8) | 0 (0) | 2 (7.7) | 3 (21.4) | 0.46 | 0.095 |
| No | 444 (92.1) | 46 (93.9) | 56 (90.3) | 43 (89.6) | 52 (85.2) | 1 (100) | 24 (92.3) | 11 (78.6) | ||
| Contact with contaminated hands | ||||||||||
| Yes | 163 (33.8) | 3(6.1) | 17(27.4) | 2(4.2) | 18(29.5) | 1(100) | 1(3.8) | 9 (64.3) | <0.001* | 0.263 |
| No | 319 (66.2) | 46(93.9) | 45(72.6) | 46(95.8) | 43(70.5) | 0(0) | 25(96.2) | 5 (35.7) | ||
| Spread of diarrheal illnesses | ||||||||||
| Air Droplets | ||||||||||
| Yes | 54 (11.2) | 2 (4.1) | 5 (8.1) | 5 (10.4) | 13 (21.3) | 1 (100) | 3 (11.5) | 2 (14.3) | 0.017* | 0.152 |
| No | 428 (88.8) | 47 (95.9) | 57 (91.9) | 43 (89.6) | 48 (78.7) | 0 (0) | 23 (88.5) | 12 (85.7) | ||
| Fecal oral route | ||||||||||
| Yes | 416 (83.3) | 41 (83.7) | 48 (77.4) | 38 (79.2) | 38 (62.3) | 1 (100) | 18 (69.2) | 10 (71.4) | <0.001* | 0.193 |
| No | 66 (13.7) | 8 (16.3) | 14 (22.6) | 10 (20.8) | 23 (37.7) | 0 | 8 (30.8) | 4 (28.6) | ||
| Respiratory secretions | ||||||||||
| Yes | 37 (7.7) | 1 (2.0) | 4 (6.5) | 5 (10.4) | 9 (14.8) | 1 (100) | 4 (15.4) | 1 (7.1) | 0.007* | 0.161 |
| No | 445 (92.3) | 48 (98.0) | 58 (93.5) | 43 (89.6) | 52 (85.2) | 0 (0) | 22 (84.6) | 13 (92.9) | ||
| Sexual intercourse | ||||||||||
| Yes | 56 (11.6) | 3 (6.1) | 7 (11.3) | 9 (18.8) | 13 (21.3) | 0 (0) | 0 (0) | 3 (21.4) | 0.064 | 0.134 |
| No | 426 (88.4) | 46 (93.9) | 55 (88.7) | 39 (81.3) | 48 (78.7) | 1 (100) | 26 (100) | 11 (78.6) | ||
| Contact with contaminated hands | ||||||||||
| Yes | 394 (81.7) | 40 (81.6) | 50 (80.6) | 37 (77.1) | 41 (67.2) | 1 (100) | 19 (73.1) | 12 (85.7) | 0.268 | 0.109 |
| No | 88 (18.3) | 9 (18.2) | 12 (19.2) | 11 (22.9) | 20 (32.8) | 0 (0) | 7 (26.9) | 2 (14.3) | ||
- * Statistically significant at p < 0.05.
3.1.2 Correlation of HCWs' experience and knowledge scores
In relation to their experience, HCWs exhibited significantly higher levels of knowledge with respect to the bloodborne dissemination of infectious organisms, such as Hepatitis C and D (p < 0.001) and HIV (p < 0.001). Moreover, their responses to the transmission of soft tissue infections through sexual intercourse (p < 0.002), respiratory route (p < 0.001), or kissing (p < 0.001) also displayed significant differences. Experienced HCWs acknowledged the importance of safeguarding vulnerable populations, such as the elderly and immunocompromised individuals, against HAIs, and the use of PPE (p < 0.002). Similarly, the general understanding of the fecal-oral route as a source of diarrheal infections was markedly high (p < 0.004) among experienced HCWs. Furthermore, their technical knowledge regarding hygiene practices, including the use of 70% alcohol (p < 0.001), proper disposal of syringes (p < 0.001), and cleaning methods preferences (p < 0.001) also varied significantly (Table 5).
