Cardiac Risk Assessment in Liver Transplant Candidates: Current Controversies and Future Directions
Abstract
In the changing landscape of liver transplantation (LT), we are now evaluating older and sicker patients with more cardiovascular comorbidities, and the spectrum of cardiovascular disease is uniquely physiologically impacted by end-stage liver disease. Cardiac complications are now the leading cause of morbidity and mortality in LT recipients, and the pretransplant risk is exacerbated immediately during the transplant operation and continues long term under the umbrella of immunosuppression. Accurate risk estimation of cardiac complications before LT is paramount to guide allocation of limited health care resources and to improve both short-term and long-term clinical outcomes for patients. Current screening and diagnostic testing are limited in their capacity to accurately identify early coronary disease and myocardial dysfunction in persons with end-stage liver disease physiology. Furthermore, a number of testing modalities have not been evaluated in patients with end-stage liver disease. As a result, there is wide variation in cardiac risk assessment practices across transplant centers. In this review, we propose a definition for defining cardiac events in LT, evaluate the current evidence for surgery-related, short-term and long-term cardiac risk assessment in LT candidates, propose an evidence-based testing algorithm, and highlight specific gaps in knowledge and current controversies, identifying areas for future research.
Abbreviations
-
- ACC
-
- American College of Cardiology
-
- AHA
-
- American College of Cardiology
-
- ASCVD
-
- atherosclerotic cardiovascular disease
-
- CAC
-
- coronary artery calcium
-
- CAD
-
- coronary artery disease
-
- CAR-OLT
-
- cardiovascular risk in orthotopic liver transplantation
-
- CCTA
-
- coronary CT angiography
-
- CMR
-
- cardiovascular magnetic resonance
-
- CPET
-
- cardiopulmonary exercise testing
-
- CVE
-
- cardiovascular event
-
- ECG
-
- electrocardiogram
-
- EF
-
- ejection fraction
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NPV
-
- low negative predictive value
-
- PET
-
- positron emission tomography
-
- QTc
-
- corrected QT
-
- SPECT
-
- single-photon emission computed tomography
-
- 6MWT
-
- 6-minute walk test
Improvements in surgical techniques, immunosuppression strategies, and management of infections has greatly reduced graft failure and infections as causes of early mortality after liver transplantation (LT) and has extended the lifespan of both the allograft and recipient. Pretransplant and posttransplant care burden has shifted primarily to detection and management of chronic diseases, such as cardiovascular disease, which is now the leading cause of death early after LT and the third leading cause of death beyond the first year.(1-3) According to the American Heart Association (AHA), the term “cardiovascular disease” encompasses conditions that affect the heart (e.g., coronary heart disease, heart failure, rhythm disorders, valvular disease) and extracardiac vasculature (e.g., venous thromboembolism, cerebrovascular disease [stroke], peripheral arterial disease, pulmonary hypertension)(4) (Fig. 1). Unfortunately, there is considerable inconsistency in the definition of cardiovascular outcomes in the LT literature, resulting in wide variation in estimated prevalence and true incidence of disease.(5) A major barrier facing optimal cardiovascular risk assessment of LT candidates is a lack of a consensus definition for clinically meaningful post-LT major cardiac and vascular events. Based on the strength of the current data in the LT population, we define cardiac events as myocardial infarction, coronary revascularization, heart failure, clinically significant arrhythmia (sustained ventricular tachycardia or atrial fibrillation/flutter), cardiac arrest, or primary cause of death due to one or more of these underlying conditions.(2, 5, 6) Vascular events are defined as clinically significant stroke or pulmonary embolism or death stemming from one of these processes. Using these definitions, approximately 1 in 3 LT recipients will experience a cardiac or vascular event within 1 year of LT, contributing to significant health care utilization after LT.(2) When considering pre-LT cardiac risk evaluation in this review, we will focus our attention to cardiac events, which are the main focus of preoperative risk assessment.(7)

The burden of pretransplant cardiac risk is rising for several reasons. First, the mean age of LT candidates is increasing,(8) and older age is a well-established risk factor for underlying cardiac disease. Second, NASH, which is associated with increased risk of incident cardiac events both before and after LT,(9, 10) is now the leading indication for LT listing.(11) Finally, cirrhosis itself is associated with unique physiology that contributes to alterations in myocardial structure and function(12) (Fig. 2). A LT operation results in substantial hemodynamic stress, and long-term immunosuppression contributes to metabolic stress, creating a perfect storm for adverse cardiac events after transplantation.
