ABIDE: An Accurate Predictive Model of Liver Decompensation in Patients With Nonalcoholic Fatty Liver-Related Cirrhosis
Abstract
Background and Aims
Nonalcoholic fatty liver disease (NAFLD) is an increasingly important cause of liver cirrhosis and subsequent complications. We retrospectively developed and validated a model to predict hepatic decompensation in patients with NAFLD and cirrhosis and compared this with currently available models.
Approach and Results
Baseline variables from an international cohort of 299 patients with biopsy-proven NAFLD with compensated cirrhosis were examined to construct a model using competing risk multivariate regression and Akaike/Bayesian information criteria. Validation was performed in 244 patients with biopsy-proven NAFLD cirrhosis from the United States. Prognostic accuracy was compared with the NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4), Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and albumin-bilirubin (ALBI)-FIB-4 score using time-dependent area under the curve (tAUC) analysis. During a median follow-up of 5.6 years (range 2.4-14.1) and 5.4 years (range 1.5-13.8), hepatic decompensation occurred in 81 and 132 patients in the derivation and validation cohorts, respectively. In the derivation cohort, independent predictors of hepatic decompensation (Aspartate aminotransferase/alanine aminotransferase ratio, Bilirubin, International normalized ratio, type 2 Diabetes, and Esophageal varices) were combined into the ABIDE model. Patients with a score ≥4.1 compared with those with a score <4.1 had a higher risk of decompensation (subhazard ratio, 6.7; 95% confidence interval [CI], 4.0-11.2; P < 0.001), a greater 5-year cumulative incidence (37% vs. 6%, P < 0.001), and shorter mean duration to decompensation (3.8 vs 6.7 years, P < 0.001). The accuracy of the ABIDE model at 5 years was good in the derivation (tAUC, 0.80; 95% CI, 0.73-0.84) and validation cohorts (0.78; 95% CI, 0.74-0.81) and was significantly more accurate than the NFS (0.72), FIB-4 (0.74), MELD (0.69), CTP (0.72), and ALBI-FIB-4 (0.73) (all P < 0.001).
Conclusions
In patients with NAFLD and compensated cirrhosis, ABIDE, a predictive model of routine clinical measures, predicts future hepatic decompensation.
Abbreviations
-
- AIC
-
- Akaike information criteria
-
- ALBI
-
- albumin-bilirubin
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BIC
-
- Bayes information criteria
-
- CI
-
- confidence interval
-
- CTP
-
- Child-Turcotte-Pugh
-
- FIB-4
-
- fibrosis-4
-
- HbA1c
-
- hemoglobin A1c
-
- H-L
-
- Hosmer-Lemeshow
-
- INR
-
- international normalized ratio
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NFS
-
- nonalcoholic fatty liver disease fibrosis score
-
- sHR
-
- subhazard ratio
Nonalcoholic fatty liver disease (NAFLD) is recognized as one of the leading causes of chronic liver disease worldwide.(1) The combination of global epidemics of diabetes and obesity has led to NAFLD becoming an increasingly important cause of liver cirrhosis, with predictions that it will become a leading cause of hepatocellular carcinoma (HCC) and liver transplantation.(2) In addition to the health burden of NAFLD, the economic cost is enormous, being related to the high prevalence of the disease with 64 million people in the United States and 52 million in Europe being affected.(1)
Approximately 7%-30% of patients with NAFLD will have nonalcoholic steatohepatitis (NASH).(1, 3-6) Of these, 20%-30% develop liver fibrosis over a 3-6 year period.(4-6) This progression may be modified by the presence of older age, type 2 diabetes, and being overweight/obesity.(7) With the development of cirrhosis, the risk of liver-related complications such as ascites, hepatic encephalopathy or variceal bleeding is 23% and 50% at 3 and 10 years, respectively.(8) The likelihood of developing decompensation once cirrhosis is present varies considerably, with a 10-year cumulative incidence between 30% and 84% in Child-Turcotte-Pugh (CTP) A5 and A6 patients, respectively.(9) The subset of patients with cirrhosis who develop liver decompensation have a reduced quality of life and a greater likelihood of hospitalization. Furthermore, the onset of liver decompensation heralds a dramatically worse outlook, with a median survival of 2 years or less.(10) Thus, predicting the onset of liver decompensation would be useful in estimating future disease burden and prognosis with implications for disease monitoring, prioritization for clinical trials, referral for liver transplantation, and counselling patients regarding their future health.
