HLA-B*35:01 and Green Tea–Induced Liver Injury
Abstract
Background and Aims
Herbal supplements, and particularly multi-ingredient products, have become increasingly common causes of acute liver injury. Green tea is a frequent component in implicated products, but its role in liver injury is controversial. The aim of this study was to better characterize the clinical features, outcomes, and pathogenesis of green tea-associated liver injury.
Approach and Results
Among 1,414 patients enrolled in the U.S. Drug-Induced Liver Injury Network who underwent formal causality assessment, 40 cases (3%) were attributed to green tea, 202 to dietary supplements without green tea, and 1,142 to conventional drugs. The clinical features of green tea cases and representation of human leukocyte antigen (HLA) class I and II alleles in cases and control were analyzed in detail. Patients with green tea–associated liver injury ranged in age from 17 to 69 years (median = 40) and developed symptoms 15-448 days (median = 72) after starting the implicated agent. The liver injury was typically hepatocellular (95%) with marked serum aminotransferase elevations and only modest increases in alkaline phosphatase. Most patients were jaundiced (83%) and symptomatic (88%). The course was judged as severe in 14 patients (35%), necessitating liver transplantation in 3 (8%), but rarely resulting in chronic injury (3%). In three instances, injury recurred upon re-exposure to green tea with similar clinical features, but shorter time to onset. HLA typing revealed a high prevalence of HLA-B*35:01, found in 72% (95% confidence interval [CI], 58-87) of green tea cases, but only 15% (95% CI, 10-20) caused by other supplements and 12% (95% CI, 10-14) attributed to drugs, the latter rate being similar to population controls (11%; 95% CI, 10.5-11.5).
Conclusions
Green tea–related liver injury has distinctive clinical features and close association with HLA-B*35:01, suggesting that it is idiosyncratic and immune mediated.
Abbreviations
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CI
-
- confidence interval
-
- DILI
-
- drug-induced liver injury
-
- DILIN
-
- Drug-Induced Liver Injury Network
-
- eMERGE
-
- the Electronic Medical Records and Genomics Network
-
- HDS
-
- herbal and dietary supplement
-
- HLA
-
- human leukocyte antigen
-
- INR
-
- international normalized ratio
-
- LD
-
- linkage disequilibrium
-
- LT
-
- liver transplantation
Herbal and dietary supplements have become common causes of drug-induced liver injury (DILI) in the United States and now are implicated in 15%-20% of cases.(1, 2) This change coincides with the increasing use of herbal medications,(3) particularly for conditions such as obesity, fatigue, and complications of aging that are poorly responsive to conventional drugs.
From a regulatory standpoint, herbal supplements are considered “generally safe” and are not regulated as drugs or subjected to rigorous assessment of efficacy and safety.(4, 5) In the past, herbal supplements were typically single botanical entities, such as ginseng, saw palmetto, or turmeric, which are widely used and have a long history of safety. A recent change has been the popularity of multi-ingredient products, containing mixtures of botanicals, vitamins, minerals, and other nutrients. In ongoing analyses of dietary supplement–associated liver injury from the U.S. Drug-Induced Liver Injury Network (DILIN), multi-ingredient products accounted for 68% of implicated dietary supplements, whereas single-component botanicals accounted for only 16%.(3) In addition, chemical analyses show that product labels of these multi-ingredient products are often inaccurate: many containing unlisted substances and not containing many of those that are listed.(6, 7)
Green tea (Camellia sinensis) is a frequent component in multi-ingredient products and has been implicated in several dozen instances of liver injury.(8-18) These reports were initially viewed with skepticism,(11, 19) given that green tea is one of the most frequently consumed beverages worldwide and has been used for centuries without known ill effects. Indeed, epidemiological surveys suggest that drinking green tea may have health benefits,(19-21) and in vivo studies in animal models show that it may protect against hepatotoxicity.(22) Importantly, most reports on hepatotoxicity have implicated tablets or capsules with green tea extract rather than its ingestion as a beverage. Aqueous/alcohol extracts from leaves of C. sinensis concentrate the polyphenolic catechins of green tea leaves and remove other components such as caffeine. The catechins in a single commercial tablet labeled as green tea extract may be equivalent to 3-10 cups of green tea.(23) Epigallocatechin gallate (EGCG) is the most common catechin in green tea and is widely believed to be responsible for liver injury.(19, 24, 25)
The aim of the current study was to better characterize the frequency, risk factors, clinical features, outcomes, and pathogenesis of green tea–associated liver injury. Using a large, prospective U.S. database, we have analyzed cases of dietary supplement–associated liver injury for the role of green tea and describe the clinical features of the injury as well as results of human leukocyte antigen (HLA) typing of patients and chemical analysis of implicated products.
