Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis
Potential conflict of interest: Nothing to report.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. Up to one third of individuals with NAFLD will develop nonalcoholic steatohepatitis (NASH), which is associated with progression to cirrhosis and is rapidly becoming the leading indication for liver transplantation. Sarcopenia is defined as a progressive and generalized loss of skeletal muscle mass, strength, and function. It is observed in up to 60% of patients with end-stage liver disease and portends a poor prognosis. Recent studies have shown that sarcopenia is a novel risk factor for developing NAFLD. Pathophysiological mechanisms relating sarcopenia and NASH may include insulin resistance (IR) and increased inflammation. IR leads to accumulation of triglycerides in both muscle tissue and the liver. It also exacerbates proteolysis and leads to muscle depletion. Chronic inflammation leads to liver injury and progression of fibrosis. The inflammatory milieu also stimulates protein catabolism. Viewing skeletal muscle as an endocrine organ that secretes various salutary myokines may help us understand its role in the development of steatosis. A better understanding of the pathophysiology will aid in developing physical and pharmacological therapeutic interventions. In this review, we will explore the complex inter-relationships between sarcopenia and NASH. We will discuss the impact of sarcopenia in patients with NASH and therapeutic options for the management of sarcopenia. (Hepatology 2017;66:2055–2065)
Abbreviations
-
- 6MWT
-
- six-minute walk test
-
- ALT
-
- alanine aminotransferase
-
- BCAA
-
- branched-chain amino acid
-
- BMI
-
- body mass index
-
- CRP
-
- C-reactive protein
-
- CT
-
- computed tomography
-
- DEXA
-
- dual-energy x-ray absorptiometry
-
- ESLD
-
- end-stage liver disease
-
- FAO
-
- fatty acid oxidation
-
- FDA
-
- U.S. Food and Drug Administration
-
- FFAs
-
- free fatty acids
-
- FFM
-
- fat free mass
-
- GH
-
- growth hormone
-
- HMB
-
- β-hydroxy β-methylbutyrate
-
- HS
-
- hepatic steatosis
-
- IGF-1
-
- insulin growth factor-1
-
- IL-6
-
- interleukin-6
-
- IR
-
- insulin resistance
-
- L3SMI
-
- L3 skeletal muscle index
-
- LT
-
- liver transplantation
-
- MetS
-
- metabolic syndrome
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NF-κB
-
- nuclear factor kappa B
-
- PPAR
-
- peroxisome proliferator-activated receptor
-
- TGs
-
- triglycerides
-
- TGFβ
-
- transforming growth factor-β
-
- TNFα
-
- tumor necrosis factor-α
-
- VDR
-
- vitamin D receptor
Background
Sarcopenia is a common complication of cirrhosis and is observed in up to 60% of patients with end-stage liver disease (ESLD).1 It is defined as a progressive and generalized loss of skeletal muscle mass, strength, and function2 and portends a poor prognosis in patients with ESLD.1 Myosteatosis, defined as infiltration of fat in the muscle, increases with age and adiposity and is associated with an increased risk of mortality,3 thus suggesting that both muscle quantity and quality are important prognostic markers. Interestingly, sarcopenia is also emerging as a novel risk factor for the development of nonalcoholic fatty liver disease (NAFLD),4 the most common cause of chronic liver disease worldwide5 and a rising indication for liver transplantation (LT). NAFLD is a spectrum ranging from benign steatosis to nonalcoholic steatohepatitis (NASH) and is associated with obesity, metabolic syndrome (MetS), diabetes, and insulin resistance (IR).5 One third of individuals develop NASH,6 which is distinguished from steatosis by the presence of hepatocellular injury and is associated with progression to cirrhosis and its complications.7 More than half of patients with NASH (56%) are obese.8 In spite of elevated body mass index (BMI), NASH patients with cirrhosis develop sarcopenia, leading to the definition of sarcopenic obesity, which is defined as the presence of sarcopenia in an individual who has a simultaneous increase in adipose tissue and is overweight (BMI >25 kg/m2).3 In patients with ESLD, the presence of NASH is associated with a 6-fold increased risk of sarcopenic obesity.9 Thus, it appears unclear whether sarcopenia is the cause or the consequence of NASH.