Noninvasive liver fibrosis tests such as the NAFLD fibrosis score (NFS), Hepascore, and transient elastography were specifically developed to predict fibrosis and can help predict patients with NAFLD at the highest risk of developing liver-related complications.(11-17) The accuracy of these models, however, may be influenced by patient factors including age, body mass index, and diabetes, potentially limiting their prognostic accuracy.(17-19) Furthermore, the majority of these studies did not include or only had small numbers of patients with liver cirrhosis caused by NAFLD who have the greatest risk of adverse outcomes. Therefore, it is currently unknown how to best predict hepatic decompensation among patients with NAFLD and cirrhosis. To fill this knowledge gap, we performed an international multicenter cohort study with the aim of developing and validating a clinical model to predict liver-related complications in patients with biopsy-proven NAFLD with cirrhosis. Secondly, we aimed to compare the predictive accuracy with currently available noninvasive models.
Patients and Methods
Data Source/Study Design and Participants
The derivation cohort for the model originated from the NAFLD progression consortium, which is an international initiative of six centers aiming to study the natural history and prognosis of biopsy-proven NAFLD with advanced fibrosis. The design and outcomes of this multicenter cohort study have been described.(9, 20) Briefly, 458 patients with NASH and bridging fibrosis or cirrhosis were assessed from April 1995 to November 2013. From this cohort, only those with cirrhosis (n = 299) were included, originating from Spain (two centers [n = 184]), Australia (two centers [n = 116]), Hong Kong (one center [n = 82]), and Cuba (one center [n = 76]). Follow-up extended to December 2016 for the derivation cohort.
All patients in the derivation cohort had provided informed consent for their medical information to be used for research with ethics committee approval from the participating centers.
The validation cohort consisted of patients retrospectively identified from Indiana University Hospital with biopsy-proven NASH cirrhosis (n = 244) assessed between April 2004 and November 2016. Follow-up extended to December 2017 for the validation cohort. The study was approved by the local institutional review board with waiver of consent. The majority of patients in the validation cohort have been described in published papers.(21, 22)
Inclusion and Exclusion Criteria
Patients were included on the basis of biopsy-proven NAFLD cirrhosis based on the NASH Clinical Research Network scoring system. The following exclusion criteria were applied to the derivation set: previous history of hepatic decompensation, Model for End-Stage Liver Disease (MELD) score ≥15, CTP ≥7, excess alcohol consumption (average >20 g/day for men or >10 g/day for women in the last 2 years or during follow-up), prior bariatric surgery, secondary causes of NAFLD or other chronic liver diseases, albumin <3.0 g/dL, total bilirubin > 2.0 mg/dL, international normalized ratio (INR) > 1.7, platelets <100,000 mm3, a diagnosis of HCC at inclusion or during first 6 months of follow-up, known human immunodeficiency virus, type 1 diabetes, and life-threatening diseases with reduced life expectancy of less than 12 months. The exclusion criteria for the validation set were similar apart from a higher cutoff for bilirubin (>2.5 mg/dL) and lower cutoff for platelet count (<40,000 mm3) to include a wider spectrum of patients with cirrhosis. All patients included in the study were CTP A with no previous episodes of clinical decompensation. Patients with varices at baseline were included; however, no patient had undergone variceal banding before inclusion.
Outcomes
The primary outcome was the first event of hepatic decompensation, defined by the occurrence of any of the following: ascites (identified or confirmed by abdominal ultrasound), upper gastrointestinal bleeding secondary to portal hypertension (confirmed by endoscopy in the presence of gastroesophageal varices or portal hypertensive gastropathy), or hepatic encephalopathy (established by clinical parameters, neuropsychological tests, or electroencephalogram). If multiple decompensated events occurred in the same patient, the patient was regarded as having one hepatic decompensation, and only the earliest event was adopted for the time-to-event analysis. Outcomes were assessed by local hepatologists after review of the patient clinical history, examination findings, and investigations.
Follow-Up
Patients were followed from the day of liver biopsy until the occurrence of death, liver transplant, or last visit. The outcome was evaluated by an experienced hepatologist in each center every 3-6 months. At each visit, a medical history, physical examination, and standard laboratory tests were performed. All patients underwent initial and subsequent upper gastrointestinal endoscopy following the recommended guidelines for patients with cirrhosis.(23) Additional endoscopies were conducted at the discretion of each investigator. Patients lost to follow-up (n = 13) were censored at the last day they were known to be alive.
