Substance-use simulation impairs driving capability in patients with cirrhosis regardless of hepatic encephalopathy
Abstract
Driving is independently affected by cirrhosis and hepatic encephalopathy (HE) and alcohol/substance use, but their concomitant impact is unclear. We aimed to determine the impact of alcohol and other substances on driving-simulator performance in cirrhosis with and without HE. Outpatients with cirrhosis and controls underwent cognitive testing and driving simulation for the following three conditions: baseline, wearing goggles simulating alcohol intoxication, and wearing goggles simulating opioid/benzodiazepine abuse. Outcomes were number of centerline crossings (CCs) and road-edge excursions (REEs). We compared controls versus patients with cirrhosis then subjects with cirrhosis with and without HE for all conditions, using generalized linear modeling (GLM). Sixty subjects (17 controls, 43 with cirrhosis [Model for End-Stage Liver Disease score, 10; 21 subjects with prior HE]) were included. Simulations showed higher CCs and REEs at baseline in patients with cirrhosis with and without HE versus controls. With alcohol- and substance abuse-impairment goggles, CCs increased but REEs decreased in cirrhosis. In the GLM, a time and group interaction was seen (p < 0.001) for CCs and REEs. Patients with cirrhosis showed higher CCs and REEs at baseline than controls (CCs, p = 0.003; REEs, p = 0.0001) and higher CCs (p = 0.03) and lower REEs (p = 0.001) with alcohol-simulating goggles. All groups were equally impaired with opioid/benzodiazepine-simulating goggles (CCs, p = 0.49; REEs, p = 0.46). Controls with alcohol-simulating goggles had similar CCs as the baseline of patients with cirrhosis (p = 0.98). conclusions: Simulating alcohol intake induces greater driving impairment in patients with cirrhosis versus controls, but similar patterns were seen with opioid/benzodiazepine-simulating goggles. At baseline, patients with cirrhosis have simulator outcomes equivalent to intoxicated controls. Driving simulation with goggles modeling substance abuse could improve insight into driving errors and enhance driving rehabilitation in patients with cirrhosis.
INTRODUCTION
Optimal driving skills require rapid processing and updating of multiple inputs in a timely manner that is influenced by host, system, and environmental factors. Prominent host factors that can influence driving capabilities include chronic diseases, alcohol abuse,[1, 2] and possibly prescription medications (opioids and benzodiazepines in particular) that tend to be prescribed more in those with chronic medical conditions.[3] Interaction of chronic medical conditions with driving performance and perception of risk is lacking[4] and is of concern in the setting of existing alcohol and prescription drug misuse.
Patients with chronic liver disease and cirrhosis are particularly prone toward driving impairment due to a combination of cognitive impairments from chronic liver disease etiologies[5, 6]; cognitive impairment, including covert or prior overt hepatic encephalopathy (OHE)[7]; and concomitant substance use (e.g., alcohol, opioids, benzodiazepines). Cognitive impairment related to minimal hepatic encephalopathy (MHE) can persist despite adequate treatment of hepatic encephalopathy (HE) and is associated with a higher number of traffic violations and motor vehicle accidents in this population when compared to the general population.[8] Additionally, It has been shown that these patients have impaired car handling and adaptation[7] and that their navigational skills are impaired,[9] thus contributing to the higher rates of accidents. Despite cirrhosis development, development of OHE, and perhaps with ongoing underdiagnosed MHE, some patients continue to misuse alcohol and/or use opioids/benzodiazepines.[10, 11] The impact of these substances over driving performance is yet unclear in patients with cirrhosis with preexisting cognitive impairment (i.e., those with prior HE or MHE).
Driving simulation is an efficient method to gauge driving skills that can be adapted to fit environmental and patient-level factors quickly compared to on-road driving and could be used to improve insight into driving impairment and effect of substance abuse.[12]It has been used extensively in the cirrhosis population in various settings. From a substance-use and research perspective, it is not ethical to provide alcohol and illegal substances to patients with cirrhosis to replicate conditions of intoxicated driving; therefore, we need to study these situations through simulation, such as simulation goggles replicating intoxication with alcohol and opioids/benzodiazepines.