| Knowledge | Years of experience (%) | ||||||
|---|---|---|---|---|---|---|---|
| <1 | 1–5 | 5–10 | 10–20 | >20 | p-Value | Cramer's v | |
| Microbes that are mainly transmitted through blood | |||||||
| Influenza | |||||||
| Yes | 25 (9.5) | 20 (7.8) | 6 (7.0) | 2 (2.3) | 1 (2.0) | 0.118 | 0.100 |
| No | 237 (90.5) | 238 (92.2) | 80 (93.0) | 84 (97.7) | 50 (98.0) | ||
| Hepatitis C and D virus | |||||||
| Yes | 180 (68.7) | 131 (50.8) | 75 (87.2) | 72 (83.7) | 44 (86.3) | <0.001* | 0.300 |
| No | 82 (31.3) | 127 (49.2) | 11 (12.8) | 14 (6.3) | 7 (13.7) | ||
| Tuberculosis causing bacterium | |||||||
| Yes | 39 (14.9) | 32 (12.4) | 11 (12.8) | 8 (9.3) | 4 (7.8) | 0.535 | 0.065 |
| No | 223 (85.1) | 226 (87.6) | 75 (87.2) | 78 (90.7) | 47 (92.2) | ||
| Chicken pox and Shingles (caused by Herpes Virus) | |||||||
| Yes | 31 (11.8) | 22 (8.5) | 8 (9.3) | 8 (9.3) | 8 (15.7) | 0.508 | 0.067 |
| No | 231 (88.2) | 236 (91.5) | 78 (90.7) | 78 (90.7) | 43 (84.3) | ||
| HIV | |||||||
| Yes | 60 (22.9) | 108 (41.9) | 3 (3.5) | 8 9.3) | 7 (13.7) | <0.001* | 0.319 |
| No | 202 (77.1) | 150 (58.1) | 83 (96.5) | 78 (90.7) | 44 (86.3) | ||
| 70% alcohol kills germs | |||||||
| Yes | 201 (76.7) | 225 (87.5) | 77 (89.5) | 81 (94.2) | 48 (94.1) | <0.001* | 0.186 |
| No | 61 (23.3) | 33 (412.8) | 9 (10.5) | 5 (5(5.8) | 3 (5.9) | ||
| Healthy person is capable of transmitting infections | |||||||
| Yes | 245 (93.5) | 237 (91.9) | 76 (88.4) | 78 (90.7) | 48 (94.1) | 0.578 | 0.062 |
| No | 17 (6.5) | 21 (8.1) | 10 (11.6) | 8 (9.3) | 3 (5.9) | ||
| Sharing of food with patients spreads viral or bacterial infections | |||||||
| Yes | 208 (79.4) | 224 (86.8) | 66 (76.7) | 37 (72.5) | 0.033* | 0.119 | |
| No | 54 (20.6) | 34 (13.2) | 20 (23.3) | 14 (27.5) | |||
| Cleaning hands while caring for patients with vomiting or diarrheal illnesses | |||||||
| Alcohol | 84 (32.1) | 34 (13.2) | 21 (24.4) | 24 (27.9) | 7 (13.7) | <0.001* | 0.226 |
| Soap | 72 (27.5) | 101 (39.1) | 7 (8.1) | 6 (7.0) | 15 (29.4) | ||
| Any of them | 106 (40.5) | 123 (47.7) | 58 (67.4) | 56 (65.1) | 29 (56.9) | ||
| Bending, shearing, breaking, or re-capping of disposable needles or removal from disposable syringes | |||||||
| Yes | 79 (30.2) | 45 (17.4) | 17 (19.8) | 13 (15.1) | 7 (13.7) | 0.001* | 0.156 |
| No | 183 (69.8) | 213 (82.6) | 69 (80.2) | 73 (84.9) | 44 (86.3) | ||
| Can we empty the contents of the sharp's disposal container into another container? | |||||||
| Yes | 54 (20.6) | 39 (15.1) | 21 (24.4) | 19 (22.1) | 8 (15.7) | 0.238 | 0.086 |
| No | 208 (79.4) | 219 (84.9) | 65 (75.6) | 67 (77.9) | 43 (84.3) | ||
| Only elderly, comorbid patients, and people with weak immune system are affected by hospital acquired illnesses | |||||||
| Yes | 80 (30.5) | 51 (9.8) | 19 (22.1) | 11 (12.8) | 9 (17.6) | 0.003* | 0.146 |
| No | 182 (68.5) | 207 (80.2) | 67 (779) | 75 (87.2) | 42 (82.4) | ||
| Wearing medical gowns during surgical procedures only | |||||||
| True | 80 (30.5) | 63 (24.4) | 10 (11.6) | 13 (15.1) | 12 (23.5) | 0.002* | 0.152 |
| False | 182 (69.5) | 195 (75.6) | 76 (88.4) | 73 (84.9) | 39 (76.5) | ||
| Transmission of skin and soft tissue infections | |||||||
| Skin contact | |||||||
| Yes | 239 (91.2) | 241 (93.4) | 80 (93.0) | 81 (94.2) | 51 (100) | 0.245 | 0.086 |
| No | 23 (8.8) | 17 (6.6) | 6 (7.0) | 5 (5.8) | 0 (0) | ||
| Sexual intercourse | |||||||
| Yes | 153 (58.4) | 114 (44.2) | 50 (58.1) | 53 (61.6) | 33 (64.7) | 0.002* | 0.151 |
| No | 109 (41.6) | 114 (55.8) | 36 (41.9) | 33 (38.4) | 18 (35.3) | ||
| Respiratory transmission | |||||||
| Yes | 83 (27.9) | 42 (16.3) | 5 (5.8) | 10 (11.6) | 4 (7.8) | <0.001* | 0.209 |
| No | 189 (72.1) | 216 (83.7) | 81 (84.2) | 7 (88.4) | 47 (92.2) | ||
| Fecal-oral route transmission | |||||||
