Cluster of Differentiation 44 Promotes Liver Fibrosis and Serves as a Biomarker in Congestive Hepatopathy
S: upported by the National Center for Global Health and Medicine (grants 29-shi-1007, 29-shi-2002, 30-shi-2001, and 20A1002 to T.K. and 30-shi-1011 to Y.O.), the Program for Basic and Clinical Research on Hepatitis (JP17fk0310119h, JP17fk0310105h, JP17fk0310106h, and 20fk0210041h0003 to T.K.), the Takeda Science Foundation (to Y.O.), and the JSPS KAKENHI (JP21K08014 to Y.O.).
Potential conflict of interest: Nothing to report.
Abstract
Congestive hepatopathy (CH) with chronic passive congestion is characterized by the progression of liver fibrosis without prominent inflammation and hepatocellular damage. Currently, the lack of reliable biomarkers for liver fibrosis in CH often precludes the clinical management of patients with CH. To explore fibrosis biomarkers, we performed proteome analysis on serum exosomes isolated from patients with CH after the Fontan procedure. Exosomal cluster of differentiation (CD)44 levels were increased in patients with CH compared to healthy volunteers and was accompanied by increases in serum levels of soluble CD44 and CD44 expression in the liver. To address the roles of CD44 in CH, we established a mouse model of chronic liver congestion by partial inferior vena cava ligation (pIVCL) that mimics CH by fibrosis progression with less inflammation and cellular damage. In the pIVCL mice, enhanced CD44 expression in hepatic stellate cells (HSCs) and deposition of its ligand hyaluronan were observed in the liver. Blood levels of soluble CD44 were correlated with liver fibrosis. The blockade of CD44 with specific antibody inhibited liver fibrosis in pIVCL mice and was accompanied by a reduction in S100 calcium-binding protein A4 expression following activation of HSCs. Conclusion: Chronic liver congestion promotes fibrosis through CD44. This identifies CD44 as a novel biomarker and therapeutic target of liver fibrosis in patients with CH.
Abbreviations
-
- α-SMA
-
- α-smooth muscle actin
-
- ALT
-
- alanine aminotransferase
-
- CD
-
- cluster of differentiation
-
- CDD
-
- choline-deficient diet
-
- CH
-
- congestive hepatopathy
-
- Coll-GFP mice
-
- transgenic mice expressing enhanced green fluorescent protein under the transcriptional control of the collagen type I α1 gene promoter
-
- cont.
-
- control
-
- DEN
-
- diethylnitrosamine
-
- ECM
-
- extracellular matrix
-
- EGFP
-
- enhanced green fluorescent protein
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- FALD
-
- Fontan-associated liver disease
-
- GFP
-
- green fluorescent protein
-
- HA
-
- hyaluronan
-
- HCV
-
- hepatitis C virus
-
- HSC
-
- hepatic stellate cell
-
- HV
-
- healthy volunteer
-
- IgG
-
- immunoglobulin G
-
- LSM
-
- liver stiffness measurement
-
- M2BPGi
-
- Mac-2 binding protein glycosylation isomer
-
- mRNA
-
- messenger RNA
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NCGM
-
- National Center for Global Health and Medicine
-
- pIVCL
-
- partial inferior vena cava ligation
-
- qRT-PCR
-
- quantitative real-time reverse-transcription polymerase chain reaction
-
- S100A4
-
- S100 calcium-binding protein A4
-
- vol
-
- volume
-
- VTQ
-
- virtual touch quantification
-
- wk
-
- week
Congestive hepatopathy (CH) is a progressive disease that eventually develops into liver cirrhosis and cancer, the fundamental mechanism of which is the continuous high pressure on the sinusoid.(1) Fontan-associated liver disease (FALD) is one of the prototypes of CH with a high risk of developing liver fibrosis because the liver is exposed to high pressure following reconstructive surgery to restore blood circulation.(2, 3) Circulatory impairment of the liver, as in sinusoidal obstruction and Budd-Chiari syndrome, also results in CH due to hepatic venous outflow obstruction.(4) In general, liver inflammation is a driving factor of liver fibrosis in inflammatory hepatopathies, including viral hepatitis and steatohepatitis. In such conditions, advanced liver fibrosis and cirrhosis are major risk factors for the development of hepatocellular carcinoma (HCC). Thus, alleviation of liver inflammation represents one of the treatment options for preventing liver cirrhosis and HCC. Evaluation of liver fibrosis is critical for the management of patients with chronic liver disease. Many investigators have reported biomarkers or indices for the assessment of liver fibrosis, most of which were established in cohorts of viral hepatitis or steatohepatitis. In contrast, in patients with CH, liver inflammation is modest and hepatocellular damage is milder than in inflammatory hepatopathies,(1, 5, 6) suggesting that the mechanisms of liver fibrosis development in CH are different from those in inflammatory hepatopathies. Therefore, most fibrosis biomarkers or indices identified based on inflammatory liver diseases are not suitable for the assessment of fibrosis in patients with CH.