Model Variables
The following variables were prospectively collected in the derivation and validation cohorts at baseline at the time of liver biopsy: demographics (age, sex, race/ethnicity), medical history (hypertension, type 2 diabetes, medications), anthropometric variables (weight, body mass index), serum biochemical parameters (fasting glucose, insulin, hemoglobin A1c [HbA1], triglycerides, total cholesterol, high-density and low-density lipoprotein cholesterol, aminotransferases, bilirubin, albumin, INR, creatinine), and others (alcohol consumption and cigarette smoking, presence of gastroesophageal varices). Alcohol consumption was classified into nondrinkers (no alcohol intake) or moderate drinkers (1-70 g/week in women, 1-140 g/week in men). The following scores were computed at baseline: MELD score, CTP score, NFS, fibrosis-4 (FIB-4) score, albumin-bilirubin (ALBI), and ALBI-FIB-4.(24-28)
Statistical Analysis
Baseline characteristics in derivation and validation cohorts were summarized and compared. Values were reported using percentage for categorical variables and as mean and standard deviation for continuous variables. The chi-squared test was applied to categorical variables, and the Wilcoxon signed-ranks test and analysis of variance test were used to compare means between groups.
Model Development
To estimate the association of baseline variables with the first event of hepatic decompensation, competing risk regression models were performed according to the method of Fine and Gray in the derivation cohort. The occurrence of nonliver death was considered as a competing event for hepatic decompensation. Subhazard ratios (sHR) with 95% confidence intervals (CIs) were calculated for each predictor. Those variables with values of P < 0.10 in univariate analysis were entered into a multivariate competing risk regression analysis. Backward stepwise selection procedures were used for variable selection.
For each multivariate regression analysis, the concordance statistics and Bayes information criteria (BIC) and Akaike information criteria (AIC) were used for the selection and final criteria for inclusion of variables in the model, respectively. Both BIC and AIC are measures of likelihood in which lower values indicate a better fit and a penalty is paid for inclusion of a new variable. Thus, this method contributes to select and design not only the most plausible variables but the most parsimonious predictive model.
Model Accuracy
Time-dependent area under the curve (tAUC) in the presence of censored data were used to evaluate model accuracy for the prediction of hepatic decompensation.(29) Model accuracy was estimated at 5 and 10 years of follow-up. The optimal model cut point was selected using the Youden and Liu criterion.(30) After model cut point selection, the cumulative incidence of hepatic decompensation for each group was reported using the cumulative incidence function after competing risk regression analysis. Differences between groups were reported by the Fine and Gray method.
Model Validation
The accuracy of the model developed in the derivation set was assessed using time-dependent receiver operating curves at 5 and 10 years of follow-up. The cumulative incidence of hepatic decompensation at 5 and 10 years was reported according to decile cut points of the model and compared using the Fine and Gray method.
Model Calibration
Two methods were used to assess model calibration (the extent to which the predicted probabilities and actual probabilities agree): the Hosmer-Lemeshow (H-L) calibration statistic as modified by D’Agostino and Nam(31) and the calibration slope.(32) H-L is a measure of calibration, and ideally no significant differences should exist between observed and predicted probabilities. Calibration slope is a robust method for censoring and ideally takes a value of 1. Both methods were calculated and reported in both derivation and validation cohorts.
Overall Model Performance
Overall model performance was tested using the Brier score, which integrates discrimination and calibration at the same time and reflects the mean squared difference between the real outcome and the predicted probability.(32) Additionally, the accuracy of the model was compared with MELD, CTP, NFS, FIB-4, ALBI, and ALBI-FIB-4 scores using tAUC values. To explore the performance of the model in different settings, subgroup analysis (diabetes vs. no diabetes, age <55 vs. age ≥55, female vs. male, presence or absence of gastroesophageal varices, use of beta blockers at baseline) was conducted in the whole cohort (n = 543). Age 55 was chosen as the median age of the entire cohort.
Statistical analyses were carried out using STATA software, release 14.2. All CIs, significance tests, and resulting P values were two-sided, with an alpha level of 0.05.
Results
Derivation and Validation Cohorts: Participant Overview
Of 512 participants assessed for eligibility in the derivation cohort, 299 patients with compensated cirrhosis fulfilled the inclusion criteria, whereas 244 of 346 patients were eligible in the validation cohort. A flow diagram outlining the patient selection is shown in Fig. 1.