| Yes | 29 (11.1) | 22 (8.5) | 10 (11.6) | 10 (11.6) | 2 (3.9) | 0.465 | 0.069 |
| No | 233 (88.9) | 236 (91.5) | 76 (88.4) | 76 (88.4) | 49 (96.1) | ||
| Kissing | |||||||
| Yes | 163 (62.2) | 183 (70.9) | 41 (47.7) | 42 (48.8) | 27 (52.9) | <0.001* | 0.189 |
| No | 99 (37.8) | 75 (29.1) | 45 (52.3) | 44 (51.2) | 24 (47.1) | ||
| Transmission of respiratory infections | |||||||
| Air droplets | |||||||
| Yes | 239 (91.2) | 237 (91.9) | 78 (90.7) | 78 (90.7) | 49 (96.1) | 0.811 | 0.046 |
| No | 23 (8.8) | 21 (8.1) | 8 (9.3) | 8 (9.3) | 2 (3.9) | ||
| Fecal oral route | |||||||
| Yes | 48 (18.3) | 33 (12.8) | 5 (5.8) | 4 (4.7) | 1 (2.0) | <0.001* | 0.172 |
| No | 214 (81.7) | 225 (87.2) | 81 (94.2) | 82 (95.3) | 50 (98) | ||
| Respiratory secretions | |||||||
| Yes | 221 (84.14) | 229 (88.8) | 78 (90.7) | 83 (96.5) | 46 (90.2) | 0.035* | 0.118 |
| No | 41 (15.6) | 29 (11.2) | 8 (9.3) | 3 (3.5) | 5 (9.8) | ||
| Sexual intercourse | |||||||
| Yes | 28 (10.7) | 21 (8.1) | 10 (11.6) | 3 (3.5) | 4 (7.8) | 0.268 | 0.084 |
| No | 234 (89.3) | 237 (91.9) | 76 (88.4) | 83 (96.5) | 47 (92.2) | ||
| Contact with contaminated hands | |||||||
| Yes | 115 (43.9) | 56 (21.7) | 10 (11.6) | 20 (23.3) | 13 (25.5) | <0.001* | 0.258 |
| No | 147 (56.1) | 202 (78.3) | 76 (88.8) | 66 (76.7) | 38 (74.5) | ||
| Spread of diarrheal illnesses | |||||||
| Air droplets | |||||||
| Yes | 41 (15.6) | 28 (10.9) | 6 (7.0) | 7 (8.1) | 3 (5.9) | 0.065 | 0.109 |
| No | 221 (84.4) | 230 (89.1) | 80 (93.0) | 79 (91.9) | 48 (94.1) | ||
| Fecal oral route | |||||||
| Yes | 202 (77.1) | 231 (89.5) | 69 (80.2) | 68 (79.1) | 40 (78.2) | 0.004* | 0.144 |
| No | 60 (22.9) | 27 (10.5) | 17 (19.8) | 18 (20.9) | 11 (21.6) | ||
| Respiratory secretions | |||||||
| Yes | 28 (10.7) | 17 (6.6) | 6 (7.0) | 7 (8.1) | 4 (7.4) | 0.533 | 0.065 |
| No | 234 (89.3) | 241 (93.1) | 80 (93.0) | 79 (91.9) | 47 (92.2) | ||
| Sexual intercourse | |||||||
| Yes | 39 (14.9) | 29 (11.2) | 11 (12.8) | 10 (11.6) | 2 (3.9) | 0.259 | 0.084 |
| No | 223 (85.1) | 229 (88.8) | 75 (87.2) | 76 (88.2) | 49 (96.1) | ||
| Contact with contaminated hands | |||||||
| Yes | 200 (76.3) | 212 (82.2) | 66 (76.7) | 73 (84.9) | 43 (84.3) | 0.249 | 0.085 |
| No | 62 (23.7) | 46 (17.8) | 20 (23.3) | 13 (15.1) | 8 (15.7) | ||
- * Statistically significant at p < 0.05.
3.2 Attitude of HCWs about HAI prevention
3.2.1 Correlation of HCWs' professional nature and attitude scores
The professional attitude of HCWs toward the prevention of HAIs was significantly favorable in this study. Specifically, HCWs demonstrated a strong disagreement with the notion of considering catheters (p < 0.001) and surgical forceps (p < 0.001) as reusable equipment. Furthermore, HCWs strongly reported the importance of using gloves while in direct contact with patients (p < 0.001), and recognized the high risk of HAIs (p < 0.001). The study also found that HCWs had a considerably high favorable attitude (p < 0.001) toward the practice of hazard prevention to ensure that others were not put at risk (Table 2).
3.2.2 Correlation of HCWs' experience and attitude scores
HCWs revealed a strong relationship between their level of experience and their attitudes toward the reuse of certain medical equipment. They strongly disagreed toward using catheters (p < 0.001), endoscopes (p < 0.001), surgical sponges (p < 0.024), and forceps (p < 0.003) as reusable equipment. Furthermore, HCWs recognized the importance of using goggles to prevent accidents, and their behaviors toward the prevention of hazards were favorable (p < 0.001). The study also found that HCWs recognized the high risk of HAIs (p < 0.001) and had a significantly favorable attitude (p < 0.001) toward preventing them (Table 6). About 72.13% of individuals had appropriate attitudes (Table 3).
