Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats
Abstract
Patients with depression showed a decrease in plasma and cerebrospinal fluid allopregnanolone (ALLO). But antidepressants increased the contents of ALLO in the rat brain. We examined the antidepressant-like effects of infusion of ALLO into the cerebral ventricle, hippocampus, amygdala, nucleus accumbens, or prefrontal cortex of learned helplessness (LH) rats (an animal model of depression). Of these regions, infusions of ALLO into the cerebral ventricle, the CA3 region of hippocampus, or the central region of amygdala exerted antidepressant-like effects. Infusion of ALLO into the hippocampal CA3 region or the central amygdala did not produce memory deficits or locomotor activation in the passive avoidance and open field tests. It is well documented that ALLO exerts its effects through GABA receptors. Therefore, we examined the antagonistic effects of flumazenil (a GABA receptor antagonist) on the antidepressant-like effects of ALLO. Coinfusion of flumazenil with ALLO into the hippocampal CA3 region, but not into the central amygdala, blocked the antidepressant-like effects of ALLO. However, coinfusion of (+)MK801 (an NMDA receptor antagonist), but not cycloheximide (a protein synthesis inhibitor), blocked the antidepressant-like effects of ALLO in the central amygdala. These results suggest that ALLO exerts antidepressant-like effects in the CA3 region of hippocampus through the GABA system and in the central region of amygdala, dependently on the activation of the glutamatergic mechanisms. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Depression has multiple biological markers. Recent studies have been directed at allopregnanolone (3α5α-tetrahydroprogesterone, ALLO), a marker of depression that is one of the neurosteroids. In clinical studies, depressed patients showed lower ALLO concentrations in the plasma and cerebrospinal fluid (CSF) than controls (Uzunova et al., 1998; Ströhle et al., 1999). Furthermore, antidepressant treatments increased ALLO levels in plasma and CSF of depressed patients (Romeo et al., 1998; Uzunova et al., 1998; Ströhle et al., 1999; Schüle et al., 2006). These studies demonstrate a possible involvement of ALLO in the pathophysiology of depression.
In animal studies, the possibility that ALLO is involved in the pathophysiology of depression is supported by a previous report that ALLO levels were decreased in the amygdala, frontal cortex and hippocampus of the olfactory bulbectomized rat (an animal model of depression, Uzunova et al., 2003). Furthermore, subchronic antidepressant treatment elevated ALLO levels in the rat and mouse brain (Uzunov et al., 1996; Griffin and Mellon, 1999; Nechmad et al., 2003). These studies demonstrate the possibility that ALLO contributes to the amelioration of depression. A previous study has reported that intracerebroventricular (ICV) administration of ALLO exerted antidepressant-like effect in the forced swim test (an antidepressants screening model) (Khisti et al., 2000).
The current study examines the effects of infusion of ALLO into the hippocampus, amygdala, nucleus accumbens, and medial prefrontal cortex of learned helplessness (LH) rats (an animal model of depression) on the conditional active avoidance test. Many clinical studies using functional magnetic resonance imaging (MRI) or postpartum victims indicated that there are several limbic brain regions that have been implicated in mood disorders, including the frontal cortex, nucleus accumbens, amygdala, and hippocampus (reviewed by Sheline, 2003; McCabe et al., 2009; Sibille et al., 2009). We chose the sites for infusion for the purpose of screening the sites where ALLO works for the treatment of stress-related depression.
Abbreviations used:
ALLO, allopregnanolone; ANOVA, one-way analysis of variance; AP, anteroposterior; BST, bed nucleus of the stria terminalis; CSF, cerebrospinal fluid; DG, dentate gyrus; DMSO, dimethyl sulfoxide; DV, dorsoventral; HPA, hypothalamo–pituitary–adrenal; ICV, intracerebroventricular; LH, learned helplessness; MRI, magnetic resonance imaging; NAcc, nucleus accumbens core; PF, prefrontal; PVN, paraventricular nucleus.
