COVID-19 vaccines and their underbelly: Are we going the right way?
Abstract
Background
Historically, a critical aetiological agent of health concern stays till eternity after its discovery, so shall it be with the COVID-19 outbreak. It has transformed human life to a ‘new normal’ with huge tolls on the social, psychological, intellectual and financial spheres.
Aim
This perspective aimed to collate numerous reported COVID-19 vaccine-associated adverse events and the predisposing factors. It focussed on the efficacy of mix-n-match (cocktail) vaccines to effectively counter COVID-19 infection to facilitate future research and possible interventions.
Material and Methods
Databases like Scopus, Pubmed and the Web-of-science were searched for published literature on ‘adverse events associated with COVID-19 vaccine’. The reports and updates from health agencies like the WHO and CDC were also considered for the purpose. The details with respect to the adverse events associated with COVID-19 vaccination and the predisposing factors were compiled to obtain insights and suggest possible future directions in vaccine research.
Results
India stood strong to manage its health resources in time and turned into a dominant global vaccine supplier at a time when healthcare infrastructure of many countries was still significantly challenged. Developing indigenous vaccines and the vaccination drive in India were its major achievements during the second and the subsequent COVID-19 waves. The fully indigenous Covaxin vaccine, primarily as an emergency intervention, was successfully rapidly launched. Similar such vaccines for emergency use were developed elsewhere as well. However, all of these reached the marketplace with a ‘emergency use only’ tag, without formal clinical trials and other associated formalities to validate and verify them as these would require much longer incubation time before they are available for human use.
Discussion
Many adverse events associated with either the first or the second/booster vaccination doses were reported. Evidently, these associated adverse events were considered as ‘usually rare’ or were often underreported. Without the additional financial or ethical burden on the vaccine companies, fortunately, the Phase IV (human) clinical trials of their manufactured vaccines are occurring by default as the human population receives these under the tag ‘emergency use’. Thus, focused and collaborative strategies to unveil the molecular mechanisms in vaccine-related adverse events in a time-bound manner are suggested.
Conclusion
Reliable data particularly on the safety of children is lacking as majority of the current over-the-counter COVID-19 vaccines were for emergency use. Many of these were still in their Phase III and Phase IV trials. The need for a mutant-proof, next-gen COVID-19 vaccine in the face of vaccine-associated adverse events is opined.
1 INTRODUCTION
COVID-19 outbreak has had a huge toll on social, psychological, intellectual and financial spheres of life, horrifying all to a “new normal.” Emerging from Wuhan city China, the virus has affected millions around the world, wave after wave.1, 2 During the cruel consecutive waves during 2020–22, nations across economic-strata were almost standstill for months to years. COVID-19 demonstrated its epidemicity in few weeks after its emergence, grappling almost half of the global population. As countries including India still struggle to cope with the post-COVID scenario, global economy is in doldrums.3, 4 Being the second largest populated country with thickly populated cities, the Indian healthcare infrastructure, public health awareness, government funding and the socioeconomy was significantly challenged.5 The pandemic also hugely contributed to depression, anxiety, domestic abuse in the society leading to an increased suicidal tendency.6 Unlike many developing nations, India indeed emerged a true warrior fighting the third COVID-19 wave valiantly, and the world witnessed and appreciated India's strong strategic planning and mass vaccination program (https://www.mohfw.gov.in/). Developing indigenous vaccines and managing the vaccination drive efficiently were the major successes during the second and subsequent COVID-19 waves here. India well-managed its health resources in time and became an influential donor of COVID-19 vaccines to the deprived economies. Developed nations too depended for drugs like Paracetamol and Remdesivir particularly during the first wave, to which India responded generously in line with its basic cultural ethos, “Vasudhaiva kutumbakam” and “Sarbe bhabantu sukhinah”; salutes for that.