Materials and Methods
The DILIN is a National Institutes of Health–funded, prospective study of incident cases of suspected DILI presenting at five to eight medical centers in the United States(26) (Supporting Table S1). The clinical protocols and methods of the DILIN have been described in detail.(26-28) In brief, patients presenting with suspected DILI are offered enrollment in a prospective, observational study (NCT00345930). After informed consent, patients undergo medical history, physical examination, and chart review for relevant data on the liver injury. Blood samples are obtained for routine liver tests and storage for future mechanistic studies. If a liver biopsy was done as a part of clinical care, unstained slides are requested for standardized central reading.(29) Starting in 2009, patients were asked to provide samples of the implicated supplements, which were sent to a repository, entered into a database, photographed, and aliquoted.(6) All details of the DILIN Prospective Study were approved by institutional review boards at participating centers and by an independent data safety and monitoring board established by the National Institute of Diabetes and Digestive and Kidney Diseases to supervise the study.
For this analysis, all cases of dietary supplement–related liver injury were reviewed for evidence from the medical history and product labels that green tea was taken. When the product label was not available, the ingredients were sought from the Dietary Supplement Label Database(30) or the Internet. All cases with suspected exposure were then re-adjudicated specifically for green tea causality using the procedures established by the DILIN, in which three investigators review the narrative history and case-report forms and score the likelihood that green tea played a role in causation, as 1 (definite, >95% likelihood), 2 (highly likely, 75%-95% likelihood), 3 (probable, 50%-74%), 4 (possible, 25%-49%), or 5 (unlikely, <25%).(26) The investigators based the scores on clinical information only, without knowledge of the chemical analyses or genetic results. Severity was graded using pre-established criteria: 1+ or mild indicating anicteric liver injury (bilirubin <2.5 mg/dL); 2+ moderate indicating jaundice but without hospitalization; 3+ moderate indicating jaundice and hospitalization; 4+ severe indicating jaundice and signs of liver failure (such as international normalized ratio [INR], >1.5); and 5+ fatal indicating death or urgent liver transplantation (LT) within 6 months of onset.(27)
Cases scored as definite, highly likely, or probable liver injury due to green tea were assessed for demographic, clinical, biochemical, and histological features(29) and for the chemical constituents found on testing product samples. Chemical analysis was performed at the National Center for Natural Products Research of the University of Mississippi (Oxford, MS) using ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry.(6)
To investigate HLA associations, patients who consented for genetic research had high-resolution class I and II HLA sequencing done on DNA extracted from whole blood using the Illumina MiSeq platform at the Vanderbilt University Medical Center Immunogenomics, Microbial Genetics and Single Cell Technologies core.(31) Two disease control groups were used: (1) patients enrolled in the DILIN with liver injury related to dietary supplements without green tea and (2) patients with liver injury attributed to conventional drugs. For population controls, the Illumina Human660W-Quad chip genotype data from the Electronic Medical Record and Genomics Study (eMERGE) network (phs000360.v3.p1) were obtained from dbGaP.(32) The eMERGE cohort consisted of persons residing in the United States of diverse racial and ethnic background, but with predominantly Americans of European ancestry. The chromosome 6 single-nucleotide polymorphisms in the major histocompatibility region were used to impute four-digit HLA alleles using the HLA Genotype Imputation with Attribute Bagging program.(33)
Statistical Analysis
Analysis of HLA class I and II associations between green tea cases, and each control group was conducted using Fisher’s exact tests. To correct for multiple testing, top alleles were selected based on a false discovery rate of <0.05.(34) The major associations were also examined separately for European-American and Hispanic subgroups of the green tea cases and population controls from eMERGE. Statistical analyses used SAS (version 9.4; SAS Institute Inc., Cary, NC) and R software (R Foundation for Statistical Computing, Vienna, Austria). Allele frequencies were also compared to those of large, ethnic-matched U.S. population cohorts reported in public databases.(35)
Results
Among 1,987 patients enrolled in the DILIN Prospective Study between September 2004 and April 2018, 1,768 had undergone follow-up and causality assessment, of which 1,414 were judged as probable, highly likely, or definite DILI. Of these verified cases, 272 (19%) were attributed to dietary supplements and 1,142 to conventional drugs (Fig. 1). Review of product labels of implicated agents for mention of green tea or its component catechins identified 70 patients as possibly having taken at least one product containing green tea.