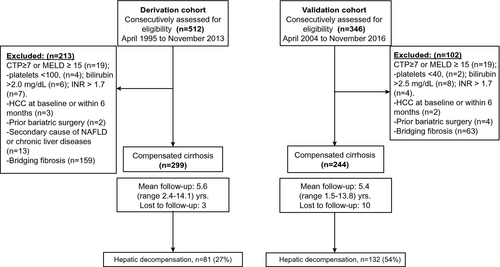
At baseline, patients in the derivation cohort were slightly older compared with those in the validation cohort (56.8 vs. 54.2 years, P < 0.001) and were less likely to be White (60.5% vs. 100%), as shown in Table 1. HbA1c levels and the prevalence of diabetes were similar between groups, whereas the validation cohort had higher baseline total cholesterol and low-density lipoprotein cholesterol levels. In comparison with the derivation cohort, patients in the validation cohort were more likely to have a history of hypertension (84% vs. 62%), esophageal varices (43% vs. 28%), and higher body mass index (37.2 vs. 32.1 kg/m2) and levels of fasting glucose (164 vs. 146 mg/dL). Liver function and MELD and CTP scores were similar between cohorts. Baseline characteristics by country of enrollment are shown in Supporting Table S1.
| Variables | Derivation Cohort (n = 299) | Validation Cohort (n = 244) | PValue | ||
|---|---|---|---|---|---|
| n or Mean | % or SD | n or Mean | % or SD | ||
| Age, years | 56.8 | 11.4 | 54.2 | 10.7 | <0.001 |
| Sex, male | 139 | 46.4 | 105 | 43.0 | 0.43 |
| Race | — | ||||
| White | 181 | 60.5% | 244 | 100% | |
| Latino | 66 | 22.0% | 0 | 0 | |
| Asian | 48 | 16.0% | 0 | 0 | |
| Black | 4 | 1.3% | 0 | 0 | |
| Type 2 diabetes | 212 | 70.9% | 176 | 72.0% | 0.41 |
| History of hypertension | 184 | 61.5% | 204 | 83.6% | <0.001 |
| Current smoker | 52 | 17.3% | 53 | 22.0% | 0.32 |
| Alcohol | 0.46 | ||||
| Nondrinkers | 250 | 83.6% | 208 | 85.2% | |
| Moderate drinkers | 49 | 16.4% | 36 | 14.8% | |
| Statin therapy | 93 | 31.1% | 91 | 37.3% | 0.07 |
| BMI (kg/m2) | 32.1 | 7.0 | 37.2 | 7.4 | <0.001 |
| MELD score | 8.2 | 2.1 | 8.5 | 2.3 | 0.11 |
| CTP score | 0.07 | ||||
| A5 | 222 | 74.2% | 195 | 80.0% | |
| A6 | 77 | 25.8% | 49 | 20.1% | |
| Gastroesophageal varices at baseline | 85 | 28.4% | 105 | 43.1% | <0.01 |
| Total bilirubin (mg/dL) | 0.95 | 0.79 | 0.95 | 0.52 | 0.99 |
| Albumin (g/L) | 4.0 | 0.42 | 3.8 | 0.43 | 0.87 |
| INR | 1.10 | 0.18 | 1.12 | 0.22 | 0.25 |
| Platelets (×109/L) | 167 | 64 | 153 | 80 | 0.10 |
| Cholesterol (mg/dL) | 176 | 52.6 | 167 | 46.3 | 0.05 |
| HDL (mg/dL) | 43.9 | 11.4 | 40.1 | 14.5 | <0.001 |
| LDL (mg/dL) | 103.3 | 45.7 | 93.7 | 35.1 | 0.01 |
| Creatinine (mg/dL) | 0.92 | 0.46 | 0.89 | 0.42 | 0.42 |
| ALT (U/L) | 62.7 | 77.6 | 44.3 | 31.7 | <0.001 |
| AST (U/L) | 61.3 | 87.2 | 52.5 | 27.5 | 0.13 |
| AST/ALT ratio | 1.10 | 0.45 | 1.33 | 0.48 | <0.001 |
| Fasting glucose (mg/dL) | 146 | 70.4 | 164 | 64.2 | 0.002 |
| Hb1A1c | 7.1 | 2.0 | 7.0 | 64.2 | 0.82 |
| NFS | 0.551 | 1.51 | 0.558 | 1.52 | <0.001 |
| FIB-4 | 3.1 | 2.24 | 3.5 | 2.13 | <0.001 |
| ALBI | −2.37 | 0.42 | −2.81 | 0.38 | <0.001 |
| ALBI-FIB-4 | −3.18 | 0.04 | −2.62 | 0.05 | <0.001 |
Note:
- Quantitative data are expressed as mean ± SD. P values for the comparison between groups. Wilcoxon signed rank test and analysis of variance test for mean comparison between groups.
- Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
During a median follow-up of 5.1 (range 2.6-8.4) and 5.4 years (range 4.5-8.8), 81 patients (27%) and 132 (54%) developed an initial hepatic decompensation event in the derivation and validation cohorts, respectively. Three patients in the derivation cohort and 15 in the validation cohort died of non-liver-related causes and were analyzed as competing events. Specific types of liver complications were ascites (n = 60), variceal hemorrhage (n = 16), and encephalopathy (n = 5) in the derivation cohort and ascites (n = 69), encephalopathy (n = 40), and variceal hemorrhage (n = 23) in the validation cohort.
Predictors of Hepatic Decompensation
The following baseline variables (outlined in Table 2) were associated with future hepatic decompensation in the derivation cohort (n = 299) using univariate competing risk regression analysis: White and Latino ethnicity, presence of esophageal varices, type 2 diabetes, INR, albumin, total bilirubin, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, and platelet levels. Multivariate analysis confirmed the presence of esophageal varices (sHR, 2.0; 95% CI, 1.18-3.61; P < 0.001), type 2 diabetes (sHR, 2.2; 95% CI, 1.20-3.58; P < 0.005), INR (sHR, 6.6; 95% CI, 2.87-10.4; P < 0.001), total bilirubin (sHR, 1.3; 95% CI, 1.14-1.67; P < 0.005), and AST/ALT ratio (sHR, 2.2; 95% CI, 1.50-3.45; P < 0.001) as independent predictors of hepatic decompensation.
| Variable | Derivation Cohort (n = 299) | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| sHR (95% CI) | P Value | sHR (95% CI) | P Value | ||
| Race/ethnicity | |||||
| Asian | Ref* | ||||
| White | 3.52 (0.99-12.4) | 0.051 | 1.44 (0.37-5.53) | 0.595 | |
| Latino | 5.64 (1.78-17.8) | 0.003 | 2.60 (0.77-8.78) | 0.122 | |
| Age, years | 0.99 (0.97-1.00) | 0.339 | — | — | |
| Sex (male vs. female) | 1.02 (0.67-1.57) | 0.895 | — | — | |
| Esophageal varices (yes) | 3.54 (2.30-5.45) | <0.001 | 2.08 (1.16-3.61) | <0.001 | |
| Currently smoking (yes) | 0.91 (0.68-1.20) | 0.522 | — | — | |
| BMI (kg/m2) | 0.99 (0.95-1.03) | 0.676 | — | — | |
| Hypertension (yes) | 0.87(0.56-1.34) | 0.541 | — | — | |
| Type 2 diabetes (yes) | 2.65 (1.23-2.76) | <0.005 | 2.25 (1.20-3.58) | <0.005 | |
| Fasting glucose (mg/dL) | 1.00 (0.99-1.00) | 0.710 | — | ||
| HbA1c (%) | 1.01(0.92-1.11) | 0.699 | — | ||
| Cholesterol (mg/dL) | 0.99 (0.99-1.00) | 0.449 | — | — | |
| Statin therapy (yes) | 0.68 (0.42-1.09) | 0.115 | — | — | |
| INR | 6.90 (2.53-9.74) | <0.001 | 6.66 (2.87-10.4) | <0.001 | |
| Albumin (g/dL) | 0.32 (0.15-0.49) | <0.001 | 0.84 (0.40-1.99) | 0.786 | |
| Total bilirubin (mg/dL) | 1.46 (1.19-1.77) | <0.005 | 1.32 (1.14-1.67) | <0.005 | |
| Creatinine | 0.76 (0.41-1.39) | 0.376 | — | — | |
| AST/ALT | 3.09 (2.27-4.19) | <0.001 | 2.28 (1.50- 3.45) | <0.001 | |
| Platelets (×109/L) | 0.98 (0.98-0.99) | <0.001 | 0.99 (0.99-1.00) | 0.922 | |
- * Ref means reference for comparison of the variable.
- Bold values represents significant association of a variable with the outcome.
- Abbreviations: BMI, body mass index; sHR: subhazard ratio.