The availability of COVID-19 vaccines within the crucial phase played the trick in altering the worrisome global scenario downside up. India showed relentless concerted efforts of healthcare professionals, biotechnologists and formulation scientists. It was indeed a very successful and strong collaboration of leading pharmaceutical biggies with the government-funded research laboratories that led to a tangible outcome, a developed vaccine. Covaxin, a fully indigenous vaccine primarily to administer as an emergency intervention (the human clinical trial is awaited), was successfully developed in a short time period. Emergency use’ vaccines Moderna of Pfizer-BioNTech, Covishield of Oxford-AstraZeneca, Sputnik V of Gamaleya, Verocell of Sinopharm, CoronaVac of Sinovac and Covaxin of Bharat Biotech were developed to counter COVID-19, and administrated worldwide.7-9 Although the likely adverse events associated with these conditionally-approved COVID-19 vaccines are reported after they were gradually observed with time, they remain as the best weapons against the pandemic.10 This article reports the challenges in vaccine development, vaccine efficacy, vaccine-associated adversities and the need of next-gen vaccines to counter all the COVID-19 variants.
2 VACCINE-ASSOCIATED ADVERSITIES
The SAGE (strategic advisory group of experts) formed and coordinated by World Health Organization (WHO) analyses the immunization and clinical trial results worldwide across various zones with the evidence of the impact, prognosis factors and probable pathophysiology of the disease. It would recommend whether and how the vaccines are used considering the regulatory guidelines, based on its internal assessment.11 GACVS (global advisory committee on vaccine safety), another wing of WHO, monitors the effects of approved vaccines on the target population. It is a leading body that identifies and collates adverse events following vaccination. SAGE and GACVS consist of independent group of experts across science disciplines to look into the scientific merits, technicalities of vaccination and authoritative delivery, and frequently recommend scientific advisories to the WHO on safe use. Although adverse events associated with approved vaccines have been reported quite likely the expert panels subsidize regulatory protocols in view global emergencies like COVID-19.9, 12
In most cases, vaccine-associated adverse events are not reported through proper channel, go unaccounted/under-reported, or are recognized as “usually rare” cases if noticed. Such cases lose focus for further scientific analyses by design. Reports of cardiac complications like myocarditis and pericarditis, mostly after the second dose, persisted with messenger RNA (mRNA) COVID-19 vaccines. Available data correlate mRNA vaccination with the symptoms.13 Following the second dose of Pfizer-BioNTech vaccine, 40.6 young males of 12–30 age group per million reported myocarditis.14 Cardiac arrhythmias along with myocarditis and pericarditis after mRNA vaccination were reported in the United States, the EU, and Israel.15-17 Although a plausible relationship between myocarditis and mRNA vaccine is claimed, it lacks validation.
Serious cases of blood clot accompanied by low platelet counts after the second dose of AstraZeneca and J&J COVID vaccines are reported.18, 19 Referred as TTS (thrombosis with thrombocytopenia syndrome), such complications were observed in the Europe and the United States within 3–30 days after vaccination.20 The United Kingdom, Germany, and Norway reported cerebral venous thrombosis and thrombocytopenia during Phase III clinical trial of AstraZeneca vaccine.21 There are reports of thrombocytopenia with low platelet counts with Vaxzevria and Covishield vaccination.22 Postural orthostatic tachycardia syndrome has been found to be mRNA vaccine associated. Bell's palsy syndrome, Gillian Barre syndrome, vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) causing life-threatening adverse reactions were observed in few cases. The GACVS subcommittee updated its statement on AstraZeneca vaccine associated blood coagulation events.23 Many vaccines were approved to be marketed in the United States and elsewhere. Only a few, predominantly, Pfizer-BioNTech, Moderna, Covishield, and J&J/Janssen dominated