| Practice | Profession (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Doctors | Nurses | Medical practitioners | Technicians | Pharmacists | Midwives | Allied health professionals | Other hospital staff | p-Value | Cramer's v | |
| Wearing masks | ||||||||||
| All the time | 326 (67.6) | 6 (12.2) | 22 (35.5) | 6 (12.5) | 21 (34.4) | 0 (0) | 8 (30.8) | 9 (64.3) | <0.001* | 0.304 |
| Wear mask at suspected places | 148 (30.7) | 41 (83.7) | 39 (62.9) | 41 (85.4) | 36 (59.0) | 1 (100) | 16 (61.5) | 4 (28.6) | ||
| Never | 8 (1.7) | 2 (4.1) | 1 (1.6) | 1 (2.1) | 4 (6.6) | 0 (0) | 2 (7.7) | 1 (7.1) | ||
| Using single needle for two pricks on a single patient | ||||||||||
| Yes | 82 (17) | 3 (6.1) | 9 (14.5) | 3 (6.3) | 7 (11.5) | 0 (0) | 1 (3.8) | 3 (21.4) | 0.123 | 0.124 |
| No | 400 (83.0) | 46 (93.9) | 53 (85.5) | 45 (93.8) | 54 (88.5) | 1 (100) | 25 (96.2) | 11 (78.6) | ||
| Using same medical equipment for another patient after sterilization | ||||||||||
| Yes | 351 (72.8) | 37 (75.5) | 39 (62.9) | 35 (72.9) | 42 (68.9) | 0 (0) | 19 (73.1) | 8 (57.1) | 0.403 | 0.099 |
| No | 131 (27.2) | 12 (24.5) | 23 (37.1) | 13 (27.1) | 19 (31.1) | 1 (100) | 7 (26.9) | 6 (42.9) | ||
| Immediate action during blood exposure accident | ||||||||||
| Don't know | 50 (10.4) | 3 (6.1) | 7 (11.3) | 1 (2.1) | 11 (18.0) | 0 (0) | 4 (15.4) | 4 (28.6) | 0.153 | 0.114 |
| Protect wound with bandage | 399 (82.8) | 39 (79.6) | 50 (80.6) | 43 (89.6) | 43 (70.5) | 1 (100) | 20 (76.9) | 10 (71.4) | ||
| Rinse the eye thoroughly with water | 33 (6.8) | 7 (14.3) | 5 (8.1) | 4 (8.3) | 7 (11.5) | 0 (0) | 2 (7.7) | 0 (0) | ||
| Frequency of washing hands | ||||||||||
| Before and after touching a patient | ||||||||||
| Yes | 366 (75.9) | 46 (93.9) | 51 (82.3) | 45 (93.8) | 53 (86.9) | 1 (100) | 24 (92.3) | 12 (85.7) | 0.003* | 0.171 |
| No | 116 (24.1) | 3 (6.1) | 11 (17.7) | 3 (6.3) | 8 (13.1) | 0 (0) | 2 (7.7) | 2 (14.3) | ||
| Before and after aseptic procedures | ||||||||||
| Yes | 332 (68.9) | 46 (93.9) | 41 (66.1) | 44 (91.7) | 37 (60.7) | 0 (0) | 24 (92.3) | 13 (92.9) | <0.001* | 0.230 |
| No | 150 (31.1) | 3 (6.1) | 21 (33.9) | 4 (8.3) | 24 (39.3) | 1 (100) | 2 (7.7) | 1 (7.1) | ||
| Only before and after having a meal | ||||||||||
| Yes | 50 (10.4) | 1 (2.0) | 3 (4.8) | 2 (4.2) | 4 (6.6) | 0 (0) | 0 (0) | 1 (7.1) | 0.183 | 0.117 |
| No | 432 (89.6) | 48 (98) | 59 (95.2) | 46 (95.8) | 57 (93.4) | 1 (100) | 26 (100) | 13 (92.9) | ||
| After body fluid exposure | ||||||||||
| Yes | 347 (72.0) | 45 (91.8) | 45 (72.6) | 45 (93.8) | 41 (67.2) | 1 (100) | 23 (88.5) | 12 (85.7) | 0.001* | 0.182 |
| No | 135 (28) | 4 (8.2) | 17 (27.4) | 3 (6.3) | 20 (32.8) | 0 (0) | 3 (11.5) | 2 (14.3) | ||
| After touching a patient's immediate surroundings | ||||||||||
| Yes | 285 (59.1) | 43 (87.8) | 42 (67.7) | 43 (89.6) | 46 (75.4) | 0 (0) | 23 (88.5) | 11 (78.6) | <0.001* | 0.243 |
| No | 197 (40.9) | 6 (12.2) | 20 (32.3) | 5 (10.4) | 15 (24.6) | 1 (100) | 3 (21.4) | 3 (21.4) | ||
- * Statistically significant at p < 0.05.
3.3 Practice of HCW in HAI prevention
3.3.1 Correlation of HCWs' professional nature and practice scores
A strong correlation was observed between handwashing and direct patient contact, both before and after (p 0.003), pre- and postaseptic procedures (p ≤ 0.001, V = 0.230), contact with immediate patient surroundings (p ≤ 0.001, V = 0.243), and exposure to body fluids (p < 0.001) (Table 6).