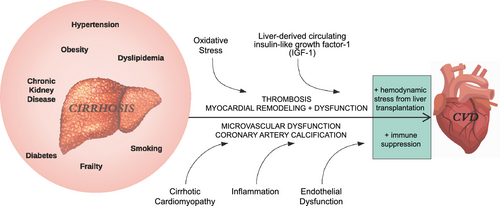
Accurate risk estimation of cardiac complications following LT is paramount to guide allocation of limited resources and to improve clinical outcomes. However, there is significant variation between transplant programs in clinical screening protocols for cardiac disease and management of cardiac pathology when identified. Much of this variation is due to poor representation of patients with cirrhosis in published clinical practice guidelines for preoperative cardiac evaluation and management in noncardiac surgeries.(13) In the following review we will summarize the rapidly growing body of literature on assessment of cardiac risk in LT candidates and identify major areas of controversy and need for future research. The management of identified cardiac conditions in these complex patients is beyond the scope of this review and has been outlined in published guidance statements from the American Association for the Study of Liver Disease (AASLD), American Society of Transplantation (AST), and the AHA and American College of Cardiology (ACC).(7, 14)
Surgical Considerations
An LT operation carries unique cardiac risk. Maintenance of preload and cardiac output is challenging due to hemorrhage, third space losses, and ascites production in patients with poor clotting ability and low oncotic pressure.(15) To avoid the decrease in preload with conventional inferior vena cava clamping, the piggyback technique may provide more stable hemodynamics, but is technically challenging due to splanchnic congestion from portal hypertension. Prophylactic fluid resuscitation is often used to prevent decreases in preload, but this can lead to undesired hypervolemia. These major shifts in hemodynamics may unmask latent cardiac dysfunction.
Intraoperative electrolyte imbalances are common during LT and can instigate cardiac dysfunction and arrhythmias. Use of citrated blood products can result in citrate toxicity, leading to hypocalcemia-induced decreases in cardiac index and stroke index.(16) Hypomagnesemia during the anhepatic stage further impairs cardiac function. The donor graft is commonly preserved in a high potassium solution, which contributes to arrhythmias and cardiac arrest during reperfusion. One mechanism to prevent reperfusion injury is to flush the portal vein or hepatic artery and use vena cava venting to decrease the amount of potassium released.(17) Finally, the time after graft reperfusion when the inferior vena cava and portal vein are unclamped represents one of the most hemodynamically unstable periods during LT. The rapid influx of blood to the right heart can lead to ventriculoarterial decoupling and cardiac arrest in an entity known as postreperfusion syndrome.(18) For these reasons, many of the guidance statements published for cardiac risk stratification in noncardiac surgeries may not apply to the unique LT population.
Framework for Cardiac Risk Assessment in the LT Candidate
Cardiac risk can be stratified into three time periods relative to LT: perioperative, early posttransplant, and late posttransplant (Fig. 3). Traditionally, risk assessment has focused predominantly on perioperative and early cardiac events, which greatly impact short-term posttransplant survival.(1) We now know that the presence and progression of cardiovascular diseases after LT also affects long-term outcomes; thus, risk stratification must consider both short-term and long-term risk. Cardiac deaths contribute to approximately 40% of all deaths within 30 days after LT.(1, 3) The cardiac event rate is estimated to range between 8.0% and 25.4%(1, 2, 5) and 15.2% and 30.0%(5, 19-21) within 30 days and 1 year following transplant, respectively. Most cardiac events at LT are nonischemic in origin and include atrial fibrillation and heart failure, which comprise nearly 70% of all cardiac events within 90 days.(2, 22) In general, cardiovascular diseases contribute to approximately 50% of all rehospitalizations in the first 30 and 90 days after LT.(2)
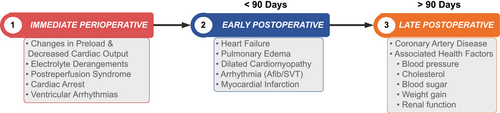
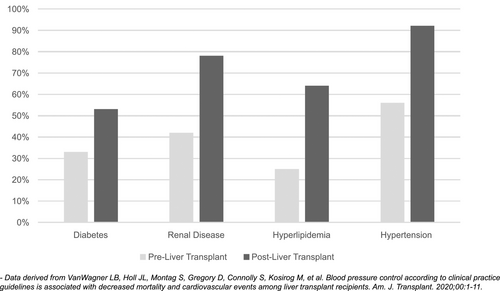