Recently, the mechanisms of liver fibrosis in CH were investigated using mice with partial inferior vena cava ligation (pIVCL).(7, 8) Mechanical stretch of liver sinusoidal endothelial cells has been shown to promote sinusoidal thrombosis formation, and mechanical stretch of hepatic stellate cells (HSCs) induces their activation, resulting in liver fibrosis. Activated HSCs undergo a change from a quiescent retinoid-storing phenotype to a contractile myofibroblast-like phenotype, with the latter producing collagen, a major extracellular matrix (ECM) component. Hyaluronan (HA), another major ECM component, accumulates in fibrotic livers of humans and mice with inflammatory hepatopathies. Thus, measurement of blood HA has been used as a noninvasive biomarker for liver fibrosis.(9, 10) HA is produced by HSCs through HA synthase 2 and mediates the fibrogenic function of HSCs through interaction with cluster of differentiation (CD)44.(9) The latter, in turn, functions as a receptor for HA and is expressed by a variety of cell types, such as immune cells and fibroblasts.(11, 12) Among the multiple isoforms, the most widely expressed isoform is the standard form (CD44s).(13) After activation, cell-surface CD44 is cleaved and the extracellular domain is released as soluble CD44, whereas the intracellular domain translocates to the nucleus to activate the transcription of various genes,(14) thus being responsible for activating a series of key signaling pathways.(15) In the liver, HSCs are one of the major cell types expressing CD44, the expression of which increases as fibrosis progresses.(16) CD44 promotes an HA-mediated invasive phenotype in lung fibroblasts and ECM production in lung fibrosis.(17) Accordingly, hepatic CD44 expression is increased in patients with liver diseases.(18) In patients with nonalcoholic steatohepatitis (NASH), CD44 expression in the liver and soluble CD44 levels in the blood are increased.(12) However, the roles of HA and CD44 in CH are largely unknown.
S100 calcium-binding protein A4 (S100A4) is released from fibroblasts, macrophages, lymphocytes, and myeloid cells and is implicated in fibrotic diseases.(19) S100A4 is increased in fibrotic liver induced by CCl4 exposure(20) or NASH,(21) and its knockdown results in a reduction of liver fibrosis following injury in mice.(22) However, the relationship between S100A4 and CD44 in CH-induced fibrosis is unclear.
Exosomes are lipid bilayer-enclosed vesicles released from various cells into the circulation. Depending on their cellular origin, exosomes carry various bioproducts as cargos, possibly reflecting the physiological and pathological conditions of these cells. Thus, exosomes are considered potential sources of biomarkers in various diseases. In this study, we explored novel biomarkers for detecting liver fibrosis in patients with CH. We isolated exosomes from the serum of patients with FALD, performed proteome analyses on them, and found that CD44 was the most prominent cargo. Next, we sought to clarify the functions of CD44 with regard to liver fibrosis development in CH using the pIVCL mouse model.
Materials and Methods
Study Approval
All analyses using human samples were approved by the institutional research ethics committees at Saiseikai Yokohamashi Tobu Hospital and the National Center for Global Health and Medicine (NCGM) Kohnodai Hospital (NCGM-G-002339-02, NCGM-G-001967-04, and NCGM-G-003231-00).