Pathophysiology
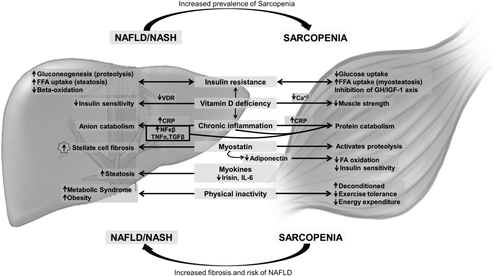
The pathophysiology of sarcopenia is complex and includes malnutrition, hypermetabolic state, increased inflammation, increased levels of myostatin, hypogonadism, and low vitamin D levels.10 Similarly, the development of NASH involves multiple factors, including metabolic, genetic, environmental, and gut microbial factors.11 Mechanisms relating sarcopenia to NASH include the following: IR; increased inflammation; myokines secreted by skeletal muscle; myostatin; adiponectin; vitamin D deficiency; and physical inactivity.12
IR
In adipose tissue, IR increases lipolysis with the consequent release of free fatty acids (FFAs) to the liver. High levels of FFA in turn inhibit the growth hormone (GH)/insulin growth factor-1 (IGF-1) axis, which normally plays a protective role in age-related muscle loss and in muscle regeneration.13 IR leads to a compensatory hyperinsulinemia, which, in the steatotic hepatocyte, leads to impaired suppression of gluconeogenesis, decreased glycogen synthesis, increased uptake of FFAs and lipogenesis, altered transport of triglycerides (TGs), and inhibition of beta-oxidation. These changes lead to accumulation of TGs in both muscle tissue (myosteatosis) and the liver (steatosis).14
The promotion of gluconeogenesis, in a state of IR, exacerbates proteolysis and leads to muscle depletion.4 IR itself can be involved in age-related muscle protein loss and sarcopenia.15 In the presence of IR, the mammalian target of rapamycin pathway remains inactive and cannot inhibit autophagy or lysosomal degradation of proteins and organelles. These effects likely contribute to accelerated muscle loss observed in diabetes.
Sarcopenia also promotes IR, independent of obesity, because the skeletal muscle is the primary tissue responsible for insulin-mediated glucose disposal.16 Similarly, myosteatosis has also been shown to be associated with IR.17 The presence of both sarcopenia and obesity acts synergistically leading to a more-severe IR and dysglycemia.16 Thus, the cycle continues, partially explaining the propagation of NASH (see Fig. 1).
SYSTEMIC INFLAMMATION
Chronic inflammation and oxidative stress are important in the development of NASH. Enhanced fatty acid oxidation (FAO) in the liver leads to generation of oxygen free radicals, which cause lipid peroxidation and induce proinflammatory cytokine synthesis. Cytokine levels, such as tumor necrosis factor-α (TNFα) and transforming growth factor-β (TGFβ), are frequently elevated in NAFLD.4 Chronic inflammation leads to direct liver injury and progression of fibrosis.14 In addition, these cytokines stimulate protein catabolism, which results in loss of muscle mass and sarcopenia.18
Chronic inflammation is present in individuals with sarcopenia, as evidenced by increased levels of C-reactive protein (CRP).19 Furthermore, elevated CRP levels correlate with liver steatosis. Patients with sarcopenia have an increased risk of fatty liver.20 Thus, inflammation is likely another shared mediator between NASH and sarcopenia.