After the first year following LT, the focus shifts from management of acute cardiac events to the identification and management of prevalent cardiac diseases, health behaviors (smoking, physical activity, diet, and weight), and health factors (cholesterol, blood pressure, renal function, and glucose control) that contribute to cardiovascular disease risk. Of all the solid-organ transplant recipients, those undergoing LT have the highest risk of incident metabolic syndrome after transplant, with prevalence of 60% within 1 year of LT.(23) Cardiac risk factor burden increases substantially after LT, largely mediated by immunosuppression and increasing age(3, 24) (Fig. 4). The leading underlying cause of cardiac events late after transplant is coronary heart disease (Fig. 3).(3)
Approach to Cardiac Risk Mitigation in LT
Pretransplant risk assessment can help prognosticate perioperative events and estimate long-term cardiac survival, enabling proper resource allocation and optimization of clinical outcomes. Established risk factors for cardiac disease in LT are found in Table 1. In addition, a variety of imaging and serum biomarkers have been reported to increase perioperative cardiac risk in LT candidates, although their ability to reclassify risk beyond established risk factors has not been confirmed.(5, 25)
| Nonmodifiable Patient Risk Factors | Modifiable Patient Risk Factors | Transplant-Related Risk Factors |
|---|---|---|
| Male sex | Hypertension | Donation after cardiac death |
| Older age | Diabetes | Cold ischemia time |
| Family history of heart disease | Obesity | Donor BMI |
| Black vs. White race | Dyslipidemia | Immunosuppression |
| Troponin level | Smoking | National (vs. local/regional) organ sharing |
| CRP value | Renal disease | MELD score |
| BNP | Frailty | Portal vein thrombosis |
| Transplant indication (NASH or HCV*) | Personal history of heart disease | Respiratory failure |
- * Whether treatment of HCV modifies cardiac risk in LT candidates or recipients is unknown.
- Abbreviations: BMI, body mass index; BNP, brain natriuretic peptide; CRP, C-reactive protein.
There are several risk-assessment scoring tools available to predict the development of cardiac disease in the general population; however, these have not been evaluated in patients with cirrhosis (Supporting Table S1). Furthermore, none of the surgical risk scores included patients undergoing LT surgery,(26) and most tools were developed to detect ischemic heart disease, which accounts for less than 10% of early complications after LT.(2, 22) Initial attempts at modeling cardiac risk in patients with cirrhosis were limited by small sample sizes.(5) In 2018, a prognostic model (CAR-OLT score) was developed to predict global 1-year risk of death or hospitalization from a major cardiac or vascular event after LT.(21) The CAR-OLT score (available at www.carolt.us) is calculated based on readily available pretransplant characteristics and can aid clinicians with point-of-care discussions about the risk of 1-year major cardiac or vascular events. Notably, CAR-OLT still requires external validation and does not address long-term cardiac risk. It should not be used to make transplant-related management decisions, but instead be used to inform risk discussions.
There is significant variability in how LT programs evaluate cardiac risk. Without standardization, it is unclear whether a specific approach to risk assessment affects outcomes before transplant. For example, guidelines and practice guidance documents from AHA/ACC, AASLD, and AST suggest that a cardiologist and/or anesthesiologist should be routinely involved in LT candidate selection.(7, 14, 27) However, literature describing cardiac risk-assessment processes, procedures, and outcomes is sparse.(28-30) Additionally, there is no definition of “routine involvement” and how level of involvement (e.g., available for consultation vs. present in the listing meeting) affects LT candidacy and subsequent outcomes. Thus, there is a critical need for LT centers to publish cardiac risk-assessment and management protocols with associated clinical outcomes for the transplant community to determine optimal risk-assessment approaches.