Our aim was to determine the impact of simulated alcohol intoxication and substance abuse (opioids/benzodiazepines) impairment on driving-simulator performance in patients with cirrhosis with and without prior HE compared to healthy controls in order to study the individualized impact on impaired driving in cirrhosis. A secondary aim was to determine the impact of cognitive impairment on these results. We hypothesized that simulating substance abuse would impair driving-simulator performance to a greater extent in patients with prior HE compared to those without HE and healthy controls, regardless of whether alcohol-simulating or opioid/benzodiazepine-simulating goggles were employed.
MATERIALS AND METHODS
Outpatients with cirrhosis and controls who were current drivers, were able to consent, and were >18 years of age were consecutively recruited. Cirrhosis was diagnosed using either liver biopsy, evidence of decompensation, or using transient elastography and/or presence of portosystemic shunts in patients with chronic liver disease. Those with prior HE needed to be on stable medications (lactulose and/or rifaximin for at least 3 months without recurrence). Healthy controls were recruited from lists generated from prior studies and were free of chronic diseases, were not drinking alcohol, and were not on any prescription medications. We excluded patients with current/recent (<3 months) alcohol misuse, those taking opioids and/or benzodiazepines, and those who were not current drivers. All subjects needed to have a valid driver's license and had to be able to drive themselves to our center. After informed consent, all subjects were administered a mini-mental status exam, and only those with scores >25 then underwent cognitive testing as detailed below.
Driving simulation with goggles
After a short training period (5–10 min depending on patient) on the driving simulator, subjects underwent the complete simulation (STISIM Drive, Systems Technology Inc., CA, USA). This was carried out under three conditions (Figure 1): (1) baseline, (2) after subjects were asked to wear goggles that simulated a blood alcohol concentration (BAC) of 0.17%–0.20%, and (3) then wearing substance abuse-impairment goggles that simulated substances, such as opioid/benzodiazepines. All patients underwent baseline driving simulation first and then simulation with either alcohol (#2) or substance abuse (opioid/benzodiazepine) goggles (#3) in a random manner. We used Fatal Vision blue goggles, which simulate being under the influence of prescription drugs, such as opioids/benzodiazepines, and Fatal Vision silver-label goggles, which simulate an estimated BAC of 0.17%–0.20% (i.e., above the legal limit of 0.07%–0.10%). Subjects were given 3–5 min to acclimate to the goggles at the start of each session.[13] The alcohol-impairment goggles impair balance/equilibrium, vision, and reaction time, while the substance abuse (opioids/benzodiazepines) goggles limit the scope of the input to impair divided attention and reduce contrast sensitivity, which reduces the ability to distinguish an object from its background in the periphery. Additionally, the substance abuse (opioids/benzodiazepines) goggles cause blurry and double vision, simulating the impairing effects of a loss of balance, poor targeting, delayed reactions, and slow judgment. The silver-label alcohol googles have been shown to change attitudes toward driving under the influence and have been used as a tool to teach drivers the impact of substance abuse on driving.[14, 15] The substance abuse (opioid/benzodiazepine) goggles have not been studied formally.
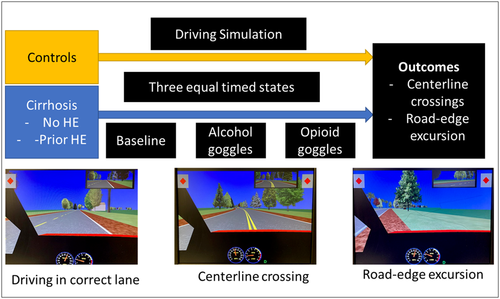
The order of goggles after baseline was as noted above, but the subjects were not told the specific characteristics of each condition beyond the baseline. All subjects were allowed to acclimate to the driving simulator with goggles before the start of each epoch for at least 3 min. Each epoch was 10 min long, and outcomes were (A) number of times crossed over the center (centerline crossings [CCs]) and (B) over the road edge (road-edge excursions [REEs]). All subjects were asked to drive at a speed limit between 45 miles per hour (mph) and 65 mph. Deceleration and acceleration beyond the limits resulted in the software prompting the driver to change speed.