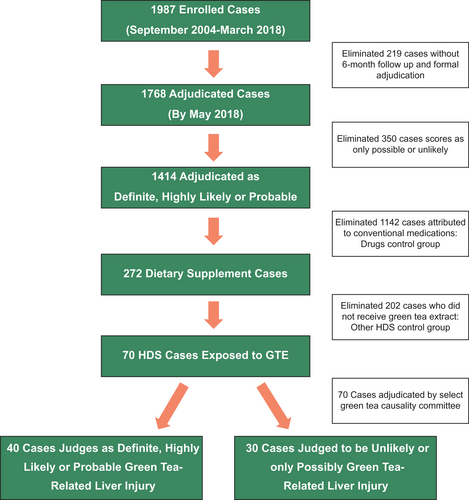
Formal adjudication of the 70 potential cases by the green tea causality assessment committee scored 3 as definite, 11 as highly likely, and 26 as probable green tea related. The 30 cases scored as only possible or unlikely included 7 for which further analysis could not demonstrate that green tea was taken, whereas in the remaining cases, alternative diagnoses were judged more or equally likely.
The demographic, clinical, and biochemical features of the 40 cases of green tea–related liver injury are given in Table 1. Three patients had a previous history of liver injury after taking a green tea–containing supplement, 2 of whom were enrolled in the DILIN twice, the second episode 1-4 years after the initial one. Thus, there were 40 episodes of liver injury in 38 different patients. Patients’ median age at time of onset was 40 years, 74% were women, most were white, and 36% were of self-reported Hispanic ethnicity. The primary implicated product was green tea extract alone in eight (20%) and a multi-ingredient product in 32 (80%) instances. The multi-ingredient supplements had 4-60 active ingredients (median = 11.5) and only seven (22%) had “green tea” in their commercial names (Supporting Table S2). Green tea was present as an extract in tablet or capsule form in all products except two, one of which was provided as a powder to make tea and the other as a liquid spray. The stated reason for taking the supplement was weight loss in 29 (73%), boosting energy in 4 (10%), promoting “wellness” in 2 (5%), body building in 2 (5%), and miscellaneous or unknown in 3 patients. Nine patients (23%) were found to be taking two separate green tea–containing products. Only 17 products (from 15 patients) had labels that provided the dose of green tea extract per serving. Total estimated daily doses ranged from 50 to 2,000 mg (median = 800). In this small subset, there was no clear correlation between total estimated daily intake and severity of injury as assessed by severity scores or peak bilirubin levels (data not shown).
| Feature | Median (Range) or No. (%) |
|---|---|
| Age, years | 40 (17-69) |
| Sex, female | 28/38 (74) |
| Race, white | 26/38 (70) |
| Hispanic ethnicity | 13/37 (36) |
| Multi-ingredient supplement | 32 (80) |
| Weight loss indication | 29 (73) |
| Symptoms, any | 38 (95) |
| Jaundice | 33 (83) |
| Rash | 5 (13) |
| Fever | 3 (8) |
| Eosinophilia (>500/µL) | 1 (3) |
| Initial ALT, U/L | 1,621 (396-4,185) |
| Initial AST, U/L | 1,298 (162-3,400) |
| Initial ALP, U/L | 155 (80-550) |
| Initial total bilirubin, mg/dL | 7.0 (0.4-30.1) |
| Peak total bilirubin, mg/dL | 13.6 (0.6-46.0) |
| Peak INR | 1.2 (1.0-7.0) |
| Antinuclear or smooth muscle antibody | 18/39 (46) |
| Initial R ratio | 24.0 (3.3-67.0) |
| Hepatocellular (R > 5) | 38 (95) |
| Mixed (R, 2-5) | 2 (5) |
| Cholestatic (R < 2) | 0 (0) |
| Severity: mild (1+: anicteric) | 5 (13) |
| Moderate (2+ or 3+: jaundice) | 21 (53) |
| Severe (4+ or 5+: INR > 1.5) | 14 (32) |
| Chronicity | 1/29 (3) |
- Two patients were enrolled twice, having two episodes of liver injury after separate exposures to green tea. All results are median (range) or number (proportion) with denominator given when there are missing data. Severity was scored in the DILIN Prospective Study as mild (1+, anicteric), moderate (2+, jaundiced or 3+, jaundiced and hospitalized), or severe (4+, jaundiced and INR > 1.5) or fatal (5+, death from liver failure or LT within 6 months of onset). R = alanine aminotransferase ÷ alkaline phosphatase, both expressed as multiples of the upper limit of the normal range.