3.3.2 Correlation of HCWs' experience and practice scores
Based on their experience, HCWs manifested a strong association between the practice of standard precautions and adherence to proper mask usage (p < 0.001) and regular handwashing before and after aseptic procedures (p < 0.001), direct patient contact (p < 0.001), exposure to body fluids (p < 0.001), and contact with patient surroundings (p < 0.001). Furthermore, avoiding the use of a single needle for more than one prick on a single patient (p < 0.001), usage of sterilized medical equipment (p < 0.001), and risk prevention during blood exposure accidents were also significantly practiced among HCWs (p < 0.001) (Table 7).
| Practice | Years of experience (%) | ||||||
|---|---|---|---|---|---|---|---|
| <1 | 1–5 | 5–10 | 10–20 | >20 | p-Value | Cramer's v | |
| Wearing masks | |||||||
| All the time | 158 (60.3) | 178 (69.0) | 20 (23.3) | 24 (27.9) | 18 (35.3) | <0.001* | 0.249 |
| Wear mask at suspected places | 98 (37.4) | 74 (28.7) | 63 (73.3) | 59 (68.6) | 32 (62.7) | ||
| Never | 6 (2.3) | 6 (2.3) | 3 (3.5) | (3.5) | 1 (2.0) | ||
| Using single needle for two pricks on a single patient | |||||||
| Yes | 57 (21.8) | 28 (10.9) | 12 (14.0) | 8 (9.3) | 3 (5.9) | 0.001* | 0.159 |
| No | 205 (78.2) | 230 (89.1) | 74 (86) | 78 (90.7) | 48 (94.1) | ||
| Using same medical equipment for another patient after sterilization | |||||||
| Yes | 161 (61.5) | 205 (79.5) | 64 (74.4) | 64 (74.4) | 37 (72.5) | <0.001* | 0.171 |
| No | 101 (38.5) | 53 (20.5) | 22 (25.6) | 22 (25.6) | 14 (27.5) | ||
| Immediate action during blood exposure accident | |||||||
| Don't know | 36 (13.7) | 28 (10.9) | 6 (7.0) | 6 (7.0) | 4 (7.8) | 0.001* | 0.137 |
| Clean disinfect and protect the wound with bandage | 205 (78.2) | 223 (86.4) | 71 (82.6) | 69 (80.2) | 37 (72.5) | ||
| Rinse the eye thoroughly with water | 21 (8.0) | 7 (2.7) | 9 (10.5) | 11 (12.8) | 10 (19.6) | ||
| Frequency of washing hands | |||||||
| Before and after touching a patient | |||||||
| Yes | 212 (80.9) | 188 (72.9) | 76 (88.4) | 77 (88.4) | 45 (88.2) | 0.001* | 0.162 |
| No | 50 (19.1) | 70 (27.1) | 10 (11.6) | 9 (10.5) | 6 (11.8) | ||
| Before and after aseptic procedures | |||||||
| Yes | 150 (57.3) | 196 (76.0) | 71 (82.6) | 74 (86.0) | 46 (90.2) | <0.001* | 0.265 |
| No | 112 (42.7) | 62 (24.0) | 15 (17.4) | 12 (14.0) | 5 (9.8) | ||
| Only before and after having a meal | |||||||
| Yes | 36 (13.7) | 12 (4.7) | 4 (4.7) | 7 (8.1) | 2 (3.9) | 0.001* | 0.154 |
| No | 226 (86.3) | 246 (95.3) | 82 (95.3) | 79 (91.9) | 49 (96.1) | ||
| After body fluid exposure | |||||||
| Yes | 169 (64.5) | 202 (78.3) | 70 (81.4) | 72 (83.7) | 46 (90.2) | <0.001* | 0.197 |
| No | 93 (35.5) | 56 (21.7) | 16 (18.6) | 14 (16.3) | 5 (9.8) | ||
| After touching a patient's immediate surroundings | |||||||
| Yes | 155 (59.2) | 157 (60.9) | 71 (82.6) | 70 (81.4) | 40 (78.4) | <0.001* | 0.207 |
| No | 107 (40.8) | 101 (39.1) | 15 (17.4) | 16 (18.6) | 11 (21.6) | ||
- * Statistically significant at p < 0.05.
3.4 HCWs practice and infection transmission risk
The binary logistic regression (BLR) model was found to be statistically insignificant based on the Hosmer and Lemeshow test, with a p > 0.05. The knowledge of transmission routes, coupled with the practice of reusing a single needle for multiple pricks, showed an odds ratio (OR) of 2.212 (95% CI: 0.956–5.119; p = 0.064). Regarding blood exposure accidents, those who did not know what to do in such situations had an OR of 1.082 (95% CI: 0.231–5.070; p = 0.921), while those who practiced protecting their wounds to prevent infection transmission showed an OR of 1.017 (95% CI: 0.269–3.843; p = 0.980). Those who frequently washed their hands to prevent infection transmission showed an OR of 1.02 (95% CI: 0.386–2.697; p = 0.967) (Table 8).