Cognitive testing
EncephalApp Stroop testing
All potential subjects who were not color blind underwent testing by a trained provider. After appropriate instructions and a mandatory trial run/test, patients were officially tested on the EncephalApp test and timings were recorded. A total of five runs were done in the off state and then five more runs were attempted on the on state. Total stage times were recorded for all groups. Standard EncephalApp metrics are times taken for five successful off-stage runs (Off Time), for five successful on-stage runs (On Time), total time taken (Off Time + On Time), extra time in on stage (On Time – Off Time), and number of runs needed to complete five off and five on runs successfully. A diagnosis of MHE was based on US norms from the multicenter North American experience.[16]
Psychometric HE score
The psychometric HE score (PHES) consists of five subtests, namely the number of connection tests (NCTs) A and B (NCT-A, NCT-B), digit symbol test, serial dotting test, and line drawing test. Tests were administered by trained providers. MHE was diagnosed on PHES for a score ≤−4 based on published norms.[16]
Statistics
We compared controls versus subjects with cirrhosis, then subjects with cirrhosis with and without HE in the three simulation conditions, using generalized linear modeling (GLM) and direct comparisons using the SAS statistical software package. Comparing subjects with cirrhosis to controls using CCs or REEs as outcome variables, a generalized mixed linear model was fit to the data. The outcome variable was modeled using a Poisson distribution, and the model included a fixed effect for group (0, control; 1, cirrhosis), condition (1, no goggles or baseline; 2, alcohol goggles; 3, opioid/benzodiazepine goggles), an interaction term between group and condition, and a random effect for subject. For the three groups, a generalized mixed linear model was fit to the data using CC or REE as outcome variables. The outcome variable was modeled using a Poisson distribution, and the model included a fixed effect for group, condition, and interaction for group (0, control; 1, cirrhosis without HE; 2, cirrhosis with HE), condition (as above), an interaction term between group and condition, and a random effect for subject.
Sample size calculation
In a prior navigation-simulator study, we found that 12.5% of controls had a negative simulator outcome compared to 85% of patients with cirrhosis. Using a two-proportion analysis with 90% power and an alpha of 0.05, we would need eight subjects per group.[9] Given that we were carrying out a related but not exactly same driving task and comparing controls versus subjects with cirrhosis without HE and controls with HE, we ensured that we would enroll at least 15 subjects per group.
RESULTS
In total, 65 subjects (19 controls, 46 cirrhosis) were enrolled. Of these, two controls and three patients with cirrhosis developed simulator sickness and were not considered further. The remaining 60 subjects (17 controls, 43 cirrhosis) had a mean driving duration of >25 years. The mean Model for End-Stage Liver Disease (MELD) score was 10 (SD, ± 6), and 21 subjects had prior HE grade 2 or higher episodes (all on lactulose, nine patients on rifaximin with lactulose). Other demographics are noted in Table 1.
| Clinical comparisons | Control (n = 17) | Without HE (n = 22) | With HE (n = 21) | p value |
|---|---|---|---|---|
| Age (years) | 52.7 ± 11.1 | 57.2 ± 11.0 | 59.0 ± 15.7 | 0.19 |
| Alcohol etiology | – | 7 (32%) | 6 (26%) | 0.15 |
| MELD score | – | 8.1 ± 4.6 | 10.1 ± 3.9 | 0.13 |
| PHES score (high, good) | 0.25 ± 1.9 | −1.5 ± 2.9 | −2.3 ± 3.7 | 0.04 |
| MHE on PHES | 0 (0%) | 15 (68%) | 18 (86%) | <0.001 |
| EncephalApp time (low, good) | 141.8 ± 28.7 | 172.1 ± 44.8 | 187.6 ± 35.0 | 0.002 |
| MHE on EncephalApp | 0 (0%) | 5 (23%) | 5 (24%) | 0.09 |
| Driving-simulator outcomes | ||||
| Centerline crossings (median) | ||||
| Baseline | 0.0 | 1.5 | 5.5 | 0.001 |
| Alcohol-simulating goggles | 5.0 | 6.5 | 17.0 | 0.04 |
| Opioid-simulating googles | 3.0 | 7.0 | 10.0 | 0.23 |
| Road-edge excursions (median) | ||||
| Baseline | 0.5 | 2.5 | 4.9 | 0.14 |
| Alcohol-simulating goggles | 0.0 | 0.0 | 0.0 | 0.23 |
| Opioid-simulating googles | 20 | 24 | 15 | 0.52 |
- Note: Data show mean ± SD or number (percentage) unless mentioned otherwise.
- Abbreviations: HE, hepatic encephalopathy; MELD, Model for End-Stage Liver Disease; MHE, minimal hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score.