All animal experiments were conducted in accordance with institutional guidelines (Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences), and the protocol was approved by the Animal Research Committee of the NCGM (20055 and 20056).
Patients
Serum samples (n = 15) and liver biopsy specimens (n = 7) were obtained from patients who had had Fontan procedures. Among them, three sets of serum and liver biopsy samples were obtained from the same 3 patients. These patients were not infected with hepatitis B virus or hepatitis C virus (HCV). Additionally, 14 sets of blood samples and liver biopsy specimens were obtained from patients who were HCV positive and who were going to receive antiviral drugs (n = 14). Blood and liver samples were obtained during a single session at our hospital. For obtaining healthy control samples, blood was donated by healthy volunteers (HVs; n = 15) who were officers at the hospitals and did not present with any abnormalities in the annual health check, including blood examination. For the inactive control for pathology, liver biopsy specimens that showed minimal abnormalities were used (n = 8). These included inactive autoimmune hepatitis and complete remission from mild hepatitis. Liver fibrosis was evaluated according to the meta-analysis of histological data in viral hepatitis (METAVIR) F stage and congestive hepatic fibrosis scoring systems.(23) Clinical data were obtained from the electronic clinical records. Clinical characteristics of the patients were determined based on blood samples and liver specimens, which are summarized in Supporting Tables S1-S3. Liver stiffness measurement (LSM) (FibroScan 502; Echosens, Waltham, MA) and virtual touch quantification (VTQ) (ACUSON S3000; Siemens, Erlangen, Germany) were performed only on patients who were HCV positive.
Animals and Treatments
Male C57BL/6J mice were obtained from Japan SLC (Shizuoka, Japan). Transgenic mice expressing enhanced green fluorescent protein (EGFP) under the transcriptional control of the collagen type I α1 (COL1a1) gene promoter (Coll-GFP mice)(24) were kindly provided by Dr. David A. Brenner (University of Calfornia, San Diego). The animals were bred for studies in the animal facility of the Research Center for Hepatitis and Immunology (NCGM) and subjected to pIVCL at 8 to 12 weeks of age, as reported.(7) Animals were anesthetized with medetomidine, midazolam, and butorphanol tartrate. The suprahepatic inferior vena cava was ligated with 6-0 silk and shrunk to 0.6 mm in diameter. Abdominal closure and atipamezole administration were performed on the animals while keeping them warm. After the operation, some mice were treated with the rat anti-mouse CD44 monoclonal antibody IM7 (BE0039; Bio X Cell, Lebanon, NH) or rat anti-human CD44 monoclonal antibody Hermes-1 (BE0262; Bio X Cell), and the effects were compared to immunoglobulin G (IgG) isotype control administration (BE0089; Bio X Cell). The antibodies were injected intravenously (100 μg/mouse) twice a week over the course of 2 weeks after the operation (administered 4 times in total). Other liver fibrosis models analyzed included mice receiving a choline-deficient diet (CDD) (8 weeks) (Research Diets, Inc., New Brunswick, NJ), common bile duct ligation (BDL) (2 weeks), or intraperitoneal CCl4 injections at 0.5 mL/kg body weight (1:5 volume [vol]/vol in corn oil, twice a week for 8 weeks) (Sigma-Aldrich, St Louis, MO). For induction of liver tumors, diethylnitrosamine (DEN) (Sigma-Aldrich) was dissolved in phosphate-buffered saline (10 g/L) and administered intraperitoneally to male mice at postnatal day 14 (0.2 mg/mouse) and at 10 weeks of age (2 mg/mouse). For the DEN-only model, the animals were euthanized 1 year after the final DEN administration. For the DEN + CCl4 model, DEN-administered mice were intraperitoneally injected with CCl4 at 0.5 mL/kg body weight (1:9 vol/vol in corn oil twice a week over the course of 12 weeks) (Sigma-Aldrich), starting at 12 weeks of age. NASH-mediated liver tumors were induced by feeding a CDD to 5-week-old male animals for 9 months. The animals were euthanized by exsanguination. The liver was immediately removed, and a part of the dissected tissue was frozen in liquid nitrogen, fixed with 10% formalin, or embedded in optical cutting temperature compound (Sakura Finetek Japan, Tokyo, Japan) for histologic analysis.