MATERIALS AND METHODS
Animal and Treatments
The procedures for animal use were in accordance with the Chiba University Graduate School of Medicine Guide for the Care and Use of Laboratory Animals and were approved by the Chiba University Graduate School of Medicine Animal Care and Use Committee. Male Sprague-Dawley rats (190–220 g) were used. The animals were housed under 12-h light/dark cycle with free access to food and water.
Surgery was performed using a stereotaxic apparatus (Kopf, Tujunga, CA) under anesthesia with pentobarbital sodium solution (50 mg/kg, intraperitoneal injection, Abbott Laboratories, Abbott Park, IL), one day after the acquisition of LH. ALLO was suspended in 20% hydroxypropyl-β-cyclodextrin (CDX; Sigma, St. Louis, MO) and dissolved in 0.9% saline to yield a working ALLO solution in 2% CDX. This solvent (2% CDX) is used in controls. Rats received bilateral microinjection of different amounts of ALLO (0.5 or 0.05 μg/side), ALLO and flumazenil (a GABA receptor antagonist, 0.05 ng in 0.8% Tween 80/side), ALLO and (+)MK801 (an NMDA receptor antagonist, 2.5 μg/side), or 0.9% saline (control) into various regions of the brain. A protein synthesis inhibitor, cycloheximide (10 μg in 5% DMSO/side) was infused bilaterally 20 min before the infusion of ALLO. The solvent (5% DMSO) is used as controls. A total volume of 1.0 μl was infused into each side over 15 min, and the injection syringe was left in place for an additional 5 min to allow for diffusion. The coordinates for the cerebral ventricle, dentate gyrus and CA3 region of the hippocampus (HIPdg, HIPca3), central and basolateral regions of the amygdala (CeA, BLA), nucleus accumbens core (NAcc), and medial prefrontal cortex (mPF) relative to bregma, according to the atlas of Paxinos and Watson (1997) were as follows: −0.3 anteroposterior (AP), ±1.2 lateral, −3.4 dorsoventral (DV) from dura (cerebral ventricle); −3.8 AP, ±2.0 lateral, −3.2 DV from dura (HIPdg); −3.6AP, ±3.6 lateral, −2.8 DV from dura (HIPca3); −2.3AP, ±4.0 lateral, −7.7 DV from dura (CeA); −2.8AP, ±4.8 lateral, −7.4 DV from dura (BLA); +2.2AP, ±1.6 lateral, −6.7 DV from dura (NAcc); +3.2AP, ±0.6 lateral, −2.8 DV from dura (mPF). The placements of injection cannula in the brain are shown in Figure 1.
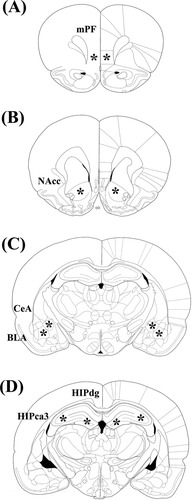
A schematic representation of microinjection sites within the medial prefrontal cortex (A), the core region of nucleus accumbens (B), the dentate gyrus and CA3 region of hippocampus (C), and the central and basolateral regions of amygdala (D). Abbreviations: mPF, medial prefrontal cortex; NAcc, nucleus accumbens core; HIPdg, dentate gyrus of hippocampus; HIPca3, CA3 region of hippocampus; BLA, basolateral amygdala; CeA, central amygdala.
LH Paradigm
To create the LH paradigm, animals are initially exposed to uncontrollable stress. When the animal is later placed in a situation in which shock is controllable (escapable), it not only fails to acquire the escape responses, but also often makes no efforts to escape the shock at all (Overmier and Seligman, 1967). This escape deficit is reversed by subchronic antidepressant treatment (Shirayama et al., 2002; Iwata et al., 2006).