Laboratory results demonstrated marked elevations in serum alanine (ALT) and aspartate aminotransferase (AST) levels, with only modest increases in alkaline phosphatase (ALP) levels so that all except 2 subjects (95%) had a hepatocellular pattern of enzyme elevations; 2 subjects having a “mixed” and none a cholestatic pattern.(25) Immunological features were not prominent; only 5 patients reported rash and 3 fever with none having both, and only 1 subject had peripheral eosinophilia, which was mild (7.3%; 511/µL). Severity of liver injury was scored as mild in 5 (13%), moderate in 21 (52%), and severe in 14 (35%) cases; 3 of the latter developing hepatic failure and undergoing LT. No patient died. Follow-up of 4-12 months after onset was available for 29 patients, 28 of whom had normal liver tests, whereas 1 had a modest increase in ALT levels (57 U/L) 10 months after onset. Overall, green tea accounted for 15% of dietary supplement–related and 3% of all cases in the DILIN Prospective Study.
Selected clinical and laboratory features of patients with green tea injury are compared to those with liver injury attributable to other herbal and dietary supplement (HDS) products (n = 202) and those with injury attributed to a conventional medication (n = 1,142) in Supporting Table S3. Patients with green tea injury differed from the other groups in being younger than patients with DILI, more likely to be women than the other HDS cases and somewhat more likely to be Hispanic compared to both groups. These differences were probably related to differences in reasons for taking the products—for weight loss rather than a specific medical condition. Most strikingly, the green tea cases were mostly hepatocellular (95%) and none were cholestatic, whereas other HDS cases and those attributable to drugs included more cholestatic (22% and 24%) and mixed cases (25% and 23%). Severity and outcome of green tea cases were somewhat worse than those of other HDS- and drug-related cases, but evidence for chronicity was less common in green tea cases (3%), particularly in comparison to those attributable to conventional drugs (18%).
Liver biopsies from 15 patients (38%) were reviewed centrally. An acute hepatitis-like pattern was noted in 12 subjects, 4 of which showed mild cholestasis. Eosinophils were prominent in 11 biopsies and mild-to-moderate steatosis in 6 cases whereas fibrosis was rare. An explant from a patient undergoing LT revealed severe hepatitis with massive necrosis. The remaining 2 patients had atypical findings with minimal inflammation and necrosis; 1 had bland cholestasis and 1 glycogenosis.
Chemical analyses were available on 16 products provided by 15 patients. Catechins typical of green tea were detected in all but one product (Supporting Table S4), the amount per serving ranging from 6.6 to 384 mg. The product without detectable catechins was a “diet spray” labeled as having green tea, but without providing its concentration.
HLA testing was performed on 36 of the 38 patients judged to have definite, highly likely, or probable green tea–related liver injury, as well as 17 possible and 12 unlikely cases, 192 control cases receiving other supplements, and 1,113 cases attributed to conventional drugs. In addition, HLA alleles were imputed for 15,094 controls from eMERGE. Four HLA alleles showed a significant association with green tea cases in comparison to the three control groups, of which HLA-B*35:01 and HLA-C*04:01 consistently ranked highest (Table 2). These two alleles are known to be in linkage disequilibrium (LD), which was also found in the green tea cases (r2 = 0.428). Because of their LD, the allele frequency of HLA-B*35:01 was similar to that of HLA-C*04:01 among green tea cases (0.42 vs. 0.46). However, because the HLA-B*35:01 allele was less prevalent than HLA-C*04:01 in population controls (0.06 vs. 0.12), its frequency in green tea cases was proportionally higher (6.8- compared to 3.7-fold) and the association had higher degrees of significance. For these reasons, HLA-B*35:01 was the focus for further analyses.