| Practice | SE. | Sig. | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Wearing masks | |||||
| All the time | 0.664 | 0.001 | 0.109 | 0.03 | 0.401 |
| Wear mask at suspected places | 0.641 | 0.058 | 0.296 | 0.084 | 1.042 |
| Using single needle for two pricks on a single patient | |||||
| Yes | 0.428 | 0.064 | 2.212 | 0.956 | 5.119 |
| Using same medical equipment for another patient after sterilization | |||||
| Yes | 0.419 | 0.949 | 0.973 | 0.428 | 2.213 |
| Immediate action during blood exposure accident | |||||
| Don't know | 0.788 | 0.921 | 1.082 | 0.231 | 5.07 |
| Protect wound with a bandage | 0.678 | 0.98 | 1.017 | 0.269 | 3.843 |
| Frequency of washing hands | |||||
| Before and after touching a patient | 0.496 | 0.967 | 1.021 | 0.386 | 2.697 |
| Before and after aseptic procedures | 0.499 | 0.394 | 0.653 | 0.246 | 1.739 |
| Only before and after having a meal | 0.685 | 0.586 | 0.689 | 0.18 | 2.636 |
| After body fluid exposure | 0.521 | 0.028 | 0.318 | 0.114 | 0.883 |
| After touching a patient's immediate surroundings | 0.482 | 0.868 | 0.923 | 0.359 | 2.373 |
- Abbreviations: CI, confidence interval; SE, standard error.
4 DISCUSSION
Infection control is a critical issue in healthcare facilities, with HCWs being the primary transmitters of infections. Therefore, this study aimed to assess the KAP of HCWs toward nosocomial infection prevention. The doctors (77.9%), medical practitioners (80%), and other hospital staff (77.2%) exhibited high knowledge scores compare to their attitude and practice toward the prevention of HAI. Kareem et al. also reported similar findings with more than half the number of doctors having less than 50% practice in evidence-based medicine (EBM). Commonly reported impediments included the attitude of colleagues, inadequate skills, insufficient time, high flow of patients, and criticism fear.20 Our study revealed significantly higher practice scores among nurses (88.7%), technicians (75.1%), and allied health professionals (85.5%). In contrast, Maurya et al. reported knowledge scores of 11.9 in their study.15 The former study is a reflection of a single hospital, whereas the current results are representing the global nurses, and this could possibly indicate the disparities between the studies. Concerning EBM, Kaseka et al. identified moderate practice scores (57.8%) among nurses and midwives in a central hospital in Malawi.21 Clinical experience and the types of hospitals were the chief contributors to these scores.15 Regarding Allied Health professions and technicians, Mukhopadhyay et al reported that the HCWs' that were directly or indirectly involved in the laboratory diagnosis of COVID-19 were more likely to exhibit good practices.22 These studies are evidence that the nature of profession and training plays a significant role in shaping the HCWs' practices.
Based on experience, the highest knowledge scores were spotted in highly experienced individuals (10–20 and >20 years). Among Nigerian HCWs, good knowledge was mainly observed in the 16–20 years experience category.23 We found a significant degree of good attitude amongst individuals having 1–5 years of experience. These results are inconsistent with hospital reports of Odisha, where higher attitude scores were recorded in ≥16 years of experience.24 The inconsistencies may be present due to the sample size distinction Concerning practice, we recorded peaked values in 10–20 years experienced HCWs, and lowest values in <1 years experienced HCWs. The overall KAP percentages were identified in HCWs with the highest experience. Indian researchers reflected a similar trend, where high practice scores were reflected in highly experienced HCWs.25
Correlational analysis manifested that a significant proportion of global HCWs exhibited high levels of knowledge concerning the transmission of microorganisms such as HCV, HDV, chickenpox, and shingles, as well as the dissemination of soft tissue infections, and direct contact spread of respiratory infections. Moreover, the respondents' understanding regarding the standard precautions of infection control was comparable to that reported in a study conducted by Engda, T in 2020, Ethiopia. Specifically, 59.5% of the respondents received training on nosocomial infections, while only 35.5% demonstrated good knowledge of nosocomial infections, and 40% exhibited good knowledge of the modes of transmission and risk factors for nosocomial infections.26 In another Ethiopian study, Bayleyegn et al. reported 90.2% of respondents with a high level of knowledge about preventing healthcare-associated infections.2 Additionally, 96.6% of participants understood the imperative need to implement standard operating procedures (SOPs) to reduce HAI incidence. The statistics from Northwest Ethiopia manifested that 84.6% of respondents were knowledgeable about infection prevention.27 Moreover, 93.33% of participants demonstrated awareness of disinfectants and antiseptic preventive measures in connection with HAIs. Comparably, Pakistan's statistics accentuated 70% (n = 115) good knowledge of standard precautions for controlling nosocomial infections.28 The variation in the percentage of knowledge regarding the prevention of HAIs among HCWs can be influenced by the availability of educational programs and training, differences in the study population, and the priority placed on infection prevention by healthcare organizations.2
Findings from our study strongly indicated that HCWs possess a robust understanding of hand hygiene in infection prevention, and this knowledge tends to increase with years of experience. This finding is consistent with the study conducted in 2020 from northeast Ethiopia. Assefa et al. reported that 95.3% of the study participants had knowledge about the role of washing hands with soap or alcohol-based antiseptics in reducing the transmission risk of nosocomial pathogens. In addition, HCWs with more than 5 years of work experience were 1.5 times more likely to have adequate knowledge compared to those with less experience. As HCWs gain experience, they become more insightful and proficient regarding infection control techniques. Thus, respondents with older age, longer work experience, and higher educational status tend to excel in infection prevention.29
Previous studies have reported substantial evidence of an association between professional work experience and knowledge regarding standard precautions. Specifically, as work experience increased, knowledge related to standard precautions also increased.28 Likewise, this study found that HCWs with greater experience were more likely to translate their knowledge into practice, as demonstrated by their adherence to infection prevention activities such as frequent hand washing before and after aseptic procedures, after touching patients' immediate surroundings, and wearing masks.