Other Experimental Procedures
Other experimental procedures are described in the Supporting Experimental Procedures. These include histologic analysis, proteome analysis of exosomes, CD44 measurement, hydroxyproline measurement, quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), exosome isolation, western blotting, HA measurement, and statistical analysis.
Results
CD44 is Increased in the Serum and Liver of Patients With FALD
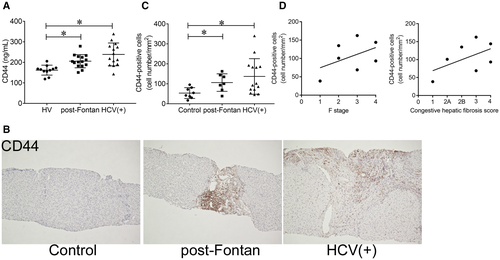
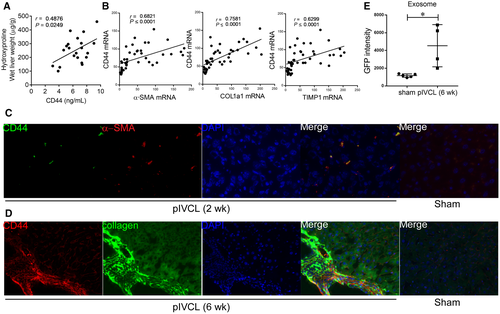
Proteome analysis was performed on serum-derived exosomes to identify biomarkers of liver fibrosis in patients with CH. Those from 3 patients after the Fontan procedure were compared with those from 3 HVs (Supporting Table S4; Supporting Fig. S1A). Although several factors were significantly changed, pathway analysis revealed no relation between these factors (Supporting Fig. S1B). Thus, we focused on CD44, the top hit in the analysis. CD44 was significantly increased in patient-derived exosomes, and a fragment of the intracellular domain of CD44 was identified by proteome analysis (Supporting Fig. S1C). In addition, serum levels of soluble CD44 were also increased in patients after the Fontan procedure and in patients who were HCV positive (Fig. 1A). Because the enzyme-linked immunosorbent assay (ELISA) kit allowed for detection of the extracellular domain of CD44, the shedding and release of CD44 was also found to be increased in these patients. In the liver, the expression of CD44 was increased in nonparenchymal cells after the Fontan procedure, mainly at perivenous sites, and in patients who were HCV positive, mainly at periportal sites, compared to specimens obtained from the controls (Fig. 1B,C; Supporting Fig. S2). These results suggest that the increase in serum levels of CD44 reflects the enhanced expression of CD44 in the liver. The number of CD44-positive cells in the livers of patients after the Fontan procedure seemed to be associated with fibrosis scores according to METAVIR F stage and congestive hepatic fibrosis scoring systems (Fig. 1D). In contrast, well-known liver fibrosis markers in the blood (HA, type IV collagen 7s, and Mac-2 binding protein glycosylation isomer [M2BPGi]) did not correlate with the fibrosis score (Supporting Fig. S3). Because we were not able to analyze a sufficient number of paired blood samples and liver biopsy specimens of patients with FALD collected at the same time (Supporting Fig. S4A), we examined the relationship between serum CD44 levels and liver fibrosis using samples from patients who were HCV positive and found that the levels were positively correlated with CD44-positive cells in the livers of patients who were HCV positive (Supporting Fig. S4B). Sirius red staining revealed collagen deposits in the livers of these patients (Supporting Fig. S4C), and the CD44 level correlated with the F stage and sirius red-positive area (Supporting Fig. S4D). In addition, LSM and VTQ values also increased with soluble CD44 levels (Supporting Fig. S4D). These results suggest that CD44 could serve as a biomarker for liver fibrosis in patients.