| HLA | Green Tea Supplements (n = 36) | Other Dietary Supplements (n = 193) | Conventional Drugs (n = 1,143) | Population Controls eMERGE (n = 15,094) | LD With B*35:01 (r2) |
|---|---|---|---|---|---|
| B*35:01 | 0.417 | 0.076 P = 5.8 × 10−12* | 0.062 P = 6.7 × 10−17* | 0.057 P = 9.7 × 10−19* | — |
| C*04:01 | 0.458 | 0.117 P = 2.3 × 10−10* | 0.132 P = 4.7 × 10−11* | 0.121 P = 1.47 × 10−12* | 0.428 |
| A*11:01 | 0.15 | 0.086 P = 0.084 | 0.052 P = 0.002* | 0.053 P = 0.001* | 0.05 |
| DPB1*04:02 | 0.22 | 0.143 P = 0.110 | 0.127 P = 0.030 | 0.096 P = 0.002* | 0.0007 |
- 1. P values from Fisher’s exact tests compare allele frequency differences between green tea–related cases and each control group. Sample sizes for each group were based on the subjects with HLA data.
- 2. LD between each top allele and HLA-B*35:01 was computed by r2 based on green tea supplement group.
- * Indicates false discovery rate <0.05.
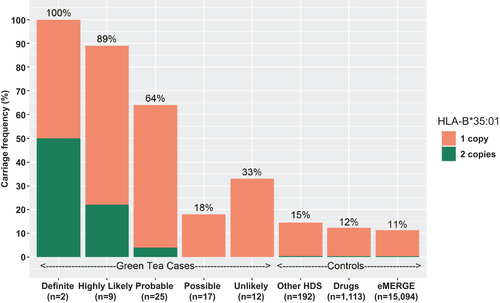
Among green tea cases, 26 had at least one copy of the HLA-B*35:01 allele yielding a carrier frequency of 72% (95% confidence interval [CI] = 58-87), a rate 5- to 7-fold higher than in control groups, including patients with non-green-tea HDS-related liver injury (15%; 95% CI = 10-20), conventional drug-related liver injury (12%; 95% CI = 10-14), and the eMERGE-derived population controls (11%; 95% CI = 10.5-11.5). Furthermore, the carrier frequency was higher in green tea cases judged to be definite or highly likely (91%) than those considered probable (64%), or only possible or unlikely (24%; P < 0.001; Fig. 2).
The 26 patients with HLA-B*35:01 included 4 who were homozygous, yielding an allele frequency of 0.417, which is 7-fold higher than the frequency among the eMERGE-derived population controls (0.057) and 5- to 19-fold higher than that in different U.S. racial groups (0.022 in Chinese Americans, 0.056 in European Americans, 0.067 in African Americans, and 0.078 in Mexican Americans) as reported in public databases.(34)
Comparison of cases of green tea–related liver injury with and without HLA-B*35:01 showed that those with the risk allele were younger (median age, 40 vs. 47 years), had a shorter time to onset (median, 66 vs. 139 days), higher initial median ALT values (2,329 vs. 696 U/L), and higher severity scores (Table 3). Those with HLA-B*35:01 were also more likely to have rash and fever (27% vs. 0%). Other features, such as race, Hispanic ethnicity, body mass index, and symptoms, were similar. Strikingly, the isolated cases with atypical features, such the 1 case in which the implicated product did not have detectable green tea, the 2 cases with a mixed rather than hepatocellular pattern of liver enzyme elevations, the 2 patients with atypical histology, the single patient with evidence of chronic liver injury during follow-up, and 4 of the 5 patients with mild, anicteric disease were HLA-B*35:01 negative.