Another key area of controversy is whether risk assessment should be repeated at regular intervals, as suggested by the guidelines for cardiac evaluation in renal transplant candidates,(7) especially when considering low versus high Model for End-Stage Liver Disease (MELD) candidates. Under allocation policies based on acuity circles, patients in long wait-time regions with a low MELD, HCC, or other exception diagnoses may end up waiting over a year for their transplant. Cardiac risk will not be static over that time period, but data are lacking regarding with which modality or how frequently assessments should be performed. For example, some programs obtain a yearly transthoracic echocardiogram or dobutamine stress echocardiogram (DSE); however, limitations of these tests for risk prediction of cardiac events in cirrhosis introduces uncertainty in their clinical utility (Table 2).(12)
| Category of Risk | Outcome of Interest | Tools Available | Diagnostic Characteristics | Pros and Cons |
|---|---|---|---|---|
| CAD | Exercise intolerance | Exercise testing | Unknown | Limited by physical function |
| CPET | Unknown | VO2 max is challenging for patients with cirrhosis to achieve | ||
| 6MWT | Unknown | 6MWD < 250 m is associated with increased risk of death; for each 100-m increase, survival increases by 42% | ||
| Stress wall motion imaging | Exercise stress ECG | Unknown | Limited by physical function | |
| Pharmacologic stress imaging | Sen: 13%-22%; NPV: 75%-80% | Limited by blunted chronotropy | ||
| DSE | Sen: 35%-37%; NPV: 77%-93% | Limited by chronic vasodilatory state | ||
| Vasodilator (adenosine) stress echocardiography | Sen: 50%; NPV: 98% | High cost, long scan time, requires center expertise; can combine with abdominal and CMR in single exam | ||
| Stress CMR | ||||
| Abnormal coronary flow/functional CAD | Myocardial perfusion imaging | Sen: 35%-37%; high false-negative rate | Limited by chronic vasodilatory state; should not be used as screening test due to high false-negative rate | |
| SPECT | Sen: 81%-89%; NPV: 87-94%* | No nephrotoxicity; not affected by chronic vasodilation; requires center expertise; not studied in LT candidates | ||
| Cardiac PET | Sen: 50%; NPV: 98% | Limited by vasodilation, high cost, long scan time; requires center expertise; can combine with abdominal and CMR in single exam | ||
| Stress CMR | Sen: 84%-89%; NPV: 84-92%(57) | Not yet studied in LT candidates | ||
| FFR by computed tomography (FFRCT) | N/A (gold standard) | FFR < 0.8 indicates clinically significant CAD | ||
| FFR on invasive angiography | ||||
| Anatomic assessment of CAD | CCTA (with or without CAC scoring) | Spec: 60%-90%; PPV: 76-88%* | Nephrotoxicity lower than that of invasive angiography; optimal in patients with healthy body habitus who are able to lie still and perform short breath-holding maneuvers; no reports comparing CCTA and invasive angiography in LT candidates | |
| Invasive coronary angiography | Sen: 95%-100%; NPV: 90%-100%* | Can be safely completed despite coagulopathy and renal dysfunction and allows for simultaneous revascularization; risk of bleeding and nephrotoxicity | ||
| N/A (gold standard) | ||||
| Heart failure | Abnormal cardiac structure | Two-dimensional ECG with myocardial strain | N/A | Screening modality for subclinical myocardial dysfunction |
| CMR | Able to identify subclinical features of cirrhotic cardiomyopathy | |||
| Impaired myocardial function | CPET | N/A | Can identify patients who may benefit from prehabilitation | |
| 6MWT | Limited to ambulatory setting | |||
| DSE | Identifies inducible systolic dysfunction | |||
| Arrhythmia | Abnormal electrical conduction | 12-lead ECG | N/A | Easy to perform |
| Single time-point screening | ||||
| QTc > 480 ms is a predictor of 30-day cardiac arrest or ventricular arrhythmia after LT(68) | ||||
| Atrial fibrillation is a major contributor to post-LT cardiac hospitalizations/events(2, 65 |
- * Compared with the gold standard of invasive angiography in the general population.
- Abbreviations: PPV, positive predictive value; FFR, fractional flow reserve; N/A, not applicable; sen, sensitivity; VO2, rate of oxygen consumption.
