An endogenous DNA adduct as a prognostic biomarker for hepatocarcinogenesis and its prevention by Theaphenon E in mice
Potential conflict of interest: Nothing to report.
Supported by the National Cancer Institute (R01-CA-190678, to F.-L.C.) and the Carlucci Family Research Award in Cancer Prevention and Early Detection (to Y. Fu).
Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer–related deaths worldwide, mainly because of its poor prognosis. A valid mechanism-based prognostic biomarker is urgently needed. γ-hydroxy-1,N2-propanodeoxyguanosine (γ-OHPdG) is an endogenously formed mutagenic DNA adduct derived from lipid peroxidation. We examined the relationship of γ-OHPdG with hepatocarcinogenesis in two animal models and its potential role as a prognostic biomarker for recurrence in HCC patients. Bioassays were conducted in xeroderma pigmentosum group A knockout mice and diethylnitrosamine-injected mice, both prone to HCC development. γ-OHPdG levels in the livers of these animals were determined. The effects of antioxidant treatments on γ-OHPdG and hepatocarcinogenesis were examined. Using two independent sets of HCC specimens from patients, we examined the relationship between γ-OHPdG and survival or recurrence-free survival. γ-OHPdG levels in liver DNA showed an age-dependent increase and consistently correlated with HCC development in all three animal models. Theaphenon E treatment significantly decreased γ-OHPdG levels in the liver DNA of xeroderma pigmentosum group A knockout mice and remarkably reduced HCC incidence in these mice to 14% from 100% in the controls. It also effectively inhibited HCC development in the diethylnitrosamine-injected mice. Using clinical samples from two groups of patients, our study revealed that higher levels of γ-OHPdG are strongly associated with low survival (P < 0.0001) and low recurrence-free survival (P = 0.007). Conclusion: These results support γ-OHPdG as a mechanism-based, biologically relevant biomarker for predicting the risk of HCC and its recurrence. (Hepatology 2018;67:159-170).
Abbreviations
-
- DEN
-
- diethylnitrosamine
-
- HCC
-
- hepatocellular carcinoma
-
- LC-MS/MS
-
- liquid chromatography-tandem mass spectrometry
-
- LEC
-
- Long-Evans cinnamon
-
- LPO
-
- lipid peroxidation
-
- NER
-
- nucleotide excision repair
-
- γ-OHPdG
-
- γ-hydroxy-1,N2-propanodeoxyguanosine
-
- WT
-
- wild type
-
- Xpa−/−
-
- xeroderma pigmentosum knockout
Genetic and epigenetic alterations in oncogenes and tumor-suppressor genes are crucial for carcinogenesis.1, 2 Somatic mutations may arise from DNA lesions that are not repaired. During a lifetime, the human genome will host a wide spectrum of mutagenic DNA lesions, induced by chemical carcinogens, viruses, and reactive oxygen and nitrogen species. This is believed to be the case for human liver as it is a major detoxifying organ that is exposed to a large number of risk factors.3-5
Primary liver cancer is the third most common cause of cancer-related death, which stems from the lack of suitable biomarkers for early detection, inadequate understanding of the molecular features, and resistance to current chemotherapy.6 There are more than 1 million newly diagnosed liver cancer cases annually, which renders liver cancer a global health care problem.7 Hepatocellular carcinoma (HCC) accounts for approximately 85% of liver cancers and is an inflammation-associated cancer.8 It is known that chronic inflammation leads to oxidative/nitrosative stress and lipid peroxidation (LPO).9-12 Upon oxidation, membrane polyunsaturated fatty acids generate highly reactive α,β-unsaturated aldehydes (enals) that can modify DNA bases, forming promutagenic cyclic DNA adducts.13-15
Oxidative stress caused by chronic inflammation has emerged as a major player in liver carcinogenesis of different etiologies, including hepatitis C and B viruses, alcoholism, and obesity.16-18 LPO-derived endogenous cyclic DNA adducts in cancer initiation/promotion have been investigated as potential markers for various types of inflammatory cancer-prone diseases (e.g., chronic pancreatitis, Crohn's disease, ulcerative colitis, alcohol-related hepatitis, Helicobacter pylori infection).19 Among the LPO-derived DNA adducts, γ-hydroxy-1,N2-propanodeoxyguanosine (γ-OHPdG) is one of the most abundant, having been ubiquitously detected in mammalian tissues.20 γ-OHPdG is mutagenic and induces predominantly G to T and G to A mutations.21, 22 One study showed that γ-OHPdG, derived from acrolein as a major constituent of cigarette smoke, occurs at the sites of the p53 gene that coincide with its mutation hot spots in lung cancer of smokers.23 These results support γ-OHPdG's role in human cancer by causing somatic mutations.