| Feature | HLA-B*35:01 Positive (n = 26) | HLA-B*35:01 Negative (n = 10) | P Value |
|---|---|---|---|
| Age, years | 40 (17-69) | 47 (24-59) | 0.29 |
| Female sex | 21 (81%) | 5 (50%) | 0.10 |
| Race: white | 18/25 (72%) | 7 (70%) | 0.05 |
| Black | 1/25 (4%) | 2 (20%) | |
| Asian | 0/25 (0%) | 1 (10%) | |
| Other | 6/25 (24%) | 0 (0%) | |
| Hispanic ethnicity | 9 (35%) | 3 (30%) | 1.0 |
| Body mass index, k/m2 | 28.1 (21-42) | 28.4 (20-32) | 0.78 |
| Time to onset, days | 66 (22-376) | 139 (35-448) | 0.08 |
| Symptoms at onset | 26 (100%) | 8 (80%) | 0.07 |
| Initial ALT, U/L | 2,329 (836-4,185) | 696 (396-3,801) | <0.01 |
| Initial ALP, U/L | 172 (80-550) | 169 (92-276) | 0.47 |
| Initial total bilirubin, mg/dL | 8.4 (0.4-22.0) | 6.6 (1.0-30.1) | 0.94 |
| Peak INR | 1.2 (1.0-7.0) | 1.2 (1.0-2.1) | 0.26 |
| Initial R ratio | 26.7 (7.5-67.0) | 15.8 (3.3-47.3) | 0.06 |
| Rash or fever | 7 (27%) | 0 (0%) | 0.16 |
| ANA or SMA | 11 (42%) | 5 (50%) | 0.72 |
| Severity score | |||
| Mild (1+) | 1 (4%) | 4 (40%) | |
| Moderate (2+ or 3+) | 15 (57%) | 4 (40%) | 0.08 |
| Severe (4+ or 5+) | 10 (39%) | 2 (20%) | |
| Hepatocellular (R > 5) | 26 (100%) | 8 (80%) | 0.07 |
| LT | 2 (8%) | 0 (0%) | 1.0 |
| Chronicity | 0/21 (0%) | 1/7 (14%) | 0.25 |
- All results are median (range) or number (proportion) with denominator given when there are missing data.
- Abbreviations: ANA, antinuclear antibody; SMA, smooth muscle antibody.
Clinical features of patients with one copy of HLA-B*35:01 (heterozygotes) resembled those with two copies (homozygotes; Table 4). All 3 patients with two episodes of liver injury after green tea exposure were heterozygous for HLA-B*35:01. The two episodes were similar in clinical and biochemical features, but time to onset of symptoms of the second episode (14, 16, and 21 days) was shorter than the first (1, 3, and 4 months), consistent with rechallenge.(8) Concise clinical summaries with the chemical analyses and HLA results of 12 cases are provided in the Supporting information.
| Feature | One Copy of HLA-B*35:01 | Two Copies of HLA-B*35:01 |
|---|---|---|
| n = 22 | n = 4 | |
| Age, years | 38 (17-69) | 42 (34-48) |
| Female sex | 18 (82%) | 3 (75%) |
| Race: white | 16/21 (76%) | 2 (50%) |
| Hispanic ethnicity | 7 (32%) | 2 (50%) |
| Body mass index, k/m2 | 28.1 (20.5-42.4) | 25.4 (20.6-40.7) |
| Time to onset, days | 66 (22-335) | 69 (39-376) |
| Initial ALT, U/L | 2,364 (968-4,185) | 1,810 (836-2,701) |
| Initial ALP, U/L | 152 (80-550) | 255 (117-391) |
| Initial total bilirubin, mg/dL | 9.3 (0.4-22.0) | 6.7 (3.3-11.6) |
| Peak total bilirubin, mg/dL | 13.0 (0.6-38.2) | 14.8 (3.7-26.7) |
| Peak INR | 1.2 (1.0-7.0) | 1.2 (1.0-1.8) |
| Initial R ratio | 29.9 (7.5-67.0) | 17.6 (10.0-35.7) |
| Hepatocellular | 22 (100%) | 4 (100%) |
| Rash or fever | 7 (32%) | 0 |
| ANA or SMA | 9 (41%) | 2 (50%) |
| Severity score | ||
| 1 (mild, anicteric) | 1 | 0 |
| 2 (moderate, jaundiced) | 3 | 1 |
| 3 (moderate, hospitalized) | 9 | 2 |
| 4 (severe, INR ≥ 1.5) | 7 | 1 |
| 5 (fatal, or transplant) | 2 | 0 |
| LT | 2 (9%) | 0 |
| Chronicity | 0/18 | 0/3 |
| Heterozygous | Homozygous |
- All results are median (range) or number (proportion) with denominator given if there were missing data. None of the differences were statistically significant.