Anti-CD44 Antibody Treatment Inhibits Liver Fibrosis Induced by Chronic Liver Congestion in Mice
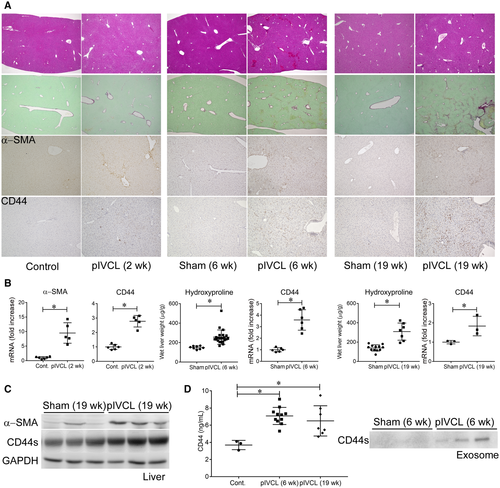
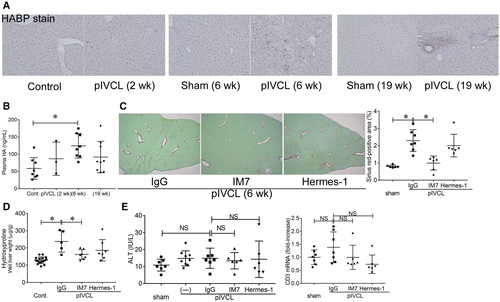
We performed pIVCL on mice to investigate the mechanisms of liver fibrosis in chronic liver congestion. Liver congestion and fibrosis were observed 6 weeks after the procedure, as indicated by hematoxylin and eosin and sirius red staining as well as by hydroxyproline measurements, and progressed chronologically (Fig. 2A). Infiltration of inflammatory cells, increase of CD3-positive T cells and F4/80-positive macrophages, hepatocellular damage, and alanine aminotransferase (ALT) elevation were inconspicuous compared to the CDD-induced NASH model (Fig. 2A; Supporting Fig. S5A), as reported.(7) An increase in α-smooth muscle actin (α-SMA), a marker of HSC activation, was already detectable 2 weeks after the operation, accompanied by increased CD44 expression in nonparenchymal cells mainly at perivenous sites (Fig. 2A-C). The apparent molecular weight of CD44 was approximately 80 kDa, according to western blot analysis (Fig. 2C), suggesting that the induced CD44 was of the standard form. Similar to the observations made for samples from patients with FALD, levels of CD44 in the plasma and exosomes were increased by pIVCL (Fig. 2D). CD44 was also found to be increased in the liver and plasma of mice reflecting other conditions, including BDL, CDD, and CCl4-induced liver fibrosis models (Supporting Fig. S5B). Among the liver tumor models, an increase in CD44 expression was observed in nonparenchymal cells of CDD and DEN + CCl4 models and was accompanied by liver fibrosis. In contrast, CD44 expression was not detectable in the DEN-only model, which does not cause liver fibrosis (Supporting Fig. S5C). These results suggest that CD44 is involved in liver fibrosis, regardless of its etiology, and that CD44 is barely expressed in hepatocytes or hepatoma cells at steady state. As with the expression of CD44, increased accumulation of HA was observed in the liver after pIVCL (Fig. 3A). In addition, plasma HA levels were also slightly increased (Fig. 3B). It has been reported that anti-CD44 antibodies prevent lung collagen accumulation in a bleomycin-induced pulmonary fibrosis mouse model.(17) To explore the role of CD44 in liver fibrosis, we administrated monoclonal antibodies against CD44, clones IM7 and Hermes-1, which recognize different epitopes(25) to the pIVCL mice. The blockade of CD44 by IM7 inhibited liver fibrosis (Fig. 3C,D), suggesting that the signaling molecules downstream of CD44 are involved in fibrogenesis during chronic liver congestion. Plasma levels of ALT and CD3 messenger RNA (mRNA) expression in the livers were not increased by pIVCL and were not affected by IM7 and Hermes-1 (Fig. 3E), suggesting that the antifibrotic effect of IM7 is not mediated by the reduction of liver injury or immune responses.