Importantly, our findings indicate that working experience is a significant factor associated with infection prevention practices. Specifically, HCWs with increased years of experience are three times more likely to engage in infection prevention activities than those with less experience. Assefa et al. in Northeast Ethiopia reported that 55% of healthcare providers had safe infection prevention practices.29 Agbana reported that only 53.4% of participants had received training on hand washing in the last 3 years of experience in Nigeria,30 while Gasabaet al. from Zimbabwe found that 79.3% of participants washed their hands and 75.9% were aware that the infection control manual guideline was available in their workplace.5 Our findings align with a study conducted in Uganda which reported that nurses' attitudes improved with greater work experience, making them better models for younger employees.31 Similarly, a study in Ethiopia found that HCWs with 10 or more years of experience were three times more likely to practice infection prevention and control than those with less than 5 years of experience. Furthermore, participants who had a positive attitude toward infection prevention and control practice were 10 times more likely to follow safe practices.22
Previous studies have reported mixed findings on infection prevention practices among HCWs. Some studies have shown good adherence to practices, such as wearing masks (83.7%) and hand hygiene (98.3%, 91.8%), while others found lower rates of compliance (45.5% in a study by SisayFogaSebro32). Additionally, a study from Bahir Dar, Ethiopia, reported 54.2% of healthcare providers practicing nosocomial infection prevention,2 and Gezie et al. in Northeast Ethiopia found less than one-fourth of the study population (23%) demonstrating good practices toward HAI prevention.6 These findings provide valuable context but should be interpreted cautiously as they may differ in population, methodology, and settings compared to our study.
The positive attitude of HCWs is an essential requirement for preventing nosocomial infections and a crucial aspect in preventing cross-infections. Our study's findings indicate that HCWs have a positive attitude toward their profession, as evidenced by their preference for using gloves (p < 0.001, Cramer's v = 0.197) during direct contact with patients, removing hazards immediately to protect others from risks, and their understanding that healthcare professionals are at high risk of nosocomial infections. Contrastingly, a study conducted in Nigeria by Babatola et al. identified only 56.7%33 of HCWs with a good attitude toward preventing nosocomial infections. Similarly, a study from Bahir Dar city in Ethiopia reported 55.6% of HCWs that exhibited a good attitude28 toward preventing such infections. Comparably, our findings are similar to a study conducted by Sai SubhakarDesu in India in 2023, which reported that 74.5% of participants had a positive attitude toward preventing nosocomial infections. Additionally, 89.2% of participants agreed that guidelines for preventing HAIs should be strictly followed, and hand hygiene measures should be taken to reduce the risk of infections after treating patients. A total of 80.8% of respondents often or always used gloves and practiced hand hygiene measures after removing gloves.34 Similarly, a study from Northeast Ethiopia by Jemal S in 2019 reported that 69.23% of HCWs had a positive attitude toward infection prevention, while 30.77% had a negative attitude. However, the majority of study participants, that is, 71.43%, did not consider that all HCWs and the community are at risk of infection.35 Engda et al. in Ethiopia revealed 36% of study participants with a good attitude toward preventing and controlling nosocomial infections.26 In contrast, Alhassan et al. from Ghana, in 2021, reported that 97.4% (p ≤ 0.001) of respondents agreed to wash their hands following the removal of gloves. About 95.5% of respondents believed that following prevention guidelines would reduce HAI rates, and 86% agreed that following the SOPs would decrease the risk of contamination.36
Furthermore, Unakal et al. conducted a study in three hospitals in Trinidad and Tobago in 2017, which indicated that 53.3% of participants had a positive attitude toward infection prevention and control. Additionally, 87.7% of HCWs agreed that a new pair of gloves should be worn for each new patient attended. This translated to a practice level of 56.0%, demonstrating the influence of attitude on practice.37 The variations in research results can be attributed to several factors, including the academic background of the participants, the sample size, and the availability and implementation of protocols for preventing HAIs. In some settings, hand hygiene may be viewed as a minor concern and may not be given sufficient attention, particularly in nonsurgical and noninvasive procedures.