Model Development
The contribution of each variable identified on multivariate analysis to predict hepatic decompensation was assessed using tAUC and information criterion (see Table 3). Each variable incrementally increased the model accuracy to a maximum tAUC of 0.79 when all five variables were included. The combination of five variables also showed the lowest information criterion (AIC 395 and BIC 406), demonstrating that the model was parsimonious and not overfitted. The final model (named ABIDE) consisted of AST/ALT ratio, Bilirubin, INR, type 2 Diabetes, and Esophageal varices, with a formula of (2.003 × INR + 0.824 × AST/ALT ratio + 0.821 × [Type 2 diabetes: 0 if absent, 1 if present] + 0.806 × [esophageal varices: 0 if absent, 1 if present] + 0.332 × total bilirubin).
| Variable | Regression Coefficient | tAUC | AIC | BIC |
|---|---|---|---|---|
| INR | 2.003 | 0.71 | 432 | 435 |
| + AST/ALT | 0.824 | 0.74 | 422 | 428 |
| + Type 2 diabetes mellitus | 0.821 | 0.76 | 403 | 412 |
| + Esophageal varices | 0.806 | 0.77 | 399 | 410 |
| + Total bilirubin | 0.332 | 0.79 | 395 | 406 |
Model Accuracy and Cut-Offs
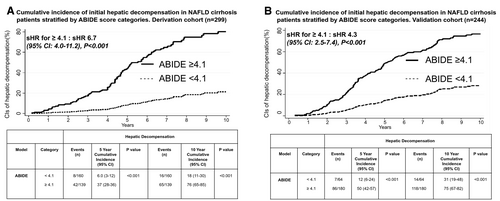
The accuracy of the ABIDE model in the derivation cohort to predict events at 5 years was good (tAUC, 0.80; 95% CI, 0.73-0.84) and similar at 10 years (tAUC, 0.78; 95% CI, 0.71-0.83), shown in Table 4 and Fig. 2. Both Youden and Liu criterion identified the optimal cut point as 4.1 based on sensitivity and specificity of 79% and 85%, respectively. Patients with an ABIDE score ≥4.1 had a higher risk (sHR, 6.7; 95% CI, 4.0-11.2; P < 0.001; Fig. 3A) and higher 5-year cumulative incidence of hepatic decompensation compared with those patients with a score <4.1 (37% vs. 6%, P < 0.001). The 10-year cumulative incidence remained divergent (76% vs. 18%, P < 0.001), as shown in Fig. 3A. The mean time to liver-related complications in the derivation cohort was 3.8 years (95% CI, 3.5-4.1) and 6.7 years (95% CI, 5.1-5.9) in patients with ABIDE scores ≥4.1 and <4.1, respectively (P = 0.01). The cumulative incidence of hepatic complications increased with each ABIDE score decile with a 2.1% 5-year incidence in the lowest decile (<2.99), compared with 58.0% in the highest decile (>5.44), as outlined in Supporting Table S2 and Fig. 4.
| 5-Year Prediction of Hepatic Decompensation | ||||
|---|---|---|---|---|
| Scores | Derivation Cohort (n = 299) | Validation Cohort (n = 244) | ||
| tAUC (95% CI) | P Value vs. ABIDE | tAUC (95% CI) | P Value vs. ABIDE | |
| ABIDE | 0.80 (0.73-0.84) | — | 0.78 (0.74-0.81) | — |
| Versus NFS | 0.72 (0.66-0.78) | P < 0.001 | 0.73 (0.67-0.79) | P < 0.001 |
| Versus FIB-4 | 0.74 (0.70-0.79) | P < 0.001 | 0.74 (0.68-0.80) | P = 0.033 |
| Versus ALBI | 0.72 (0.62-0.84) | P < 0.001 | 0.69 (0.62-0.76) | P < 0.001 |
| Versus ALBI-FIB-4 | 0.73 (0.66-0.79) | P < 0.001 | 0.72 (0.60-0.83) | P < 0.001 |
| Versus CTP | 0.72 (0.64-0.79) | P < 0.001 | 0.67 (0.63-0.75) | P < 0.001 |
| Versus MELD | 0.69 (0.66-0.75) | P < 0.001 | 0.68 (0.62-0.80) | P < 0.001 |
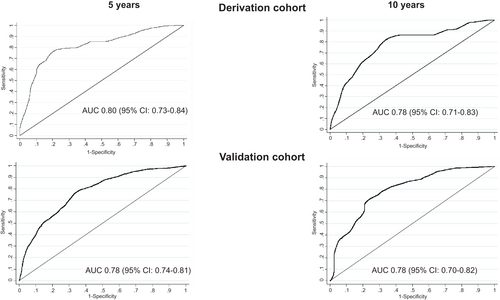

Model Validation
In the validation cohort (n = 244), the accuracy of the ABIDE model remained satisfactory at 5 years (tAUC, 0.78; 95% CI, 0.73-0.84) and 10 years (tAUC, 0.76; 95% CI, 0.70-0.82), as shown in Table 4, Fig. 2, and Supporting Table S3. Patients with a score ≥4.1 had a higher risk of hepatic decompensation (sHR, 4.3; 95% CI, 2.5-7.4; P < 0.001), whereas those with a score <4.1 had minimal risk (sHR, 0.22; 95% CI, 0.13-0.39; P < 0.001), as shown in Fig. 3B. Time to hepatic decompensation was shorter in those with ABIDE score ≥4.1 (mean 4.2 years; 95% CI, 3.9-4.6) compared with those with ABIDE score <4.1 (mean 6.5 years; 95% CI, 4.6-5.3; P = 0.03).