CD44 Expression is Increased in Activated HSCs in the Congestive Liver
As observed in the patients who were HCV positive, plasma CD44 had a positive correlation with liver fibrosis examined by hydroxyproline measurement in the pIVCL mice (Fig. 4A). mRNA expression of CD44 was correlated with that of HSC activation factors (Fig. 4B), whereas no correlation was found with endothelial cell marker CD31 or T-lymphocyte marker CD3 (Supporting Fig. S5C), suggesting that CD44 is involved in the activation of HSCs. Indeed, the CD44-specific signal colocalized with that of α-SMA (Fig. 4C) and collagen-expressing cells (Fig. 4D), suggesting that CD44 expression is increased in activated HSCs in congestive livers. In addition, the GFP intensity of the exosomes isolated from the Coll-GFP mice was increased in response to pIVCL (Fig. 4E). Thus, the release of exosomes from activated HSCs may have been increased in these pIVCL mice.
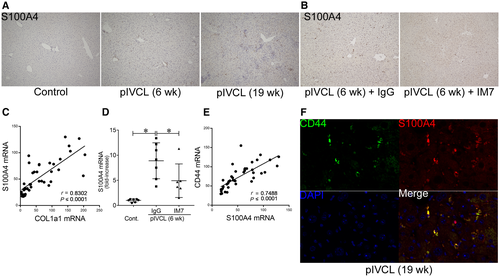
We also examined the relationship between CD44 and S100A4 in order to investigate the mechanisms of CD44-induced liver fibrosis. S100A4 expression was increased in the livers of pIVCL mice (Fig. 5A), which further correlated with collagen expression (Fig. 5C). The induction of S100A4 in pIVCL mice was reduced by CD44 blockade with IM7 (Fig. 5B,D). The expression of CD44 mRNA was positively correlated with that of S100A4 (Fig. 5E). In the pIVCL liver, CD44 expression overlapped with that of S100A4 (Fig. 5F). These results suggest that CD44 is involved in S100A4 expression to activate HSCs.
Discussion
In this study, we found that CD44 is elevated in the liver and blood of patients with CH after the Fontan procedure. Using a pIVCL mouse model of CH, we showed that a blockade of CD44 could reduce liver fibrosis induced by this procedure. These findings show that CD44 is a useful biomarker with functional capability for liver fibrosis in CH.
In patients with CH, some blood markers, including aspartate aminotransferase, ALT, alkaline phosphatase, bilirubin, and HA levels as well as prothrombin time, do not correlate with the degree of liver fibrosis.(1) Although it was reported that dysfunctional liver sinusoidal endothelial cells in fibrotic livers reduce HA uptake resulting in its retention in the blood,(26) no correlation was found in our study. Type IV collagen is a major component of the basement membrane and has been reported to increase in patients from the early phase of the Fontan procedure.(27) M2BPGi is also increased in various types of liver fibrosis by dual activation of HSCs and liver macrophages.(28) Type IV collagen 7s and M2BPGi are recognized as feasible fibrosis markers in inflammatory hepatopathies; however, no correlation was found with the fibrosis scores in our study. In the pIVCL liver, the macrophage marker F4/80 was not increased, and the induction of tumor necrosis factor-α, which reflects macrophage activation, was weak (data not shown), as reported.(7) In clinical practice, methods for liver stiffness assessments, such as LSM, VTQ, or magnetic resonance elastography, cannot distinguish liver congestion from fibrosis because congestion causes an increase in liver stiffness.(1) Thus, the fibrosis markers currently available are not suitable for the evaluation of liver fibrosis in CH. We note that our results may be due to the low number of patients used in the study.