Driving simulation with goggles
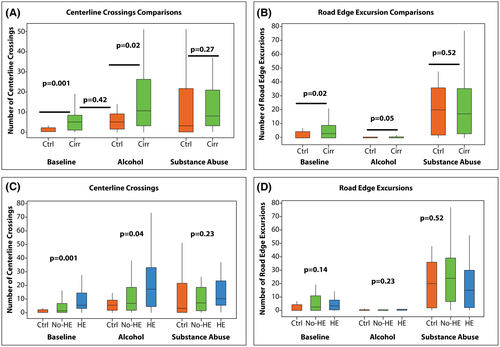
Within the control group, there was a significantly higher CC rate (baseline mean ± SD, 1.53 ± 2.62; vs. alcohol, 8.47 ± 11.40; vs. substance abuse, 11.82 ± 13.44; p = 0.04) and REEs (baseline mean ± SD, 2.35 ± 3.71; vs. alcohol, 0.4 ± 0.79; vs. substance abuse, 19.06 ± 17.90; p < 0.0001) in alcohol- and substance abuse-simulating goggles versus baseline. We found higher CCs and REEs at baseline between controls versus subjects with cirrhosis, especially in those with HE (Figure 2A,B; Table 1). With alcohol-simulating goggles, CCs increased but REEs decreased in cirrhosis groups as subjects tended to steer toward the middle of the road. For example, subjects tended to center their vehicle in the road, increasing CC error but limiting the potential for REE error. Substance abuse (opioid/benzodiazepine)-impairment goggles had a similar driving-simulation error impact regardless of whether or not subjects had cirrhosis or prior HE (Figure 2A,B; Table 1). Using the three groups, we found worse outcomes in patients with prior HE at baseline for CCs but not REEs. This worsened with respect to CCs with alcohol-simulating goggles, but no differences between groups were seen on substance abuse-impairment (opioid/benzodiazepine) goggles (Figure 2C,D). When dividing those with MHE or not, there were no differences (Table 2).
| Simulation | EncephalApp Stroop | PHES | ||||
|---|---|---|---|---|---|---|
| Without MHE | With MHE | p value | Without MHE | With MHE | p value | |
| CC baseline | 4.3 ± 7.1 | 6.7 ± 11.5 | 0.34 | 6.3 ± 11.2 | 4.2 ± 3.9 | 0.30 |
| CC alcohol | 11.2 ± 15.4 | 17.8 ± 20.6 | 0.18 | 15.1 ± 18.7 | 18.7 ± 22.5 | 0.65 |
| CC substance abuse | 13.4 ± 15.1 | 13.9 ± 17.5 | 0.90 | 13.5 ± 17.2 | 14.9 ± 14.9 | 0.80 |
| REE baseline | 5.0 ± 7.0 | 5.8 ± 10.2 | 0.73 | 4.6 ± 6.6 | 10.3 ± 16.9 | 0.32 |
| REE alcohol | 0.7 ± 1.2 | 1.7 ± 5.2 | 0.26 | 0.8 ± 1.6 | 4.4 ± 9.9 | 0.28 |
| REE substance abuse | 20.7 ± 15.3 | 21.5 ± 20.1 | 0.87 | 19.8 ± 17.6 | 28.1 ± 22.3 | 0.29 |
- Note: Data show mean ± SD.
- Abbreviations: CC, centerline crossing; MHE, minimal hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score; REE, road-edge excursion.
GLM analysis
Comparing cirrhosis with controls
When analyzing CCs, the model resulted in a statistically significant interaction between group and condition (F2,169 = 28.14, p < 0.001). Post hoc tests indicated that there was no difference between groups in the substance abuse-impairment condition (t = −0.70, p = 0.49) but that patients with cirrhosis were significantly worse than controls in both the no-goggle condition (t = −4.07, p = 0.0001) and the alcohol-impaired condition (t = −2.33, p = 0.0237). In addition, when controls in the alcohol-simulating goggles condition were compared to patients with cirrhosis at baseline, there was no difference between groups (t = −0.03, p = 0.9778). For REEs, the model again showed a statistically significant interaction between group and condition (F2,169 = 18.55, p < 0.001), but post hoc tests indicated no statistically significant difference among the three groups in the substance-abuse-impairment goggles condition (t = −0.74, p = 0.4606). However, similar to CC results, patients with cirrhosis had higher REEs than controls at baseline (t = −3.04, p = 0.0033) and in the alcohol-simulating goggles condition (t = −3.23, p = 0.0015). Controls in the alcohol-simulating goggles condition performed better than patients with cirrhosis at baseline (t = −5.68, p < 0.0001).