Coronary Artery Disease
Cardiac risk assessment in LT candidates has traditionally focused on assessment of coronary artery disease (CAD), despite most perioperative and early cardiac events being nonischemic in origin. However, CAD prevalence is increasing among LT candidates, and asymptomatic moderate CAD is present in nearly 25% of LT candidates.(31) The strongest predictors of obstructive CAD in LT candidates are NASH, renal dysfunction, and two or more traditional AHA/ACC cardiac risk factors (age >60 years, hypercholesterolemia, hypertension, diabetes, tobacco use, prior cardiovascular disease, and left-ventricular hypertrophy).(13, 32) Patients with NASH or renal dysfunction are more likely to have a higher burden of CAD and more critical coronary stenosis.(10, 33)
The identification of CAD in LT candidates is especially important, as recent studies have demonstrated that proper revascularization mitigates the previously identified negative impact of CAD on posttransplant survival.(31, 34, 35) There are a number of modalities available for the assessment of CAD risk, each with their own limitations.(13) Historically, functional imaging in LT candidates has focused on stress wall motion imaging with stress echocardiography.(27) However, LT candidates often have limited physical function and blunted chronotropy to achieve target heart rates on exercise stress testing and pharmacologic (dobutamine) stress testing, respectively, resulting in low sensitivity (13%-22%) and low negative predictive values (NPVs) (75%-80%) of these tests.(36, 37) Furthermore, patients with cirrhosis are in a chronic vasodilatory state, leading to inaccurate pharmacologic vasodilator (e.g., adenosine or regadenoson) testing (sensitivity 35%-37%; NPV 77%-93%), thus limiting its predictive value for CAD in this patient population.(38, 39) Recently, stress imaging with cardiac magnetic resonance (CMR) has been proposed; however, the utility of CMR for the diagnosis of CAD is limited, with a sensitivity of 50% and NPV of 98%.(40) At present time, coronary angiography, either noninvasive or invasive, is the most accurate test for identification of CAD in most patients with cirrhosis.(7)
Noninvasive coronary angiography includes assessment of severity of coronary stenosis with coronary computed tomography angiography (CCTA) with or without coronary artery calcium (CAC) scoring. Assessment of functional CAD includes myocardial perfusion imaging by single-photon emission computed tomography (SPECT), positron emission tomography (PET), or CMR. CAC score > 400 Hounsfield units predicts the need for revascularization and early complications after LT.(41, 42) Furthermore, the NPV (95%-100%) and sensitivity (90%-100%) of CCTA in the general population is excellent for excluding significant CAD.(43) However, performance of CCTA requires a normal body habitus and the ability to lie still and perform breath-holding maneuvers, which may be difficult in patients with cirrhosis and, in particular, with ascites. These potential issues may be mitigated by newer CT scanners with high temporal resolution.(44) Importantly, CCTA alone does not provide information on coronary blood flow and specificity for ischemia-causing lesions ranges from 60%-90% with positive predictive value of 76%-88% in the general population.(45)
Functional testing with SPECT has low sensitivity (35%-37%) for detecting obstructive CAD in patients with end-stage liver disease with a high false negative rate,(46) and therefore should not be used as a screening modality for CAD in LT candidates.(38, 39, 47) There are emerging data on the role of cardiac PET with calculation of coronary flow reserve in the general population, which demonstrated superior diagnostic accuracy (sensitivity 81%-89%, NPV 87%-94%) compared with CCTA for coronary ischemia.(48) Although PET has not been studied in patients with cirrhosis, it has the potential to be a very useful tool, as it has no nephrotoxicity and is not affected by the chronic vasodilation that plagues other modalities of functional CAD testing.
There are limited data evaluating how noninvasive coronary angiography directly compares to invasive coronary angiography for the detection of CAD in the LT population. Invasive coronary angiography has been shown to be safe in LT candidates,(49) especially if using a transradial approach.(50) Although both invasive angiography and CCTA are associated with nephrotoxicity, the risk of nephrotoxicity is less with CCTA.(51) Coronary angiography (invasive or noninvasive) can be performed in patients with renal dysfunction after consultation with nephrology and steps to minimize contrast-induced nephropathy.(13)
Perhaps the most important aspect of CAD detection in the LT candidate is that revascularization has been shown to improve outcomes after LT.(35) In the general population, there are fairly robust data to argue against revascularization of asymptomatic obstructive CAD.(52) However, in the LT candidate, asymptomatic lesions are associated with an unacceptably high rate of myocardial injury and 30-day mortality after LT(53) and should be treated if the degree of obstructive burden would prohibit LT.(13, 14) Thus, invasive coronary angiography should be the last procedure performed in the workup before listing for LT after a patient has already been deemed an acceptable transplant candidate. Most importantly, multidisciplinary discussions are crucial before the patient undergoes invasive angiography, to ensure that there is agreement as to the medical plan if disease is found.