Comparison With Other Models and Sensitivity Analysis
Other noninvasive fibrosis models (NFS, FIB-4) and prognostic cirrhosis models (MELD, CTP, ALBI, and ALBI-FIB-4) were also predictive of future decompensation (Supporting Figs. S2-S11). However, the ABIDE model had better discriminative power at 5 and 10 years compared with NFS, FIB-4 and also when compared with MELD, CTP, ALBI and ALBI-FIB-4 scores, in both the derivation and validation cohorts (Table 4). Notably, less than 10% of the validation cohort had low FIB-4 or NFS scores, demonstrating a limited ability to discriminate individuals with favorable prognosis, whereas <5% of the cohort had a high ALBI score, demonstrating limited ability to detect individuals who were at greatest risk of decompensation.
The accuracy of the ABIDE score was not impacted by the presence of type 2 diabetes, age, or sex. The accuracy of the model was marginally lower in the presence of varices (Supporting Table S4). A subanalysis in patients with varices showed equivalent accuracy of the model at 5 and 10 years in patients both with and without beta blockers at baseline (Supporting Table S5).
Model Calibration and Performance
Using the H-L goodness-of-fit test, the model calibrated well with no differences between the observed and predicted probability of developing hepatic decompensation at 10 years in both derivation (H-L 11.0; P = 0.23) and validation (H-L 6.73; P = 0.56) cohorts. Additional calibration (calibration slope, calibration plot) and overall accuracy of the model by means of the Brier score were also evaluated (Supporting Fig. S1). The ABIDE model demonstrated good calibration in both the derivation (calibration slope, 1.08) and validation cohorts (calibration slope, 1.00). Furthermore, the Brier score was 0.12 and 0.07 for derivation and validation cohorts, respectively, showing satisfactory overall performance of the model.
Discussion
In clinical practice, risk stratification of patients affected by chronic liver disease is important because transition from a compensated to a decompensated state is associated with a dramatic reduction in survival as well as significant increases in health care utilization. Based on data from two independent cohorts from multiple centers and countries, we have developed and validated a predictive model of hepatic decompensation in patients with NAFLD and compensated cirrhosis. The model will be available as a web-based calculator at http://gihep.com/. The ABIDE model has good predictive accuracy for hepatic decompensation at both 5-year and 10-year time points. The model discriminated and calibrated well and was more accurate at predicting future hepatic decompensation in comparison with two noninvasive fibrosis markers (NFS and FIB-4) and two established prognostic scores in liver cirrhosis (MELD and CTP).
Accurate methods to predict future liver decompensation in patients with cirrhosis have been widely sought.(11, 16, 33-35) Standard liver function tests have limited accuracy, and thus, alternative methods have been examined, including magnetic resonance elastography, hepatic-wedge pressure gradient, and genetic risk scores; however, these have been limited by their invasiveness, complexity, or cost.(34, 36) The ABIDE model is composed of simple and readily available parameters (AST/ALT ratio, bilirubin, INR, diabetes, and presence of esophageal varices). Not surprisingly, several studies have shown that bilirubin and INR (indicative of liver function) and esophageal varices (a marker of portal hypertension) act as independent predictors of hepatic decompensation in patients with chronic liver disease.(8, 37-39) For example, among patients with cirrhosis caused by chronic hepatitis C infection, the 6-year cumulative incidence of hepatic decompensation increases from 26% to 66% once varices are present.(39) We found the presence of varices were associated with a 2-fold increase in risk in our cohort of patients with NAFLD cirrhosis, which was increased to a 4-6–fold increase when combined with the other covariates within the ABIDE model.