Comparing controls with cirrhosis with HE and without prior HE
The model for CCs results in a statistically significant interaction between group and condition (F4,166 = 17.80, p < 0.001). Post hoc tests indicated no difference among the three groups in the substance-abuse-impairment goggles condition but both cirrhosis groups were significantly worse than the controls at baseline (control vs. cirrhosis without HE, t = −2.45; p = 0.02; control vs. cirrhosis with HE, t = −4.81; p < 0.0001). In addition, when controls in the alcohol-simulating goggles condition were compared to subjects with cirrhosis without HE or with HE in the baseline conditions, there were no differences between groups (t = 1.34, p = 0.19; t = −1.22, p = 0.23, respectively). Similarly for REEs, the model resulted in a statistically significant interaction between group and condition (F4,166 = 10.23, p < 0.001). Post hoc tests indicated no difference among the three groups in the substance-abuse-impairment goggles condition but both cirrhosis groups were significantly worse than controls in the baseline condition (control vs. cirrhosis without HE, t = −3.09; p = 0.003; control vs. cirrhosis with HE, t = −2.18; p = 0.03). In addition, when controls in the alcohol-simulating goggles condition were compared to subjects with cirrhosis without HE and to those with cirrhosis and prior HE in the baseline condition, there was a significant difference between groups (t = −5.62, p < 0.0001; t = −4.90, p < 0.0001, respectively).
DISCUSSION
This is the first study using goggles simulating substance abuse to study the impact of alcohol and substances, such as opioids/benzodiazepines, on driving in patients with cirrhosis and healthy controls. In our study, we found that patients with cirrhosis, especially those with prior HE, had worse driving outcomes when goggles simulating an alcohol-intoxication condition were used when compared to healthy controls. With alcohol- and substance-abuse-simulating goggles, we found driving conditions worsened in healthy controls as well. Controls with alcohol-simulating goggles continued to do better or the same as the baseline condition of patients with cirrhosis, indicating the profound driving challenges in cirrhosis.
Several factors determine whether a person can drive optimally. While other substance misuse, MHE, prior HE, and alcohol intoxication can independently affect driving, these have the potential to further impair driving in patients with cirrhosis.[17] The intricate coordination required between stimulus perception and processing and constant updating during driving needs intact attention, visuomotor coordination, psychomotor speed, and response inhibition as well as personal insight into driving capability.[4] Although the impact of each of these conditions may be unique, the baseline from which the subject starts also determines the overall impact. The goggles induced worse driving outcomes in controls, which speaks to the impairment that can be wrought with these goggles even in healthy people.
Driving under the influence despite increased public awareness is still a public hazard, and per the National Highway Traffic Safety Administration statistics, everyday 28 people die from traffic accidents related to drunk driving.[18] Unfortunately, many patients continue to use alcohol despite knowing that they have underlying chronic liver disease.[19] This is further worsened from the provider standpoint with the need to improve questioning and documentation of alcohol-use disorder in clinics.[20] Alcohol use and its link to fatal crashes have been well established with another recent, large, paired-matched study highlighting this association.[21]
Our study noted that simulating alcohol intake with BAC >0.17% and driving under simulation induces greater CCs but lower REEs in patients with cirrhosis, especially in those with prior HE. With alcohol-simulating goggles and greater underlying impairment (cirrhosis or prior HE), CCs increased while REEs decreased. This indicated a tendency to steer toward the middle of the road and could be due to the greater visibility of the color change at the road edge rather than the center that was even visible through goggles. CCs are associated with distracted driving or could be considered by subjects a necessary compensation for staying on the road itself. This is concerning because even within 10 min, there were major changes in REEs and CCs, which are usually precursors for crashes.