Learned helplessness behavioral tests were performed using the Gemini Avoidance System (San Diego Instruments, San Diego, CA). This apparatus was divided into two compartments by a retractable door. On days 1 and 2, rats were subjected to 30 inescapable electric footshock [0.65 mA, 30-s duration at random intervals (mean 30 s, average 18–42 s)]. On day 3, a two-way conditioned avoidance test was performed as a postshock test to determine if the rats would show the predicted escape deficits. This screening session consisted of 30 trials in which electric foot shocks [0.65 mA, 6-s duration at random intervals (mean 30 s, average 18–42 s)] were preceded by a 3 s conditioned stimulus tone that remained on until the shock was terminated. Rats with more than 25 escape failures in the 30 trials were regarded as having reached criterion and were used for further experiments. Approximately 65% of the rats met this criterion. On day 4, rats received bilateral microinjections of ALLO and/or other chemicals (flumazenil, (+)MK-801, cycloheximide) as described above. On day 8 (4 days after surgery), a two-way conditioned avoidance test was performed. This test session consisted of 30 trials in which electric foot shocks [0.65 mA, 30-s duration at random intervals (mean 30 s, average 18–42 s)] were preceded by a 3 s conditioned stimulus tone that remained on until the shock was terminated. The numbers of escape failures and the latency to escape in each 30 trial were recorded by the Gemini Avoidance System.
For antidepressant treatment, imipramine (20 mg/kg, once per day) or saline (0.9%) was administered i.p. for 12 days after the postshock screening test until 1 day before the conditioned avoidance test.
Open Field Test
Four days after surgery, an open field test was performed in a square area (76.5 × 76.5 × 49 cm3) using a standard procedure (Lacroix et al., 1998). The open field was divided into two areas, a peripheral area and a square center (40 × 40 cm2). The test room was dimly illuminated (60 W light, indirect). Rats were allowed to explore for 30 min. A computer software program (Be Trace: Behavioral and Medical Sciences Research Consortium, Hyogo, Japan) calculated the velocity of movement, the distance of travel, and the time spent in the center of the open field. These parameters are thought to reflect locomotor activity and fear or anxiety, respectively.
Passive Avoidance Test
The passive avoidance test was conducted according to standard procedures with the following modifications (Ferry et al., 1999). The apparatus was divided into two compartments by a retractable door: a lighted safe compartment and a darkened shock compartment (Gemini Avoidance System). Four days after surgery, the test animal received a single inescapable foot shock (0.80 mA; 4 s duration). Twenty-four hours later, each rat was placed in the lighted safe compartment and the latency until reentry into the darkened shock compartment was recorded as the measure of retention.
Statistical Analysis
Statistical differences among more than three groups were estimated by a one-way analysis of variance (ANOVA), followed by Tukey's test. For comparison of the mean values between the two groups, statistical evaluation was done using the two-tailed Student's t-test. The criterion of significance was P < 0.05.
RESULTS
Effects of ALLO Infusion Into the Cerebral Ventricle or Other Regions of LH Rat Brain
Subchronic treatment of LH rats with the tricyclic antidepressant imipramine produced a significant improvement in the conditioned avoidance test (Fig. 2A). This demonstrates that the LH paradigm is responsive to antidepressant treatment, as reported previously (Shirayama et al., 2002; Iwata et al., 2006).

Influence of ALLO infusion into the brain on the LH paradigm. Imipramine administration (12 days) for comparison (A). ALLO or saline was administered via bilateral infusion into the cerebral ventricle (B), the medial prefrontal cortex (C), the nucleus accumbens core (D), the dentate gyrus of hippocampus (E), the CA3 region of hippocampus (F), the basolateral amygdala (G), or the central amygdala (H). Animals were subjected to conditioned avoidance test 4 days later. Escape failure and latency to escape were determined. The results are expressed as mean ± standard error of mean (SEM). The number of animals is listed under each column. (A) Top, t = 5.668, P = 0.0001; bottom, t = 4.830, P = 0.0005; (B) top, t = 2.517, P = 0.0286; bottom, t = 2.288, P = 0.0429; (C) top, t = 0.168, P = 0.8698; bottom, t = 0.258, P = 0.8020; (D) top, t = 0.905, P = 0.3915; bottom, t = 0.535, P = 0.6067; (E) top, t = 1.206, P = 0.2585; bottom, t = 0.8648, P = 0.4074; (F) top, F (2,13) = 4.560, P = 0.0316; bottom, F (2,13) = 4.076, P = 0.0422; (G) top, t = 2.150, P = 0.0638; bottom, t = 2.163, P = 0.0625; (H) top, F (2,14) = 4.028, P = 0.0415; bottom, F (2,14) = 3.862, P = 0.0462. *P < 0.05; ***P < 0.001 when compared with saline-injected controls (Student's t-test or ANOVA followed by Tukey's test). Abbreviations: ICV, cerebral ventricle; mPF, medial prefrontal cortex; NAcc, nucleus accumbens core; HIPdg, dentate gyrus of hippocampus; HIPca3, CA3 region of hippocampus; BLA, basolateral amygdala; CeA, central amygdala.