Type 2 diabetes was included in the final model as an important independent predictor of a future hepatic decompensation. Diabetes and glucose intolerance among patients with NAFLD has been described as increasing the risk of overall and liver-related death(40, 41); however, our data extend these observations to the subset of patients with NAFLD-related cirrhosis. Notably, the presence of type 2 diabetes has been noted to increase the risk of liver-related death or decompensation in other liver diseases, including chronic hepatitis B and C and alcohol-associated liver disease.(42-48) This raises the possibility that the ABIDE score may also be accurate in predicting outcomes in patients with cirrhosis caused by non-NAFLD etiologies. Further validation studies will be needed to examine this hypothesis.
The NFS and FIB-4 were developed to detect advanced liver fibrosis and have been demonstrated to predict future liver-related events in patients with NAFLD.(11) However, once patients are identified as being “at risk” on the basis of their fibrosis stage (i.e., as having cirrhosis), we found that the accuracy of these noninvasive fibrosis tests was inferior to the ABIDE score. We additionally compared the ABIDE model with established prognostic scores in patients with liver cirrhosis, namely MELD and CTP scores. Although these scores are accurate predictors of mortality outcomes across the severity spectrum of cirrhosis,(49, 50) we found that they had limited prognostic ability when restricted to patients with compensated cirrhosis and were not superior to ABIDE in the prediction of hepatic decompensation at either 5 or 10 years. Although the ABIDE score shares similarities with both CTP, ALBI, ALBI-FIB-4, and MELD scores (namely bilirubin and INR), the inclusion of other important independent predictors of hepatic decompensation (type 2 diabetes, esophageal varices) in NAFLD cirrhosis increased the prognostic significance of our model.
In clinical practice, ABIDE may be a useful tool to assess the initial risk of hepatic decompensation following a diagnosis of cirrhosis in patients with NAFLD. Very low scores (<3.43) in the bottom quintile were associated with <5% risk of hepatic decompensation at 5 years, whereas a score in the top quintile (>4.92) was associated with >50% chance of decompensation at 5 years and almost 100% at 10 years. This information is important in counselling patients regarding their prognosis and may influence monitoring strategies or treatment of other comorbid medical conditions. Furthermore, this information has implications for the design of phase-three clinical trials in NAFLD, in which liver decompensation is a current long-term endpoint.
Our study has a number of strengths: the model design was based on a large international multicenter cohort of patients with biopsy-proven NAFLD and liver cirrhosis. The patients have a long-term follow-up with sufficient events, and few were lost during the study period. The variables included in the model were based on competing risk analysis, and the validity of the predictive model was confirmed in a large independent cohort. This study, however, is not exempt of limitations: our cohorts included only histologically confirmed cirrhosis, which provided robustness to the diagnosis but may bias toward atypical or more severe disease. Further external validation of the accuracy of ABIDE will be useful to determine its generalizability. In addition, the retrospective nature of the data collection, particularly for the validation cohort, may introduce selection bias; however, we feel that the comprehensive nature of the data capture from medical and pharmacy records with negligible missing data minimizes this possibility. Lastly, increasing the use of Baveno VI criteria may reduce the endoscopic assessment of varices, limiting availability of this model covariate. However, given the high negative predictive value of a liver stiffness measurement <20 kPa and platelet count >150/mm3, it is possible that this may provide equivalent accuracy to an endoscopic assessment. Further study is required to explore this.
In conclusion, the prediction of complications related to liver cirrhosis in patients with NAFLD may be accurately predicted using a scoring system including routine clinical measures. The calculation of risk of long-term liver-related complications using the ABIDE model has relevance to patients and their clinicians and can be readily incorporated into clinical practice.
Acknowledgment
The preliminary results of this study were partially presented in the annual meeting of the American Association for the Study of Liver Diseases on November 11, 2018, in San Francisco, CA, USA. We express our gratitude to Timothy Imler for the design and implementation of the web-based calculator tool.
Author Contributions
L.A.A., L.C.-B., E.V.-G., V.W.-S.W., J.G., M.R.-G., N.C., R.A.F., M.C., and G.L.-H.W. were responsible for the concept and design. All authors were responsible for the acquisition and interpretation of data. L.C.-B. was responsible for analysis. L.C.-B., E.V.-G., and L.A.A. were responsible for drafting of the manuscript. L.A.A., V.W.-S.W., J.G., M.R.-G., L.C.-B., G.P.J., A.P.D., E.V.-G., and N.C. were responsible for critical revision of the manuscript for important intellectual content.