Exosomes carry various bioproducts that reflect the physiological and pathological status of their cells of origin. Based on our data on Coll-GFP mice, the release of exosomes from HSCs seemed to be increased after the pIVCL procedure, suggesting that the analysis of exosomes provides a valuable tool for determining the activation status of HSCs in the liver. In support of this notion, CD44 was increased in exosomes and liver tissues after pIVCL, and soluble CD44 in plasma was increased in parallel. In addition, CD44 was elevated in the liver and blood samples of patients after the Fontan procedure and in those with HCV infection. A positive correlation was observed between CD44 levels in the blood and liver fibrosis in patients who were HCV positive as well as in various liver fibrosis mouse models. It is therefore plausible to consider that the levels of CD44 are indicative of the degree of liver fibrosis. In pIVCL mice, CD44 expression was increased before the development of liver fibrosis, and CD44-specific signals colocalized with α-SMA expression and collagen-expressing cells. Thus, CD44 is an activation marker of HSCs rather than an indicator of liver fibrosis. Because CD44 is a well-known marker in stem cells and cancer stem cells, soluble CD44 in the blood has been reported as a potential marker for several types of cancer.(29, 30) Based on our data, soluble CD44 is a potential biomarker of HSC activation in patients with CH, independent of the impacts of liver congestion or inflammation.
In the pIVCL liver, HA and CD44 were increased, and the neutralization of CD44 by specific antibody inhibited the development of liver fibrosis. Similarly, renal(31) or lung(17) fibrosis was found to be reduced in CD44-knockout mice. CD44-deficient fibroblasts exhibit fewer stress fibers and focal adhesion complexes.(32) HA activates HSCs through CD44 in vitro.(9) In addition to HSCs, CD44 is known to be expressed in immune cells, such as leukocytes and macrophages. Neutralization of CD44 expression in immune cells decreases macrophage infiltration in NASH mice.(12) However, CD44 expression was not conspicuous in immune cells or hepatocytes of pIVCL livers and post-Fontan livers, suggesting that the HA–CD44 axis of HSCs contributes to fibrogenesis in CH. In most of the animal models used to study fibrosis, which have focused on CD44, a significant inflammatory phase preceded the progression to fibrosis. Thus, it cannot be excluded that the effects of blocking or deleting CD44 resulted from an influence on inflammatory cells. Because the pIVCL model does not reflect inflammatory hepatopathy, our results indicate that therapeutic targeting of CD44 could be a direct approach for regulating HSCs instead of regulating immune cells for the treatment of liver fibrosis. With regard to the downstream pathways, CD44 has been reported to activate a series of key signaling molecules, including Rho guanosine triphosphatase (GTPase), Ras-mitogen-activated protein kinase, phosphatidylinositol 3-kinase/AKT, Notch1, epidermal growth factor receptor, signal transducer and activator of transcription 3, and Wnt/β-catenin pathways.(9, 15, 33-36) Following proteome analysis of exosomes, plakophilin-1, a Wnt signaling regulator,(37) was the second most strongly increased factor after CD44 (Supporting Table S4). CD44 small interfering RNA blocks activation of β-catenin in HSCs.(38) S100A4 is controlled by cyclic adenosine monophosphate response element-binding protein binding protein/β-catenin(20, 21) and promotes liver fibrosis.(22) CD44 may, at least partially, contribute to liver fibrosis in an S100A4-dependent manner through a β-catenin-related pathway in CH.
In addition to HA, other ligands, such as osteopontin, fibronectin, collagen, laminin, and matrix metalloproteases, bind to CD44; their roles are unknown with respect to their contribution to the pathogenesis of CH. The question remains whether CD44-mediated mechanisms are involved in HCV-induced liver fibrosis. Further studies are needed to resolve the roles of CD44 in the exosomes and the mechanistic links between CD44 and S100A4 elevation.
In conclusion, CD44 is increased in the liver and blood of patients with CH and contributes to HSC activation and liver fibrosis. Hence, we propose CD44 as a novel biomarker of liver fibrosis in CH. CD44 represents a therapeutic target for preventing and/or treating liver fibrosis in patients with congestive liver disease, such as FALD.
Acknowledgment
We thank Dr. David A. Brenner and Dr. Tatiana Kisseleva for providing the Coll-GFP mice.