LH rats that received bilateral microinjections of ALLO into the cerebral ventricle demonstrated a significant improvement in the conditioned avoidance test relative to saline-treated controls (Fig. 2B). Infusion of ALLO into the infralimbic prefrontal cortex or nucleus accumbens core failed to produce the antidepressant-like effects (Figs. 2C,D).
LH rats that received bilateral microinjection of ALLO into the CA3 region, but not the dentate gyrus, of the hippocampus demonstrated a significant improvement in the conditioned avoidance test relative to saline-treated controls (Figs. 2E,F). Infusion of ALLO into the central, but not the basolateral, region of the amygdala of LH rats significantly decreased escape failure in the conditioned avoidance test (Figs. 2G,H).
Effect of Infusion of ALLO Into CA3 Region of Hippocampus or Central Region of Amygdala on Locomotor Activity or Passive Avoidance
Infusions of ALLO into the CA3 region of the hippocampus or the central region of the amygdala failed to affect the time spent in the center, distance traveled, or velocity in the open field test (Figs. 3A,B). This would not be the result expected if a general increase in locomotor activity contributed to the effect of ALLO on conditioned avoidance in the LH models of depression.
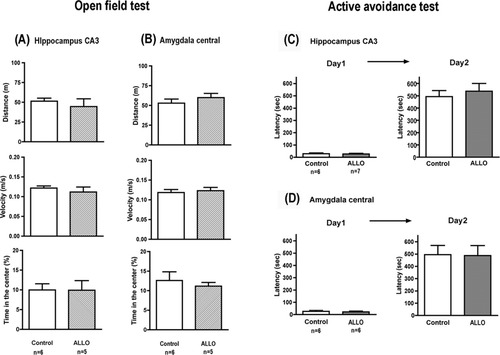
Influences of ALLO infusion into the hippocampus or amygdala on locomotor activity or passive avoidance. ALLO or saline was infused into the CA3 of hippocampus (A, C) or central amygdala (B, D), and 4 days later, the distance traveled, velocity, and time spent in center in an open field were determined or passive avoidance test was conducted. The results are the mean ± SEM of the number of animals indicated under each column. (A) Top, distance, t = 0.6821, P = 0.5124; middle, velocity, t = 0.7700, P = 0.4610; bottom, time in the center, t = 0.0238, P = 0.9815. (B) Top, distance, t = 0.9464, P = 0.3687; middle, velocity, t = 0.4033, P = 0.6961; bottom, time in the center, t = 0.5686, P = 0.5835. (C) Day 1, t = 0.2849, P = 0.7810; Day 2, t = 0.5328, P = 0.6047. (D) Day 1, t = 0.4395, P = 0.6696; Day 2, t = 0.0753, P = 0.9414.
Infusions of ALLO into the CA3 region of the hippocampus or the central region of the amygdala did not alter the length of time spent in the darkened compartment in consecutive retention tests (Figs. 3C,D). These results suggest that ALLO infusions do not cause a deficit in learning, which could result in the effects observed in the LH paradigms.
Effects of Coadministration of GABA Antagonist Flumazenil With Infusion of ALLO Into CA3 Region of Hippocampus or Central Region of Amygdala of LH Rats
It is well documented that ALLO exerts its effects through GABA receptors. Coadministration of flumazenil (a GABA receptor antagonist) and ALLO into the CA3 region of the hippocampus blocked the antidepressant-like effects of ALLO (Fig. 4A), whereas coadministration of flumazenil and ALLO into the central region of the amygdala failed to block the antidepressant-like effects of ALLO (Fig. 4B). Infusion of flumazenil alone into the hippocampal CA3 region failed to exert any effects on the conditioned avoidance test (Fig. 4A). This indicates that ALLO exerts antidepressant-like effects through GABA receptors in the CA3 region of the hippocampus, but independent of the GABA system in the central region of the amygdala.