MYOKINES
Skeletal muscle is an endocrine organ secreting peptides called myokines. Myokines affect other metabolic tissues, including the liver.21 In transgenic mice with mildly elevated muscle peroxisome proliferator-activated receptor (PPAR)γ coactivator-1 α, synthesized in muscle, there is resistance to age-related obesity, diabetes, and a prolonged life span.22 Interleukin-6 (IL-6), one of many myokines, has a protective effect on the development of NAFLD in inflammation-prone animal models.23 Irisin, an exercise-inducible myokine, causes a significant increase in total body energy expenditure whereby, reducing body weight, thus obesity and IR.24 It is inversely associated with the degree of hepatic steatosis (HS) in obese individuals.25 Irisin also involves the PPARα through downstream signaling,25 which plays a crucial role in fatty acid β-oxidation in the liver26 and in the regulation of lipid metabolism.27 It leads to improvement of HS and insulin sensitivity by up-regulating fibroblast growth factor 21 gene.28 Hence, it is plausible that muscle could play a causative role for fatty liver through reduced secretion of various salutary myokines in the context of sarcopenia.
MYOSTATIN AND ADIPONECTIN
Myostatin, a TGFβ superfamily member, is an inhibitor of protein synthesis and regeneration. Levels of myostatin are elevated in patients with cirrhosis.29 Myostatin activates two of the major skeletal muscle proteolytic pathways—the ubiquitin-proteasome pathway and autophagy-mediated proteolysis.30 Its receptor, Activin IIbr, has been found in hepatic stellate cells.12 This finding raises the question of whether fatty liver results in sarcopenia (through activation of myostatin in the skeletal muscle) or whether sarcopenia promotes liver injury in patients with NASH (by stimulating stellate cells).
Adiponectin receptors in the muscle regulate insulin signaling and increase FAO.31 Obesity and adipose tissue inflammation are accompanied by low levels of adiponectin. Myostatin also increases adipose tissue mass and leads to decreased adiponectin secretion. The interplay between adiponectin and myostatin within the muscle, liver, and adipose tissue are complex and worthy of further investigation.
VITAMIN D DEFICIENCY
Vitamin D deficiency contributes to IR, MetS, and NAFLD.5 Vitamin D regulates expression of the insulin receptor in pancreatic beta cells32 and in peripheral target tissues (including the liver).33 Vitamin D mediates its intracellular signals through its receptor, vitamin D receptor (VDR), which is constitutively expressed in the liver.34 It may improve insulin sensitivity by improving systemic inflammation.35 Animal studies have shown that vitamin D plays an important role in the regulation of oxidative stress, the production of proinflammatory cytokines, and liver fibrosis.5 Vitamin D deficiency likely contributes to worsening NAFLD through an inflammation-mediated pathway.5 Patients with NAFLD have lower levels of vitamin D.5 VDR expression on hepatocytes is inversely correlated with NAFLD activity score, independent of BMI, IR, or adiponectin levels, and is strongly associated with a diagnosis of NASH.36
VDRs are also expressed in skeletal muscle, and vitamin D signaling plays an important role in myogenesis, myoblast proliferation and differentiation, skeletal muscle growth, and muscle inflammation.37 In animal studies, myofibrillar protein degradation is increased with vitamin D deficiency. Vitamin D deficiency may affect muscle protein turnover by inducing hypocalcemia and decreasing insulin secretion.38 Individuals with sarcopenia have significantly lower levels of vitamin D.20 Studies in the elderly have shown that lower levels of vitamin D are associated with lower muscle strength, poor muscle function, and an increased risk of sarcopenia.39 Furthermore, supplementation with vitamin D results in improved muscle strength and function in this population.40
PHYSICAL INACTIVITY
Sarcopenia leads to physical disability and functional decline,2 hence these patients tend to be more sedentary. It is presumed that this sedentary lifestyle causes a reduction of energy expenditure, which may result in obesity and HS. Indeed, studies have shown that patients with sarcopenia have an increased body fat mass, more components of MetS, and higher CRP levels.41 Furthermore, studies have shown that sarcopenia and visceral obesity in the elderly may synergistically increase their effect on physical disability, metabolic disorders, and cardiovascular diseases.42 It is well known that a sedentary lifestyle increases the risk for obesity, MetS, and NAFLD.