The determination of who should receive invasive versus noninvasive angiography remains an elusive one; thus, centers vary widely in their approach to CAD risk assessment and diagnosis. Risk stratification should consider both the anatomic and functional consequences of CAD in LT candidates. Notably, functional microvasculature disease may produce undetected cardiac symptoms in evaluations that only use an anatomic evaluation of epicardial coronary arteries and may contribute to risk for type 2 myocardial infarction after LT.(54) The ideal situation would be to obtain anatomic and functional information in a single, noninvasive modality (a one-stop shop). The recent development of noninvasive fractional flow reserve by computed tomography may make this a reality in the future.(48, 55-57) Additionally, Reddy et al. demonstrated feasibility of combining liver and cardiac risk stratification using a single abdominal and CMR protocol.(40) However, there are major limitations to widespread implementation, including prolonged scan time, high cost, lack of broad center expertise in CMR, and low sensitivity of stress CMR (50%) for CAD detection in LT candidates.
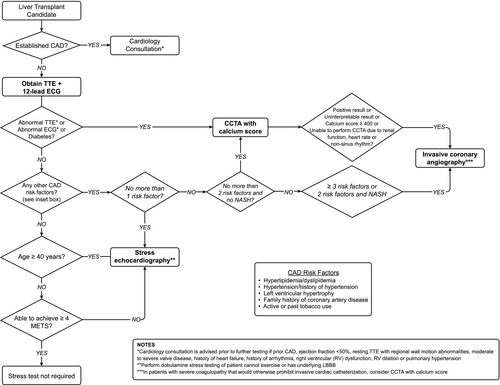
There are limited data regarding how center-specific approaches to CAD risk stratification affect cost, health care use, patient-reported outcomes, and clinical outcomes. Some centers perform invasive angiography in most, if not all, LT candidates.(49, 58) A recently published protocol from Spain used a risk-based stratification approach in which low-risk candidates underwent no pretransplant testing for CAD. However, indications for coronary anatomy evaluation were highly inclusive (three or more cardiac risk factors [male sex, age > 50 years, smoking, dyslipidemia, and hypertension), diabetes, ejection fraction (EF) <50%, symptomatic angina, or calcium deposits in large abdominal vessels). CCTA was obtained in patients with coagulopathy; otherwise, patients underwent direct coronary angiography. In addition, they applied strict criteria for revascularization.(28) There was no difference in coronary events or survival between patients who did and did not undergo CAD evaluation. The main limitation of this single-center study was its generalizability to a US population due to a low prevalence of NASH (1.5%) and a high smoking prevalence (59%). However, publication of protocols linked to hard clinical outcomes, such as this one, are important for the transplant community to compare practice patterns and hopefully standardize assessment and management of CAD risk in LT candidates. Figure 5 presents a proposed algorithm for assessment of CAD risk in LT candidates based on currently available evidence. Finally, risk for atherosclerotic cardiovascular disease (ASCVD) is highest > 1 year after LT.(5, 19) Thus, repeated ASCVD risk assessment at least yearly is recommended among LT recipients to address modifiable ASCVD risk factors (e.g., blood pressure, lipids), to reduce long-term ASCVD events.
Heart Failure
There is a high burden of cardiac dysfunction after LT leading to 25% of readmissions within 90 days of LT(2) and a mortality risk of 15%.(59) Etiology-specific interactions between the liver and heart, and the unmasking of subclinical cirrhotic cardiomyopathy in the setting of major surgery, likely explain a significant proportion of heart-failure events after LT.(12) Both heart failure with preserved or reduced left-ventricular function are described after LT; however, it is not known whether clinical outcomes differ based on the type of heart dysfunction present.
Although there are no established EF cutoffs to preclude LT, it is generally accepted that an EF <40% is an absolute contraindication and <50% is a relative contraindication to transplant.(13) An EF <50% or impaired global longitudinal strain on tissue Doppler echocardiography should raise suspicion for possible underlying cirrhotic cardiomyopathy.(12) Currently, echocardiography is the primary modality to screen for subclinical myocardial dysfunction.(12) DSE may additionally identify inducible systolic dysfunction, which can indicate impaired aerobic capacity.(12) Cardiopulmonary exercise testing (CPET) can be used to identify a high-risk population for clinical heart failure events. Reduced aerobic capacity on CPET is associated with increased wait list and 90 days mortality after LT.(60, 61) Increasing distances achieved on the 6-minute walk test (6MWT) is associated with improvement in survival after LT.(62) However, these tests include the need for specialized equipment (CPET), time (6MWT), and can only be performed in ambulatory patients. CMR is the gold standard for assessment of cardiac morphology and function. In patients with cirrhosis, imaging features on CMR associate with clinical outcomes after LT.(63) Stress CMR may also demonstrate reduced improvement in cardiac output and myocardial strain, a feature characteristic of cirrhotic cardiomyopathy.(12)
The identification of subclinical myocardial dysfunction in patients with cirrhosis is an area of substantial controversy and development over the past decade.(12) Although EF describes global cardiac systolic function, myocardial strain imaging by echocardiography or CMR measures local longitudinal tissue function and is a marker of subclinical systolic dysfunction.(12) Patients with cirrhosis have statistically significant but not clinically significant less myocardial tissue deformation on echocardiography compared with healthy controls, even in the setting of a normal EF.(64) Notably, advanced echocardiographic measurements for the assessment of LV systolic and diastolic dysfunction in cirrhosis are now included in the new proposed criteria for defining cirrhotic cardiomyopathy.(12) Whether these criteria predict clinical outcomes after LT is unknown.