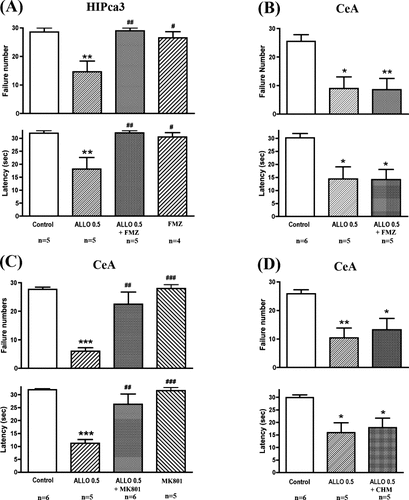
Influence of flumazenil (GABA receptor antagonist), (+)MK-801 (NMDA receptor antagonist), or cycloheximide (protein synthesis inhibitor) on the antidepressant-like effects of ALLO infusions into the hippocampus or amygdala in the LH paradigm. Coinfusion of flumazenil blocked the antidepressant-like effects of ALLO in the CA3 region of hippocampus (A), but not in the central amygdala (B). Coinfusion of (+)MK-801 (C), but not cycloheximide (D), blocked the antidepressant-like effects of ALLO infusions into the central amygdala in the LH paradigm. Animals were subjected to conditioned avoidance test 4 days later. Escape failure and latency to escape were determined. The results are expressed as mean ± SEM. The number of animals is listed under each column. (A) Top, F (3,15) = 8.653, P = 0.0014; bottom, F (3,15) = 7.066, P = 0.0035. (B) Top, F (2,13) = 8.579, P = 0.0042; bottom, F (2,13) = 7.591, P = 0.0065. (C) Top, F (3,18) = 16.07, P < 0.0001; bottom, F (3,18) = 15.61, P < 0.0001. (D) Top, F (2,13) = 9.053, P = 0.0040; bottom, F (2,13) = 7.254, P = 0.0086. *P < 0.05; **P < 0.01; ***P < 0.001 when compared with saline-injected animals; #P < 0.05; ##P < 0.01; ###P < 0.001 when compared with the ALLO-injected group (ANOVA followed by Tukey's test). Abbreviations: HIPca3, CA3 region of hippocampus; CeA, central amygdala; FMZ, flumazenil; CHM, cycloheximide.
Effects of Coadministration of NMDA Antagonist (+)MK-801 With Infusion of ALLO Into Central Region of Amygdala of LH Rats
Coadministration of (+)MK-801 (an NMDA receptor antagonist) and ALLO into the central region of the amygdala blocked the antidepressant-like effects of ALLO (Fig. 4C). Infusion of (+)MK-801 alone into the central amygdala failed to exert any effects on the conditioned avoidance test (Fig. 4C). This indicates that ALLO exerts antidepressant-like effects through the activation of glutamatergic mechanisms in the central region of the amygdala.
Effects of Preinfusion of Protein Synthesis Inhibitor Cycloheximide on Antidepressant-Like Effects of ALLO Infusion Into Central Amygdala
Preadministration of cycloheximide (a protein synthesis inhibitor) failed to block the antidepressant-like effects of ALLO in the central region of the amygdala (Fig. 4D). This indicates that ALLO does not produce antidepressant-like effects through a genomic-based mechanism.
DISCUSSION
The primary finding of the present study is that infusions of ALLO into the cerebral ventricle, CA3 region of hippocampus, or the central region of amygdala, but not into the dentate gyrus of hippocampus, basolateral region of amygdala, nucleus accumbens, and medial prefrontal cortex, produced antidepressant-like effects in LH rats, an animal model of depression. Thus, the antidepressant action of ALLO on brain is the region-specific.
The open field test did not demonstrate any significant differences in distance traveled, velocity, or time spent in the center, suggesting that the antidepressant-like effects of ALLO did not contribute to enhanced locomotion or modulation of anxiety. Furthermore, the results of the passive avoidance test did not show any differences between the ALLO and control groups, indicating that the antidepressant-like effects of ALLO did not contribute to the memory deficits. Thus, the antidepressant-like effects of ALLO were not because of nonspecific actions.