Assessment of Sarcopenia in NASH
Traditionally, the term sarcopenia has been used to define loss of muscle mass in the aging population.2 The European Working Group on Sarcopenia in Older People recommends using both low muscle mass and low muscle function to diagnose sarcopenia.2 However, most studies focus on objective measures of muscle mass alone to diagnose sarcopenia. Many consider skeletal muscle cross sectional imaging with computed tomography (CT) or magnetic resonance imaging to be a gold-standard tool.43 There is heterogeneity in the literature with regard to definition of sarcopenia and methods for diagnosis. Some methods used for diagnosis include using the psoas muscle area, psoas muscle thickness, total muscle, and fat area in the third lumbar (L3) region and the L3 skeletal muscle index (L3SMI). L3SMI is the most commonly used method; in a recent study, it was found to be superior to psoas muscle measurements in predicting survival in patients with ESLD.44 The following cutoffs for L3SMI have been proposed in patients with ESLD awaiting LT: <50 cm2/m2 in men and <39 cm2/m2 in women.44 However, because imaging is not widely available and exposes patients to radiation, studies are ongoing to find better alternatives. Dual-energy x-ray absorptiometry (DEXA)-measured appendicular lean mass has been used to diagnose sarcopenia. In one study, a lower prevalence of sarcopenia (42%) was found when using DEXA appendicular lean mass to diagnose sarcopenia when compared to CT (76%).45 The researchers in this study concluded that DEXA-measured appendicular lean mass is not a suitable alternative to cross-sectional imaging. In a recent study, the combination of BMI and thigh muscle thickness (measured by ultrasound) identified sarcopenia almost as well as cross-sectional imaging with a receiver operating characteristic area under the curve of 0.78 in men and 0.89 in women.46 Further studies validating these results are needed. Functional measures of sarcopenia include hand grip and the six-minute walk test (6MWT). Recent studies have shown that both measures have prognostic importance. Hand-grip strength is a surrogate for muscle strength in the lower arms or legs and is predictive of clinical outcomes.2 Similarly, a 6MWT distance of less than 250 m identifies patients with an increased risk for mortality pre-LT (sensitivity 90%).47 In addition, for every 100-m improvement in 6MWT, the mortality rate is halved.47 Thus, the 6MWT allows for objective measurement of sarcopenia and is a useful tool to monitor responses to interventions. Additionally, myosteatosis may have a greater impact on muscle function than muscle mass itself.48 In older hospitalized patients, myosteatosis is linked to longer hospital stay and increased mortality.49 Whether this translates into increased mortality in the NASH or patients with cirrhosis population remains to be determined.
Impact of Sarcopenia in NASH
The majority of studies assessing the relationship between sarcopenia and NAFLD have been done in Asian populations (Table 1).4, 19, 20, 42 The first study showed that there was an inverse correlation between skeletal mass and cardiovascular risk factors, including MetS.42 There was also an inverse correlation between skeletal mass and total body fat mass, TG, and alanine aminotransferase (ALT) levels. Patients with sarcopenia had an increased risk of having NAFLD, with an odds ratio of 5.88 (95% confidence interval, 2.33-14.84; P = 0.002) for those in the 25th percentile.42
| >Reference | Title | Study Design | Assessment of NASH and Sarcopenia | Conclusion |
|---|---|---|---|---|
| Hong et al.42 | Relationship between sarcopenia and non-alcoholic fatty liver disease: the Korean sarcopenic obesity study | Cross-sectional study of 526 Korean adults (62% women) |
NAFLD was diagnosed using CT. LAI was used to define NAFLD. Sarcopenia was diagnosed using DEXA. It was defined as SMI 1 SD below the sex-specific mean for a young reference group (39.8% men; 34.1% women). |