The link between hemochromatosis patients with iron overload and p53 mutation in HCC development and the correlations of inflammatory cells, LPO-derived protein adduct, and oxidized DNA bases as general oxidative markers in liver tissues of hepatitis C virus–associated HCC patients underlies the role of oxidative stress in liver carcinogenesis.24 Epidemiological studies have associated antioxidants with reduced risk of certain types of cancer.25 However, conflicting results have been obtained in intervention trials.26, 27 A mechanism-based biomarker is crucial for identifying the population that will actually benefit from taking antioxidants.
To date, there has been no report of a specific LPO-derived DNA adduct as a predictive biomarker for HCC. In this work, we studied γ-OHPdG as a biomarker of hepatocarcinogenesis in animal models, including xeroderma pigmentosum group A knockout (Xpa−/−) transgenic mice, diethylnitrosamine (DEN)–exposed mice, and Long Evans cinnamon (LEC) rats, and its prevention by antioxidants. Furthermore, we demonstrated in two sets of liver specimens from HCC patients after surgical resection that γ-OHPdG is a highly reliable predictor of survival and HCC recurrence.
Materials and Methods
CHEMICALS AND ENZYMES
All reagents purchased were of analytical or high-performance liquid chromatography grade.
ANIMALS
The details of the study design are listed in the Supporting Information.
QUANTIFICATION OF γ-OHPdG
The liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been described.28
PATIENTS AND DATABASE
Paraffin-embedded liver biopsies or HCC specimens from patients who had liver biopsies or curative resection of HCC as part of standard medical care were obtained from Georgetown University Medical Center. Informed consent was obtained from all patients under an approved institutional review board protocol (1992-048). A secure database with proper safeguards was constructed for the management of patient data. An institutional review board approved the ethical, legal, and social implications of the project.
WHOLE-EXOME SEQUENCING
Genomic DNA extraction, library preparation, and whole-exome sequencing were performed by Otogenetics (Norcross, GA). Genomic mouse DNA samples were constructed into libraries and hybridized to Agilent SureSelect Mouse All Exon 51 Mb probes (Santa Clara, CA). Paired end sequencing was executed on the Illumina HiSeq 2500 (San Diego, CA), and raw data files were pushed through the DNAnexus standard exome analysis pipeline to complete alignment, quality control, coverage analysis, and variant calling.
IMMUNOHISTOCHEMICAL STAINING
Immunohistochemical detection of γ-OHPdG in healthy and cancerous tissues was performed using tissue microarrays (HLiv-HCC060CD-01 and HLiv-HCC180Sur-02; US Biomax, Rockville, MD), paraformaldehyde-fixed, and paraffin-embedded blocks of tumor tissue from cases operated at Georgetown University. All patients gave informed consent, and the study was authorized by the respective hospital ethics committees. Immunoscores were calculated by adding intensity and region of staining: 0, 1, 2, 3 correspond to no, weak, moderate, and high intensity staining, respectively, and 0, 1, 2, 3 correspond to no, focal, regional, and diffuse region staining, respectively.
STATISTICAL ANALYSIS
Differences in adduct levels were compared using the Student t test. Fisher's exact test was used to analyze categorical data. The Kaplan-Meier method was used to analyze the survival data, and the log-rank test was used to compare the survival between different groups. A z test was employed to calculate the P values of tumor incidences. P < 0.05 was considered statistically significant. The SAS software (SAS Inc., Cary, NC; version 9.3) was used for statistical analysis.
Results
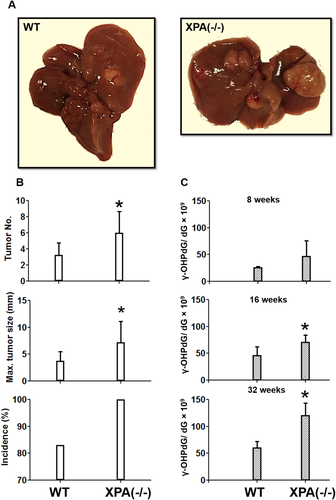
INCREASED LEVELS OF γ-OHPdG ARE ASSOCIATED WITH HEPATOCARCINOGENESIS IN Xpa−/− MICE
The mutagenicity of γ-OHPdG has been demonstrated using in vitro assays,29 but the evidence of its role in carcinogenesis in vivo is elusive. We first examined the association between γ-OHPdG and liver tumorigenesis in Xpa−/− mice deficient in nucleotide excision repair (NER).30 γ-OHPdG is the only endogenous DNA adduct known to be repaired by NER.31 Xpa−/− C57/B6 mice are a model for skin carcinogenesis by ultraviolet-B irradiation. Intriguingly, these mice also develop spontaneous liver tumors.32 We proposed that the cumulated γ-OHPdG levels, due to the lack of its repair, contribute to hepatocarcinogenesis. To further enhance the spontaneous HCC, we backcrossed Xpa−/− C57/B6 mice with C3H/HeNCrl mice, which have a high rate of spontaneous liver cancer (Supporting Fig. S1). Compared to wild-type (WT) controls, Xpa−/− mice not only developed a higher incidence of liver tumors but also showed significantly larger tumor sizes and increased multiplicity (Fig. 1A,B). γ-OHPdG levels in liver DNA were quantified by LC-MS/MS. The data showed that it increased age-dependently in Xpa−/− mice (P < 0.05; Fig. 1C). Mouse gender had no influence on γ-OHPdG levels (Supporting Fig. S3). We were able to see a statistically significant difference in the hepatic γ-OHPdG levels and liver cancer development between Xpa−/− and WT mice (Fig. 1). Unlike Xpa−/− mice, LEC rats are inflicted with increased LPO because of abnormal copper accumulation, mimicking that of human Wilson's disease.33 As a result, LEC rats develop acute hepatitis, followed by chronic hepatitis and eventually HCC. We found that γ-OHPdG levels in the livers of LEC rats were significantly higher than those of the WT Long-Evans rats (Supporting Fig. S4). These results provide evidence that the elevated liver γ-OHPdG levels are associated with an increased risk of HCC.