the US market. The CDC suggested restricted emergency use of J&J/Janssen COVID-19 vaccine owing to safety concerns. The occurrence of adverse effects was about 4–6 people in every million vaccinated, although data on symptoms show that the impact varied between age group, sex, and geographical location. However, a due diligent collection of clinical data is in question. While available data suggest an increased risk in younger adults, further research to identify the exact mechanism behind TTS and the role of COVID vaccines across various age groups is underway. Long-term data analyses to decipher COVID-19 vaccine-related myocarditis and pericarditis and investigating into specific biological mechanisms behind such acute cardiac complications following vaccination need to be prioritized.17
The Indian experience of Sputnik V vaccination reports swelling of throat, difficulty in breathing, increase in blood pressure and convulsions as pathological conditions.24 Common side-effects of Covishield and Covaxin vaccination in India were swelling at injection site, abdominal pain, fever, irritability, headache, etc.,25 although Indian reports on adverse effects of COVID-vaccines are sketchy. The side-effects associated with Covaxin and Covishield vaccines seem much similar differing in their manifestation and severity. The data presented during a meeting of the National AEFI Committee of the Ministry of Health and Family Welfare, Government of India showed a total of 617 Covaxin, Covishield and Sputnik V vaccination associated seriously adverse events, after the first dose. A total of 180 adverse events ultimately resulted in death following the second dosage.26 A survey noticed that more than 50% vaccinated individuals experienced musculoskeletal side-effects. Out of a total 1729 vaccinated, 80.7% were Covishield vaccinated while 17.8% Covaxin. Among them, 38.9% had received only a single dose while 61.1% received both the doses.27 A series of adverse events at an average of 136 cases in every 1000 vaccinated within a week of the first Covishield dose was reported in a survey in rural health training centers around Faridabad, Haryana. Out of all vaccinated, 97 recipients reported serious issues of which about 76.3% reported it in 24 h post the vaccination.28 Another survey reported 413 (74.82%) adverse cases out of 1112,29 the adverse experiences being majorly in 18–40 years age group. The majority (913; 82.10%) received Covishield among the 1112 vaccinated cases surveyed, while 199 (17.90%) received Covaxin. Among the observed recipients, “breakthrough” infections were observed in 7.91% cases, where males had a higher (57.96%) risk than the females (42.04%).29 Vaccine-associated adverse events and the predisposing factors are presented in Table 1.30-32
| Vaccine type | Vaccine name | Mild adverse event | Severe adverse event | Predisposing factor |
|---|---|---|---|---|
| mRNA vaccine | Pfizer-BioNTech (BNT162b2) | Sore throat, headache, fatigue, myalgia, joint pain, nausea, muscle spasm, chills, fever, sweating, dizziness, flushing, feelings of relief, localized swelling, itching, tingling, brain fogging, anorexia, sleep issues, diarrhoea, nasal stuffiness, and palpitations30-32 | Injection site pruritus for more than a week and pain (excluding headache, muscle and joint pain), severe persistent lymphadenopathy, Bell's palsy30-32 | Previous history of allergy to food, drugs or vaccines like the ARV and influenza vaccines30-32 |
| Moderna (mRNA-1273) | Similar reactions as above, and predominance of fatigue30-32 | Non-anaphylaxis allergic reactions, anaphylaxis reactions30-32 | Second dose of vaccine30-32 | |
| Viral vector vaccine | Oxford-AstraZeneca/Covishield (ChAdOx1 nCoV-19) | Injection site pain, feverish feeling, muscle ache and headache30-32 | abdominal cramps, postural drop in blood pressure, loose stool, syncopal attack; fever above 40°C30-32 | People below 55 years of age, first dose30-32 |
| Janssen (Ad26.COV2-S [Recombinant]) | Headache, injection site pain, fatigue, muscle ache, nausea30-32 | Tachycardia-associated anxiety, chest pain, hyperventilation, dyspnea, paresthesia, light-headedness, hypotension, headache, pallor, syncope, clotting disorder30-32 | Not reported | |