There was a negative correlation between skeletal mass and cardiovascular risk factors. There was a negative correlation between skeletal mass and total body fat mass, TGs, and ALT levels. Patients with sarcopenia (25th percentile) had a 5-fold increased risk of NAFLD. |
| Lee et al.20 | Sarcopenia is associated with non-alcoholic fatty liver disease independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011) | Cross-sectional study of 15, 132 Korean adults (63% women) |
NAFLD was defined using prediction models HIS, CNS, LFS. Sarcopenia was diagnosed using DEXA. It was defined as SMI 1 SD below the sex-specific mean for a young reference group (32.2% men; 25.5% women). |
Sarcopenia was associated with NAFLD independent of obesity, IR, or MetS. Sarcopenia was associated with a 2.3- to 3.3-fold increased risk of NAFLD. |
| Lee et al.4 | Sarcopenia is associated with significant liver fibrosis independently of obesity and IR in non-alcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011) | Cross-sectional study of 2761 Korean adults (55% women) |
NAFLD was defined using prediction models HIS, CNS, LFS. Sarcopenia was diagnosed using DEXA. SI was calculated by dividing ASM by BMI. The following cutoffs were used for SI (<0.79 men; <0.52 women). |
Sarcopenia was associated with significant liver fibrosis, independent of obesity and IR. Sarcopenia was associated with a 2-fold increased risk of fibrosis. |
| Koo et al.19 | Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis | Cross-sectional study of 309 Korean adults (53% women) |
NAFLD was defined as ≥5% macrovesicular steatosis on liver biopsy. The diagnosis of NASH was based on histopathology. Sarcopenia was defined as ASM 2 SD below sex-specific mean for healthy population (<29 men; <22.9 women). ASM was estimated using BIA. |
The prevalence of sarcopenia increased with progression of disease from no NAFLD (8.7%) to NAFL (17.9%) to NASH (35%). Individuals with sarcopenia had more-severe grades of steatosis and fibrosis. Forty-six percent of individuals with sarcopenia had significant fibrosis compared to 25% of those without sarcopenia. In those with NAFLD, sarcopenia was associated with a 2.5-fold increased risk of NASH and significant fibrosis, independent of obesity and IR. |
| Issa et al.51 | The presence of sarcopenia (muscle wasting) in patients with non-alcoholic steatohepatitis | Retrospective study of 75 American adults |
NASH was diagnosed using histopathological criteria. Sarcopenia was diagnosed using psoas muscle and paraspiral muscles in the lumbar (L4) region using CT. |
The prevalence of sarcopenia increased with progression of disease from controls to NASH to NASH cirrhosis. |
| Petta et al.50 | Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease | Retrospective study of 225 Italian adults (37% women) |
NAFLD was defined as ≥5% macrovesicular steatosis on liver biopsy. The diagnosis of NASH was based on histopathology. Sarcopenia was diagnosed by SI. ASM was assessed using BIA. The following cutoffs were used for SI (≤37 men; ≤28 women) |
The prevalence of sarcopenia increased with progression of disease. In those without fibrosis, 22% had sarcopenia, and in those with advanced fibrosis (≥F3) 67% had sarcopenia. Individuals with sarcopenia had a 2-fold increased risk of fibrosis. |
- Abbreviations: ASM, appendicular skeletal muscle mass; BIA, bioelectrical impedance analysis; CNS, comprehensive NAFLD score; HSI, – hepatic steatosis index; LAI, liver attenuation index; LFS, NAFLD liver fat score; SI, sarcopenia index; SMI, skeletal muscle index.
Subsequently, it was shown that sarcopenia was associated with NAFLD independent of obesity, IR, or MetS.4, 20 Those with sarcopenia had a 2.3- to 3.3-fold increased risk of NAFLD20 and a 2-fold increased risk of fibrosis, independent of obesity or IR.4 Interestingly, in one of these studies, exercise was beneficial only in NAFLD patients without sarcopenia,20 suggesting that exercise-induced release of healthy myokines may play a key role in the amelioration of NAFLD.