It is noteworthy that infusions of ALLO into the central amygdala as well as into the CA3 region of hippocampus exerted antidepressant effects. Previous studies showed that chronic administration of antidepressant drugs increased the number of Fos-positive neurons in the central nucleus of the amygdala (Duncan et al., 1996; Veening et al., 1998; Morelli et al., 1999). Other studies demonstrated that uncontrollable stress (a kind of learned helplessness) caused extracellular signal-regulated kinase phosphorylation in the hippocampus, basolateral, and central amygdala (Yang et al., 2008), and that the central amygdala of Flinders sensitive line rats (a genetic animal model of depression) did not respond to acute restraint stress without increased mRNA expression of corticotropin-releasing hormone (Zambello et al., 2008). ALLO levels were decreased in the amygdala, frontal cortex, and hippocampus of olfactory bulbectomized rat (an animal model of depression) (Uzunova et al., 2003). Taken together, the findings indicate that the central amygdala and hippocampus could be involved in the pathophysiology of depression and the mechanism of antidepressant effects of ALLO.
The second finding is that coinfusion of flumazenil (a GABA receptor antagonist) with ALLO into the hippocampal CA3 region blocked the antidepressant-like effects of ALLO. ALLO is a positive allosteric modulator of the GABAA receptor, and thus this finding indicates that ALLO exerts antidepressant-like effects through GABAA receptors in the CA3 region of the hippocampus. Forced swim stress increased ALLO expression in the brain and plasma (Purdy et al., 1991) and decreased function of GABAA receptors in the rat brain (Drugan et al., 1989). This increase in ALLO levels has been postulated as a homeostatic mechanism to restore decreased GABA system functions. In contrast to acute stress, social isolation induced decreases in both ALLO levels and GABAA receptor function in the cortex and hippocampus of rats (Serra et al., 2000). Chronic stress altered mRNA expression of glutamate decarboxylase, a GABA synthesizing enzyme, in the hippocampus (Bowers et al., 1998) and chronic exposure to stress levels of corticosterone altered GABAergic function in the hippocampus of rats (Orchinik et al., 1995). Social isolation decreased 5α-reductase, an enzyme necessary for ALLO synthesis, in the hippocampus, amygdala, and prefrontal cortex of mouse (Agís-Balboa et al., 2007). It is likely that long-term mild stress finally decreases ALLO levels, which follow the decreased GABAA receptor function. Therefore, it may be that ALLO exerted antidepressant effects by compensating for the reduction of ALLO and restoring GABA function in the hippocampus of the LH rats. An alternative hypothetic mechanism might be that ALLO attenuates GABAergic neurotransmission through GABAA receptors on the GABAergic interneurons, in turn enhancing the glutamatergic transmission in the CA3 region of the hippocampus. Further study will be needed to elucidate the involvement of GABAergic interneurons in the antidepressant-like effects of ALLO in the hippocampus.
The third finding is that coinfusion of flumazenil (a GABAA receptor antagonist) with ALLO into the central amygdala failed to block the antidepressant-like effects of ALLO. This suggests that the GABAA receptors involved in the antidepressant-like effects of ALLO infusions into the central amygdala are insensitive to benzodiazepine modulation because flumazenil works as a benzodiazepine-sensitive GABAA receptor antagonist. It was reported that chronic administration of ALLO increases expression of the α4 subunit of the GABAA receptor, whereas α4 subunit-containing GABAA receptors are insensitive to benzodiazepine modulation (Smith et al., 2007). Hence, the antidepressant-like effects of ALLO in the central amygdala might be mediated by benzodiazepine-insensitive α4 subunit-containing GABAA receptor. Otherwise, the benzodiazepine-insensitive GABAA receptor might be exprasynaptic δ subunit-containing GABAA receptor (Lambert et al., 2009). Therefore, it remains possible that ALLO exerts its effects through benzodiazepine-insensitive GABAA receptors in the central amygdala.