| Gamaleya-Sputnik V | General discomfort, headache, chills, fever, arthralgia, myalgia, asthenia, and local reactions like injection-site tenderness, hyperaemia and swelling30-32 | Not reported | People below 55 years of age30-32 | |
| Inactivated vaccine | Sinopharm-Verocell | Injection site pain, while systemic AEFI are headache, fatigue, myalgia, fever, arthralgia, dyspnea, cough, nausea, diarrhea and pruritus30-32 | Not reported | After first dose30-32 |
| Sinovac-CoronaVac | Injection site pain, headache, fatigue, myalgia, arthralgia30-32 | Serious allergic reaction with urticaria 48 h after the first dose30-32 | Predominant in females30-32 | |
| Covaxin | Injection site pain, fever, headache, fatigue, nausea30-32 | Not reported | First dose30-32 |
In every million vaccinated, 457 deaths were reported from Covishield and 20 from Covaxin. Both Covaxin and Covishield vaccines manifested reacted adversely at about 0.01% rate. As the data seem insignificant, it seems many cases might have gone unnoticed/unregistered. Among Indian states, Maharashtra registered the highest (4521) vaccination-associated adverse events followed by Kerala (4074), Karnataka (2650) and West Bengal (1456).33 Many casualties were classified as coincidental, unclassifiable or undefined in case reports published by governments that failed to determine the root cause, exposing the underbelly of healthcare systems. Such reports may need serious investigation to decipher the cause-effect relationship of vaccination adverse effects.
3 CHALLENGES IN VACCINE DEVELOPMENT
The efforts do not end as COVID-19 is still round the corner with new variants that exhibit unexplored mutation phases with new treatment/prevention challenges. With the passage of time, emerging forms like Omicron and its variants are threatening the world wherein the potency of the existing vaccines is questioned. Omicron variant, with rapid mutation rate, encodes more than 37 amino acid substitutes in the spike protein, which indeed challenges vaccine development strategies.34 Many questions on the Phase III clinical trials during the early approval of vaccines including the rationality of subject selection, bypassing technical regulatory, dearth of post-marketing surveillance reports, etc. are unanswered. Historically, an etiological agent of serious health concern stays ever after its discovery, and in light of this fact the human civilization is at crossroads to adopt living with COVID-19 following the COVID-appropriate norms, the “new normal.”
4 BIVALENT COVID-19 BOOSTER AND COCKTAIL VACCINES
The available bivalent COVID-19 vaccines include the original virus strain and the Omicron components. Developed by Pfizer and Moderna, the two leading vaccine manufacturers, these are preferred as boosters allegedly to protect against Omicron. In the face of rapid alteration in viral genetic makeup, it is really difficult to assess whether these boosters would really be effective against the infection.35 To counter COVID-19 infection effectively, vaccine mix-n-match (vaccine cocktail) gained popularity. SAGE too has advocated in favor of a third dose of Pfizer-AstraZeneca (heterogeneous) vaccine cocktail in case of unavailability of original vaccine. However, the potency, safety, cell-specific interaction or the long-term effect/risks of such cocktail on larger population need validation.35
The GACVS subcommittee recommended that all countries should monitor the safety of the approved COVID-19 vaccines, and could promote reporting of the suspected adverse events. Such recommendations seemingly are not taken seriously by most government regulatory agencies. While data on the Phase IV clinical trials of Moderna, Vaxzevria and Covishield vaccines are available, post-market surveillance reports on other vaccines, especially from the low income countries, are scanty. In the face of such apprehensions, few queries that need to be urgently addressed are, like, whether the vaccine mix-n-match strategy technically sound, whether the clinical safety of the vaccine-cocktail evaluated, whether the vaccines are safe for women with periods, lactation or pregnancy, whether the vaccines are safe for children and whether ample of multi-centric Phase III clinical data available to support it, and what is the specific roadmap for people on chronic medication or are moderately immunocompromised.