Organizational support factors, such as the presence of an infection control team, supply of disinfectants, and visible information (leaflets and posters) about HAIs, play a significant role in shaping behavior. It puts HCWs into a reminder about the threats and the impact of HAIs in line with the health beliefs model, increasing the “willingness to act.” The majority of HCWs in our study wore gloves while having direct contact with patients, indicating a positive trend toward safe practices. In contrast, a study from Italy reported that only 57.3% of HCWs changed gloves to prevent the transmission of infection.38 The availability of gloves may reflect these inconsistencies, as improved availability allows more accessibility. Adherence to safe infection prevention practices is mandatory for both patients and HCWs, as it reduces the incidence of HAIs. However, a recent study reported unsatisfactory levels of good practice, indicating the need for continued efforts to improve infection prevention and control measures.2
It is important for hospital administrators to foster a culture that prioritizes adherence to recommended hand hygiene practices and infection prevention measures. This can be achieved by providing visible support and adequate resources for continuing education programs that are tailored to the specific needs of healthcare personnel. Despite the fact that most healthcare providers in our study had good knowledge and a favorable attitude toward HAI prevention, there is still room for improvement. Therefore, ongoing efforts are needed to ensure that infection prevention practices are consistently implemented in healthcare settings.
5 LIMITATIONS
There are certain limitations to our study, such as the use of a self-administered survey questionnaire as it may introduce the potential for response bias (participants may not respond truthfully or accurately due to social desirability bias, where they provide socially acceptable answers rather than their true perceptions), and the exclusion of housekeeping staff who may also be a source of transmitting HAIs. Additionally, social desirability bias may have influenced the results to some extent. To address these limitations, large-scale multicenter studies are needed. The predominance of doctors in the study sample may lead to an overrepresentation of their perspectives, and not knowing the specific departments where HCW work could limit the generalizability of findings to different healthcare settings. Additionally, cultural norms and beliefs shaping attitudes toward practices like mask-wearing or glove use, resource availability affecting adherence to guidelines in resource-limited settings, communication barriers hindering information dissemination, and organizational support may impact infection control programs' effectiveness, leading to disparities in training opportunities and cultural attitudes toward infection control, thereby affecting KAP levels. Understanding and addressing these factors are crucial for designing targeted strategies to enhance infection prevention practices among HCWs globally, ultimately reducing nosocomial infections.
6 SUGGESTIONS
- 1.
Regular Training Programs: Implement regular and comprehensive training programs on infection prevention for all HCWs, including doctors, nurses, technicians, and allied health professionals. These programs should be evidence-based, interactive, and tailored to the specific needs of each professional group.
- 2.
Simulation and Role-Modeling: Utilize simulation-based training and role-modeling to demonstrate best practices in infection prevention. Practical demonstrations can be more effective in reinforcing the importance of adherence to guidelines.
- 3.
Infection Control Champions: Designate infection control champions within each department or unit to act as leaders and advocates for infection prevention. These champions can lead by example, provide guidance, and promote a positive culture of infection control.
- 4.
Regular Feedback and Audits: Conduct regular audits and provide timely feedback to HCWs on their infection prevention practices. Feedback can help identify areas for improvement and motivate HCWs to adhere to best practices.
- 5.
Multidisciplinary Training: Foster a multidisciplinary approach to infection prevention training, where different healthcare professionals collaborate and learn from each other's experiences. This can enhance teamwork and promote a unified approach to infection control.
7 CONCLUSION
In summary, our study accentuated the impact of HCWs' knowledge regarding the mode of infection transmission on their infection prevention practices. The study provides valuable insight into the KAP scores of HCWs, based on years of experience and profession. The study findings indicate that HCWs globally, including doctors, medical practitioners, nurses, and other hospital staff, have a good understanding and positive attitude toward the prevention of HAIs or nosocomial infections. Moreover, their knowledge was observed to be reflected in their practices. However, it is recommended that educational seminars and awareness programs be conducted annually to ensure the retention of knowledge, attitudes, and practices among HCWs of different categories. These educational programs would help improve adherence to barrier protection measures such as hand washing, the use of gloves, and hand disinfection. It is also important for every institution to have proper guidelines for HCWs, and a regular system of monitoring infection rates and disseminating data to form a link between the management and the HCWs to improve strategies for HAI prevention.
AUTHOR CONTRIBUTIONS
Elham M. Khatrawi: Conceptualization; data curation; formal analysis; investigation, Visualization; writing—original draft; writing—review and editing. Priyadarshi Prajjwal: Conceptualization; data curation; formal analysis; investigation; visualization; writing; original draft; writing—review and editing. Muhammad Farhan: Formal analysis; investigation; visualization; writing—original draft; writing—review and editing. Pugazhendi Inban: Formal analysis; investigation; visualization; writing—original draft; writing—review and editing. Shraddha Gurha: Visualization; writing—original draft; writing—review and editing. Saud M. S. Al-ezzi: Writing—original draft; writing—review and editing. Mohammed D. M. Marsool: Writing—original draft; writing—review and editing. Prerna Ahuja: Writing—original draft; writing—review and editing. Mohammed A. Mateen: Writing—original draft; writing—review and editing. Felix O. Aina: Writing—original draft; writing—review and editing. Omniat Amir: Writing—original draft, writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Elham Mohammed Khatrawi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings are available at Zenodo under title: Analyzing the knowledge, attitude, and practices of healthcare workers toward the awareness of nosocomial infections: A global survey. doi: 10.5281/zenodo.7845444.