Discussion
In an ongoing study of DILI in the United States, green tea was judged to be the most likely cause in 40 cases, making it the major single cause of herbal supplement–related liver injury(1) and the sixth-most common among all causes.(28) The clinical, biochemical, and histological features of green tea liver injury were remarkably consistent and resembled those of acute viral hepatitis with hepatocellular injury, marked serum ALT and AST elevations, and variable degrees of severity and clinical outcomes. Rate of death or LT was typical for acute, icteric hepatocellular DILI (3 of 33 hepatocellular and jaundiced cases; 9%) as would be predicted by Hy’s law.(36)
Green tea was usually being taken for weight loss and most commonly as a component of a multi-ingredient product without green tea in its name. Average daily and total accumulated doses were difficult to estimate given that most product labels did not provide concentrations of green tea extract or catechins, and patients were often uncertain about dates of starting and stopping and number of pills taken daily. Finally, 9 subjects were taking two green tea containing supplements concurrently. Chemical analysis indicated that catechins and ECGC were present in most products. Three patients with documented recurrence of liver injury after taking the same (1 case) or another (2 cases) green tea–containing product had similar clinical features and outcome, but with a shorter latency, as would be expected from an immune-mediated reaction to rechallenge.
A striking finding in the analysis was a close association of green tea–related liver injury and the HLA allele B*35:01. This allele is carried by 5%-15% of U.S. populations, rates being lowest in Asian Americans, intermediate in European and African Americans, and modestly higher in Hispanics.(35) In contrast, this allele was found in 72% of patients with green tea–associated liver injury and >90% of those in whom the injury was judged as highly likely or definite. Furthermore, cases carrying HLA-B*35:01 more closely fit the typical phenotype: a latency of 1-6 months; occurrence of rash or fever; a hepatocellular enzyme pattern; and a moderate-to-severe course with jaundice. The 2 subjects with atypical histology on liver biopsy, the 2 with “mixed” rather than hepatocellular enzyme elevations, the 1 with evidence of chronic liver injury in follow-up, and the 1 taking a product without detectable catechins were all negative for HLA-B*35:01. Importantly, in this study, green tea causality was scored before the chemical analyses or the HLA typing results were known. The accumulated findings suggest that the presence of HLA-B*35:01 is a more reliable indicator of green tea liver injury than expert opinion, at least in cases not considered to be definite or highly likely.
HLA associations have been made with several forms of liver injury from conventional drugs such as flucloxacillin, amoxicillin-clavulanate, terbinafine, and fenofibrate.(31, 37, 38) Interestingly, HLA-B*35:01 has been associated with another herbal cause of liver injury, reported to be present in 89% of 27 Chinese patients with Polygonum multiflorum–induced liver injury compared to 5% of controls.(39) In addition, the phenotype of the injury resembled that of green tea, with a mean age of 45 years, latency of 1-6 months, and hepatocellular injury. Thus, this allele may be a risk factor for liver injury from several herbal components.
Studies of rodent models of green-tea-extract–associated liver injury have generally focused on its direct hepatotoxicity when given in high doses,(24, 25) which, however, were far higher than those used in humans.(17, 19) Furthermore, the clinical features of green tea–associated liver injury suggest that it is idiosyncratic.(18, 40) In a recent clinical trial of green tea extract for prevention of breast cancer, monitoring of ALT levels revealed de novo elevations in 8.6% of treated patients compared to 1.8% of placebo-treated controls.(41) Elevations were mild-to-moderate in degree, were not associated with symptoms or jaundice, arose a median of 3 months (range, 1-12) after starting green tea, resolved upon stopping the supplement, and recurred rapidly after restarting, features typical of idiosyncratic and immune-mediated liver injury.
In summary, analysis of cases of DILI from a prospective study in the United States has identified green tea as the major cause of dietary supplement–related liver injury. The clinical phenotype was an acute-viral-hepatitis–like syndrome arising 1-6 months after starting green tea that was occasionally severe, but otherwise self-limiting in course. Green tea–related liver injury was strongly associated with carriage of the HLA B*35:01 allele, a risk factor that may be helpful in diagnosis and which is likely to provide insights and directions for research into the nature of drug- and dietary supplement–induced liver injury.
Acknowledgment
The authors acknowledge the invaluable help of Hoss Rostami, B.S.M.S.E., for administration of the green tea causality assessment process and identifying product labels.
Author Contributions
Substantial contribution to concept and design: J.H.H., H.L.B., E.J.P., H.B., V.J.N. Substantial contribution to acquisition of data or analyzing and interpretation of data: All authors. Drafting the article or revising it critically: J.H.H., H.L.B., E.J.P., V.J.N., I.K., D.E.K., Y.J.L. Final approval of the version to be published: All authors.