5 NEED OF NEXT-GEN COVID-19 VACCINES
Populous countries like India are turning into epicenters of particularly lifestyle diseases like hypertension, diabetes, cancer, etc. Thus, issues like vaccine-drug compatibility/relations and adverse reactions if any need to be addressed meticulously to support evidence-based prescription. COVID-19 vaccines reportedly exhibited adverse effects in various forms, particularly among the young. Vaccination nevertheless was recommended by the scientific communities and healthcare professionals despite such observation, with an argument that the benefits of COVID-19 vaccines on target population continue to outweigh the associated risks. However, further evidence of vaccine safety remains to unravel. Emerging SARS-CoV-2 mutants could capably escape neutralizing antibodies rendering the over-the-counter vaccines ineffective.36-38 Analysing mutation patterns to facilitate developing mutant-proof, next-gen vaccines against variants seem to be even more rational. The need to work on the significantly mutation-proof, multi-epitopic, next-gen COVID-19 vaccines is emergent.36
6 FUTURE RESEARCH AND POSSIBLE INTERVENTIONS
Given the fact that most COVID-19 vaccines were approved for emergency use, and many were still in their Phase III/IV trials as they were released for over-the-counter sell, reliable vaccine-safety data is lacking. Vaccine safety especially among children is a major hurdle in successful immunization. A recent report compiled at least 27 cases assessing vaccine safety among babies and adolescents between 6 months and 18 years age-group in 10 countries including India, the United States, and China, with comparatively high adversity rates with mRNA vaccination.39 The European region study noticed higher immunological disorder incidents like multisystem inflammatory syndrome and autoimmune diseases in kids and adolescents.40 Based on the available medical literature and published reports, Covishield and Covaxin as inactivated vaccines were recommended by the Indian Association of Pediatricians (IAP) and the Academy of Pediatrics Advisory Committee on Vaccination and Immunisation Practices (ACVIP) as safe to administer also to pregnant and lactating/breastfeeding women. Yet, foolproof clinical trials are still needed to be addressed.41, 42
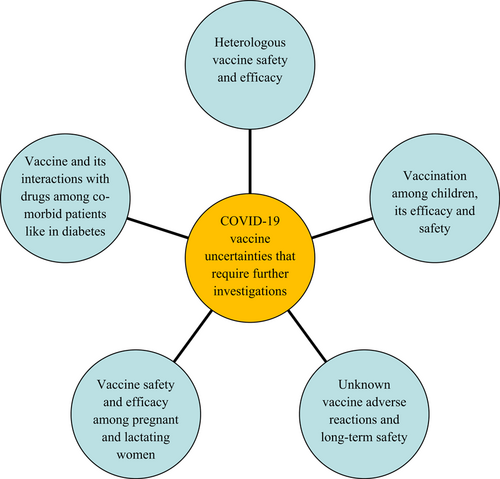
The effect of COVID-19 vaccination on comorbid like the diabetic for potential vaccine-drug interactions was explored. Antidiabetic drugs appear to restrict SARS-CoV-2 infection. Among other benefits, these help reduce inflammation and viral replication. Thus, COVID-19 vaccine may be beneficial for the diabetic.43 Contrastingly, antidiabetic medication like insulin and dipeptidyl peptidase-4 inhibitor (DPP-4i) affected the course of COVID-19 and associated mortality adversely.44 Even though COVID-19 pandemic has existed for more than 3 years, the emerging novel variants and sub-variants have complicated the issue of combating the pandemic even with the launch of “emergency use” vaccination. Vaccination hesitancy due to the mixed responses wherein individuals either voluntarily accepted or did not receive vaccination could have contributed to the rolling spread with variants and sub-variants. Adverse vaccination experiences like thrombosis with TTS and vaccine-induced immune thrombotic thrombocytopenia (VITT) could have triggered vaccination hesitancy even among the learned or informed section.45 Figure 1 depicts the COVID-19 vaccines focussed future research directions in a nutshell.