The data are compelling because patients with HE included in our study were all adherent with lactulose/rifaximin, were free from HE for >3 months before testing, and were all active drivers. Our data show that patients with cirrhosis with and without HE have worse outcomes than controls at baseline, but that there is statistical equivalence or superiority of controls on alcohol-simulating goggles compared to patients with cirrhosis at baseline. This indicates that the baseline impairment in a person with cirrhosis is relatively equivalent to or worse than a healthy person who is driving while drunk. While the mechanism is unclear, this may be due to the baseline lack of attention found in patients with cirrhosis that reduces the ability to update stimuli as well as from impaired psychomotor speed/coordination.
Prescription drugs have also been shown to correlate with a higher rate of driving impairment or motor vehicle-related accidents through perceptual visuomotor changes.[21] Prescription drug use can result in cognitive impairment and sedation[22] and increased incident HE[23] in patients with cirrhosis. Interestingly, we did not note any difference in driving simulation parameters (i.e., in CCs or REEs among controls, those with cirrhosis with HE, and those with cirrhosis without HE) while using the substance-abuse-impairment goggles. This demonstrates the specificity of individual goggles on CCs and REEs rather than being the result of a general unfamiliarity with the process.
It is important to note that the presence of prior HE rather than just cognitive impairment had a greater influence on driving outcomes in the alcohol- or substance abuse-simulating goggles condition. This could be due to the persistent impact of prior HE on cognitive function, which is characterized by impaired neuropsychological and perceptual motor dysfunction and which is likely to overshadow cognitive impairment alone, and the relatively small sample size.[24] Our results could also be explained by the relative short duration of the driving-simulator sequences and the focus on CCs and REEs rather than speeding and collisions compared to prior studies where cognitive performance had greater correlations with driving simulation.[9, 25] Ultimately, our goal was to analyze prior HE on these outcomes compared to the remaining groups with differing simulations using goggles.
The implication of these data in the era of polypharmacy, alcohol misuse, and increasing prevalence of chronic liver disease is that these goggles can be used as surrogate training modules for both patients and providers. Driving simulation without using goggles can be used to improve the poor insight into driving errors that patients with cirrhosis have, but this experience with goggles could extend that in patients with cirrhosis who continue to drink.[12, 26] Given the importance of driving impairment and resultant crashes that can be potentially prevented,[1] these training sessions and data could also initiate and facilitate conversations about alcohol cessation in cirrhosis with broader implications for public health and safety. With the corona virus disease 2019 pandemic, there has been an increase in the severity and number of alcohol-associated liver disease admissions and severity,[27] adding further relevance to this study.
Our study is limited due to the small sample size, but the data patterns were relatively consistent. We used REEs and CCs as the outcome rather than crashes because our simulation did not include oncoming cars. The simulator had built-in prompts to warn the drivers who were stalling or going very fast, which could have resulted in fewer outcomes noted. We only used 10-min epochs due to the difficulty in tolerating the goggles for longer periods. However, this design and time duration was able to show differences among groups. Although simulations do not have the real-world feeling, results are often linked with real-world outcomes.[28] For the alcohol simulation, we used a higher BAC than the legal limit, and it is possible that these findings might not hold true at lower levels of BAC. Unlike the alcohol-related goggles, the other impairment goggles reflect the visuomotor changes that occur with generic substance abuse but have not been modeled on specific drug levels. As acknowledged above in the Materials and Methods section, the substance-impairment goggles have not been formally validated in cirrhosis and controls. Finally, it must also be considered that the substance abuse goggles could appear to have a deleterious effect on all subjects as shown in controls, but this impairment was greater in patients with cirrhosis and HE. We conclude that driving performance of healthy controls after alcohol-intoxication simulation is equivalent to that of patients with cirrhosis at baseline, underlining the major cognitive impact of cirrhosis on driving performance. Simulating substance abuse intoxication in a safe manner using these goggles and driving simulation could improve insight into driving errors; facilitate an open dialogue between patients, addiction medicine specialists, and hepatology specialists; and could be used as a training tool. A nonjudgmental approach, including hepatology, neuropsychology, mental health, and occupational therapy, may be needed to manage this important multidisciplinary issue of driving in patients with cirrhosis.
ACKNOWLEDGMENT
We appreciate the CTSA at VCU.
FUNDING INFORMATION
VA Merit Review, Number: 2I0CX001076; National Center for Advancing Translational Sciences, Number: R21TR003095, UL1TR002649; McGuire Research Institute.
CONFLICT OF INTEREST
RS received grants from Abbott, Roche, AbbVie, and Gilead. JSB's institution has received grants from Bausch and Grifols.