Furthermore, coinfusion of (+)MK-801 (an NMDA receptor antagonist) with ALLO into the central amygdala blocked the antidepressant-like effects of ALLO. Since the anatomical fact showed that the central region of amygdala receives glutamatergic projections from the basolateral region of amygdala and sends the outputs to the brainstem and hypothalamus (Pitkanen et al., 1997), the antidepressant-like effects of ALLO could be dependent on the activation of glutamatergic mechanisms. It was reported that NMDA receptors could mediate regular synaptic transmission in GABAergic interneurons in the lateral amygdala (Szinyei et al., 2003). An electrophysiological study has demonstrated that ALLO reduces GABAA receptor-mediated inhibitory postsynaptic currents in the central nucleus of the amygdala via an NMDA receptor-mediated mechanism (Wang et al., 2007). Although direct effects of ALLO on glutamatergic receptors have never been reported, other sulfated neurosteroids have modulatory effects on the NMDA receptors (Elfverson et al., 2008; Sedlacek et al., 2008). These support the assumption that GABAA receptor function is modulated by the NMDA receptor in the central amygdala.
The present result cannot be simply attributed to an amnesiac effect because of an enhancement of GABAergic function by ALLO because memory function was intact in the passive avoidance test (Fig. 3). Moreover, ALLO exerted not only memory-inhibitory action (Ladurelle et al., 2000; Johansson et al., 2002) but also memory-facilitatory action, probably with relation to glutamatergic systems (Cheney et al., 1995). Further study will be needed to elucidate the relevance of memory modulation by ALLO to the antidepressant-like effects of ALLO in the LH rat.
LH has a kind of matter of learning and extinction, which may be related to the function of the hippocampus and amygdala. It could be that LH rats have a difficulty in starting new reasonable behaviors because of obsession by past experience (Overmier and Seligman, 1967). The matter of memory in the LH rats may be consistent with the memory deficits in human depression. ALLO was found to inhibit the glucocorticoid receptor-mediated gene transcription in vitro (Basta-Kaim et al., 2007). Therefore, the gene transcription in the central amygdala induced by increased glucocorticoid during the process attaining LH might be the cause of depression. Learning requires long-term memory, which needs gene transcription. However, the present study showed that preadministration of cycloheximide (a protein synthesis inhibitor) into the central amygdala failed to block the antidepressant-like effects of ALLO infusion into the central amygdala of LH rats, indicating that the antidepressant-like effects of ALLO were not exerted through the gene transcription in the central amygdala.
The preferable mechanism is that bed nucleus of the stria terminalis (BST) is a switching relay point because BST has the outputs from both stress-inhibitory structure hippocampus and stress-excitatory region CeA (reviewed by Ulrich-Lai and Herman, 2009). In accordance with this, the effects of ALLO were blocked by antagonism of GABAergic system in the CA3 of hippocampus and antagonism of glutamatergic system in the CeA. The paraventricular nucleus of the hypothalamus (PVN) receives projections from brainstem, BST, and CeA and participates in hypothalamo–pituitary–adrenal (HPA) activation. Thus, BST and PVN could be key modulators for HPA activation to stress conditions driven by LH, although this is speculation. Otherwise, the central nucleus of the amygdala sends projections to the VTA, locus coeruleus, and dorsal raphe (Wallace et al., 1992; Gonzales and Chesselet, 1990). The precise mechanism remains to be elucidated.
In summary, infusion of ALLO into the CA3 region of hippocampus or the central region of amygdala produced antidepressant-like effects in LH rats. Coadministration of flumazenil (a GABA receptor antagonist) with ALLO into the CA3 region of the hippocampus, but not into the central amygdala, blocked the effect of ALLO. However, coinfusion of (+)MK801 (an NMDA receptor antagonist) with ALLO into the central amygdala blocked the antidepressant-like effects. Finally, infusion of ALLO into the amygdala or hippocampus did not alter memory ability or locomotion. These results demonstrate that the antidepressant-like effects of ALLO in the CA3 region of hippocampus are produced through the GABA system, and in the central amygdala the effects are contingent on activation of the glutamatergic mechanisms.