Cellular and humoral immune responses to both mRNA and viral vector vaccines in the immunodeficient individuals were unprotective, suggesting a heterologous vaccine strategy among such population.46 Yet, heterologous booster dose does not guarantee improved protection, study identifies.47 The protection levels of booster dose of various vaccines varied. Vaccine cocktails like bivalent original prototype virus+omicron BA.4/5 subvariants and original prototype virus+omicron BA.1 subvariant vaccine combinations demonstrated positive results in clinical trials, and are approved for human use (https://healthcare-in-europe.com/en/news/cocktail-vaccines-protect-future-covid-19-variants.html).
7 RECOMMENDATIONS
Considering the currently approved commercial vaccines as a panacea against COVID-19 and not considering the adverse fall-outs will indeed be a blunder in the long run. It is high time to instil adequate awareness among healthcare experts engaged in vaccination and the vaccine recipients to acknowledge the signs/symptoms of the associated adversities. Critical TTS and myocarditis cases need serious analyses. Clinicians also should carefully analyse the persistent symptoms like abdominal pain, severe headache, palpitations, dyspnea, etc. post the vaccination till about a week, that are still considered as insignificant medical conditions. The platelet counts and thrombolytic events too need to be closely monitored. These events might be precursors to myocarditis, especially in young males.
As the majority of the current over-the-counter COVID-19 vaccines worldwide are approved for emergency use only, their efficacy and safety features need to be ascertained. There seems to be a silver-lining in this for the scientific insight in general and clinical trial in particular. That is, the Phase IV (human) clinical trial of these is happening by default with no financial or ethical regulations burden on the vaccine development companies. Taking the advantage, proper documentation, detailed scientific analyses and timely reporting of the adverse fallouts of vaccination may be prioritized to unravel the facts of therapeutic incompatibility and cell-specific interactions. The national-level regulatory agencies may set standard framework to identify, analyse and report all cases with postvaccination adverse clinical symptoms. If need be, an IoT-based national grid may be put on job. Open, transparent and evidence-based communication about the potential benefits and risks of COVID-19 vaccines is essential to ensure trustworthiness. Focused and time-bound collaborative strategy may be tailored for a foolproof understanding of the facts and unveil the molecular mechanism behind vaccine-related adversities. Rigorous studies on the alternate data sources, robust study designs including comparing the vaccinated and nonvaccinated population and a monitoring system for long-term follow-up shall help attaining the prerogatives.
8 CONCLUSION
The mRNA-based COVID-19 vaccination reported cardiac (myocarditis and pericarditis) complications, mostly after the second dose. Serious blood clot cases and low platelet counts with AstraZeneca and J&J COVID vaccine dosing especially after second dose are reported. Indians experienced throat swelling, breathing difficulty, high blood pressure and convulsion as pathological conditions after Sputnik V vaccination. Considering the currently approved commercial vaccines as the panacea, under-evaluation of the adverse fall-outs will be blunder for sure. Vaccine-associated adverse events are often underreported or are considered as “usually rare,” and the scientific focus for further investigations is lost by design. The Phase IV (human) clinical trial is occurring by default without financially or ethically burdening the concerned companies. A focused and time-bound collaborative strategy to unveil molecular mechanisms of the vaccine-related adverse events may, therefore, be set.
AUTHOR CONTRIBUTIONS
Bhabani Sankar Satapathy: Conceptualization; Data curation; Formal analysis. Gurudutta Pattnaik: Data curation; Writing—original draft. Rudra Narayan Sahoo: Conceptualization; Formal analysis. Sovan Pattanaik: Writing—review & editing. Ashish K Sarangi: Conceptualization; Writing—original draft. Venkataramana Kandi: Conceptualization; Data curation; Writing—original draft. Snehasish Mishra: Writing—original draft. Ali A Rabaan: Writing—review & editing. Aroop Mohanty: Formal analysis; Writing—review & editing. Ranjit Sah: Writing—original draft; Writing—review & editing. Ranjan K Mohapatra: Formal analysis; Validation; Writing—original draft; Writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Ranjit Sah, Ranjan K. Mohapatra affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
No primary data was collected or associated with this article.