Another study showed that there was a step-wise increase in the prevalence of sarcopenia with worsening liver inflammation and fibrosis, such that those with NASH had the highest prevalence of sarcopenia followed by patients with NAFLD and the controls had the least (35% vs. 17.9% vs. 8.7%; P < 0.001; Fig. 2).19 This study also confirmed the relationship between fibrosis and sarcopenia. Patients with sarcopenia were more likely to have fibrosis.
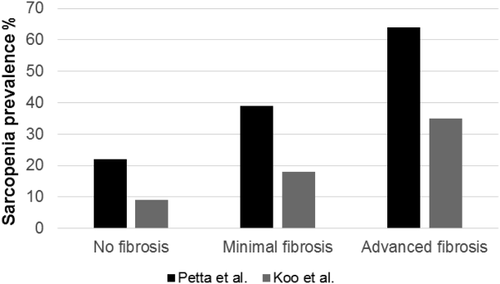
Thus far, there are only two studies looking at the association between sarcopenia and NAFLD in non-Asian populations.50, 51 An Italian study showed that sarcopenia was associated with both visceral obesity and IR.50 This study also showed an increase in the prevalence of sarcopenia with worsening fibrosis, independent of obesity and IR. Furthermore, those with sarcopenia were more likely to have fibrosis when compared to their counterparts; this association was independent of obesity and IR. Fibrosis was present in 22% of nonsarcopenic patients versus 60% of those with sarcopenia (P = 0.002). The second study was done in North America and was relatively small (50 NASH patients),51 showing an increased prevalence of sarcopenia with worsening liver disease.
Management Issues in Sarcopenia
LIFESTYLE INTERVENTION
Weight loss, achieved by diet and exercise, is the mainstay of NASH management. The amount of weight loss is directly proportional to the degree of improvement in liver histology. A minimum of 7%-10% weight loss is necessary for histological improvement,8 including improvement in steatosis, lobular inflammation, and ballooning.8
The recommendation for weight loss in patients with sarcopenia, however, should be carefully balanced with their nutritional status. Those who have sarcopenic obesity may benefit from caloric restriction,52 whereas those with low to normal BMI may be harmed by this recommendation. Management of sarcopenia is thus complex in this patient population. If managed improperly, weight loss by means of caloric restriction alone could further lead to sarcopenia because it leads to loss of both fat tissue (75%) and fat free mass (FFM; 25%).53 Dietary intervention should focus on reducing fat intake, simple carbohydrates (sugar), and starch while ensuring adequate protein consumption. Consumption of 1.3 g/kg of protein per day has been recommended to prevent sarcopenia.54
Patients with cirrhosis have an accelerated state of catabolism with fasting55 and should be encouraged to snack frequently. Late evening snacks providing an extra 200-250 kcal, leading to beneficial results by reducing fasting time and muscle and lipid catabolism.1 Supplementation with protein-based calories or branched-chain amino acid (BCAA) has been shown to improve muscle mass and reduce protein catabolism.56 Supplementation with BCAA has also been shown to improve survival.56 Leucine-enriched essential amino acids have also been used for treatment of sarcopenia because they stimulate muscle synthesis.57 β-hydroxy β-methylbutyrate (HMB), a downstream metabolite of leucine, has been shown to reduce protein degradation and up-regulate protein synthesis,58 In one study, older rats supplemented with HMB had a leaner, stronger body phenotype compared to controls.59 Vitamin D supplementation also improves muscle mass and strength and acts synergistically with HMB supplementation.60 Further studies evaluating the efficacy of leucine-enriched essential amino acids and HMB in humans are needed.