The optimal timing of repeated assessment of cardiac function both before transplant and after transplant is not known. The Cirrhotic Cardiomyopathy Consortium recommends repeated comprehensive echocardiography at a minimum of 6-month intervals before transplant and at 6, 12, and 24 months following transplant in all patients with any degree of pretransplant systolic or diastolic dysfunction.(12) Identification of subclinical or overt heart failure may allow tailored pretransplant and posttransplant interventions. For example, a patient with stage B or C heart failure can be co-managed with cardiology and appropriately placed on goal-directed medical management to stabilize, and perhaps improve, cardiac function going into LT.
Arrhythmia
Previously, arrhythmias were felt to be a result of the LT operation itself (see “Surgical Considerations”) and thus a predictor of poor outcome. However, arrhythmias are now known to be a marker of underlying cardiac pathology, and possible causes should be investigated further.(13) It is recommended that a 12-lead electrocardiogram (ECG) be performed as part of routine pretransplant evaluation to rule out underlying pathology.(7, 27) Atrial fibrillation is the most common arrhythmia, accounting for 43% of all cardiac events after LT.(2, 34) Prevalent atrial fibrillation in LT candidates is associated with increased intraoperative and postoperative cardiac complications, graft dysfunction, and mortality.(2, 65)
There are numerous risk factors present in patients with cirrhosis that may prolong the corrected QT (QTc) interval, including electrolyte derangements, pH-dependent calcium binding to albumin, alkalosis, elevated sympathetic tone, and medications.(66) Long QT syndrome is defined as a QTc > 440 ms and is not associated with increased risk of sudden cardiac death in cirrhosis.(66) However, posttransplant long QTc has been linked with increased risk of death.(67) As patients with pre-existing ventricular arrhythmias are not considered for LT, there are little data regarding its impact on posttransplant outcomes. In 2020, Koshy et al developed a point-based cardiac arrest risk index for prediction of periprocedural (30-day) cardiac arrest and ventricular arrhythmias.(68) Prolonged QT was the strongest predictor of post-LT cardiac arrest or ventricular arrhythmia (OR, 5.2), suggesting that prolonged QTc may be a marker for subclinical cardiac dysfunction that can be unmasked by LT.
Assuming appropriate management of pretransplant arrhythmias, the transplant operation itself introduces several additional considerations, including management of anticoagulation, consideration of transcutaneous or transvenous pacing, electrical cardioversion, or defibrillation and/or antiarrhythmic medications to reduce arrhythmogenic risk. There are no data to suggest that LT recipients are at higher risk than the general population for development of arrhythmias long after LT; thus, routine ECG monitoring following LT is not recommended.
Conclusions
Patients with cirrhosis are at high risk for cardiac disease–related morbidity and mortality, both before and after transplant. As such, significant attention is needed to identify patients at high risk for cardiac events, and to improve management of associated cardiac risk factors surrounding LT. There is a significant knowledge gap in the understanding of the evolution of major cardiac and vascular outcomes in patients undergoing LT, including optimal timing of repeat assessments, especially when considering patients with low versus high MELD scores (Table 3). As such, standardized evidence-based approaches are needed to appropriately risk-stratify patients before transplant and improve identification of cardiac disease and risk for intraoperative and short-term and long-term cardiac events after transplant.
| Research Area | Selected Gaps in Knowledge |
|---|---|
| Risk mitigation |
|
| CAD |
|
| Heart failure |
|
| Arrhythmia |
|
Author Contributions
P.M.B. and L.B.V. were both responsible for the manuscript draft, editing, and oversight.