Exercise has been shown to enhance functional capacity in older adults and in those with chronic disease.61, 62 Exercise is a potent stimulant for muscle growth and should be part of the management of sarcopenia.52 In one pilot study, exercise significantly increased muscle mass in patients with cirrhosis.63 In this study, patients performed aerobic exercise for 12 weeks at moderate intensity (heart rate, 60%-70% of maximum) and received leucine supplementation.63 In a recent study, aerobic exercise performed for 12 weeks, without any nutritional supplementation, led to a significant decrease in body fat (P = 0.003), an increase in lean mass (P = 0.01), and an increase in functional capacity.64 Furthermore, aerobic exercise combined with caloric restriction attenuated loss of FFM from 25% to 11%.52 Thus, in patients with sarcopenic obesity, management should include both caloric restriction and exercise. It is possible that addition of resistance training may further reduce loss of FFM.52 However, more studies are needed to assess the safety, type, and impact of exercise in sarcopenic patients.
PHARMACOLOGICAL TREATMENT
The management of patients with NAFLD involves the treatment of the liver disease and the associated metabolic comorbidities (hyperlipidemia, IR, and diabetes). Although several drugs have been evaluated in clinical trials for the treatment of NASH, there are currently no U.S. Food and Drug Administration (FDA)-approved therapies.
Like in NASH, there are no FDA-approved therapies for sarcopenia. Antagonists of myostatin for treatment of sarcopenia are of interest. Animal studies have shown that myostatin antagonists reduce these levels without affecting liver function.1 Moreover, these agents appear to protect mice from fatty liver and improve IR.65 One phase 2 randomized, controlled trial showed that the use of humanized myostatin antibody led to a small increase in muscle mass in elderly patients with sarcopenia.66
Testosterone and GH have both been shown to inhibit myostatin.1 Patients with cirrhosis have low levels of testosterone and have a relative state of GH resistance.67, 68 Furthermore, GH deficiency has been shown to increase the risk of MetS, cardiovascular disease, and fatty liver. Up to 21% of individuals with GH deficiency have NASH, and replacement of GH leads to improved liver enzymes and histology.69 Testosterone injections in men with cirrhosis with low testosterone levels have also been shown to significantly improve muscle mass,70 but further studies are needed.
Hyperammonemia, on the other hand, contributes to sarcopenia.71 In animal studies, hyperammonemia leads to increased myostatin expression through activation of p65/nuclear factor kappa B (NF-κB).71 Ammonia can also activate autophagy by way of mitochondrial dysfunction and generation of reactive oxygen species.71 However, skeletal muscle turnover is a slow process and likely requires long-term ammonia-lowering strategies to reverse the metabolic changes leading to sarcopenia. Ammonia-lowering agents include lactulose, rifaximin, and novel agents, such as α-ketoglutarate esters, that remove ammonia as glutamine.71 Further studies evaluating agents for long-term ammonia removal are needed before these agents can be used for management of sarcopenia.
SURGICAL INTERVENTION
LT is the ultimate treatment in patients with ESLD. However, currently there is no consensus on the effects of LT on sarcopenia. The limited number of extant studies shows little improvement in sarcopenia post-LT (6%-28%).72, 73 The presence of risk factors, including immunosuppression use, hospitalization, post-LT complications, renal failure, and disease recurrence, likely contributes to lack of normalization of muscle mass following LT.74 Further studies assessing the natural history of sarcopenia and the impact of LT are needed.
Conclusion
The overlap in the pathophysiology of NASH and sarcopenia make it challenging to determine whether sarcopenia is a risk factor for NASH or whether it is a complication of NASH. The two entities are so intricately intermeshed that the presence of either one increases the risk for the other. IR and increased inflammation play a key role in the development of both conditions. Furthermore, understanding the role of myokines secreted by skeletal muscle may provide a novel area in pharmacological development.31 Importantly, patients who have both entities are at a higher risk of worsening liver disease and its complications as well as an increased cardiovascular risk. Further research in diverse populations, delineating the course of disease, complications, and mortality risk in patients with both conditions, is needed.
Clinicians need to have an increased awareness of sarcopenia to diagnose it in patients with NASH and should intervene early in these high-risk patients. Lifestyle intervention is the cornerstone of management for both conditions. Further studies are needed to assess exercise intervention in sarcopenia and the development of pharmacological intervention that addresses both conditions.