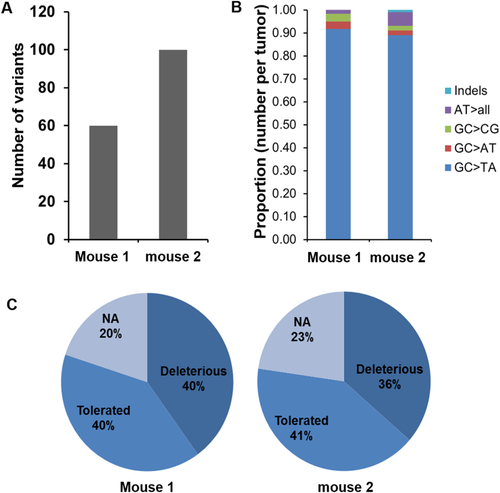
GC>TA IS THE DOMINANT SOMATIC MUTATION IN HCC OF Xpa−/− MICE
Studies have shown that γ-OHPdG causes predominantly GC>TA and GC>AT mutations.21, 22 We reasoned that the increased γ-OHPdG in the livers of Xpa−/− mice can lead to a somatic mutation pattern in which GC>TA and GC>AT mutations are the most frequent alterations. We obtained the mutation frequencies through comparing liver tumor nodules versus adjacent normal liver tissues from two Xpa−/− mice using whole-exome next-generation sequencing. Whole-exome sequencing produced a mean yield of 23.8 million reads or 2.5 gigabases of data per sample with 94.1% >Q30. Samples were sequenced to a mean coverage of 29X, and 99.4% of the reads were mapped to the target regions. We found 60 and 100 variants in the two liver nodules (Fig. 2A), with GC>TA mutation as the dominant alteration, accounting for 92% and 86% mutations, respectively (Fig. 2B). While examining the Sorting Intolerant from Tolerant prediction scores, we also noted that >35% of the variants in both samples were predicted as deleterious mutations (Fig. 2C). The high GC>TA mutation frequency in the Xpa−/− mouse liver tumors implies that γ-OHPdG may play a role in the mutagenesis of HCC development. Different from other solid tumors in which CG>TA transitions are the highest variations in the mutation spectrum, with the exception of lung cancer, variants in human HCC also showed an overrepresentation of GC>TA transversion.1 We identified a number of mutant genes within mouse liver nodules that were reported in human HCC, including ABCA1, CSMD1, LAMA2, TRRAP, and TRANK1,1 suggesting that this model is relevant to human liver carcinogenesis (Supporting Table S1).
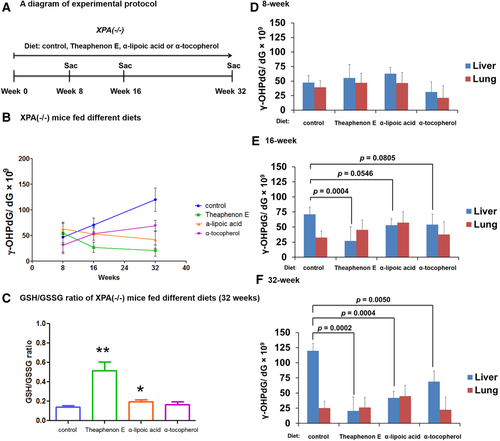
ANTIOXIDANTS SUPPRESS LIVER γ-OHPdG LEVELS AND HCC DEVELOPMENT IN Xpa−/− MICE
To further assess the role of γ-OHPdG in HCC development, we next studied if suppression of γ-OHPdG formation in the mouse liver would inhibit hepatocarcinogenesis. Xpa−/− mice yield 100% liver cancer incidence at the end of the 72-week bioassay. Three antioxidants (Theaphenon E, α-lipoic acid, and α-tocopherol) were used.34 To determine whether these antioxidants suppress liver γ-OHPdG levels, Xpa−/− mice were fed diets containing antioxidants: α-lipoic acid (2 g/kg), Theaphenon E (20 g/kg), and α-tocopherol (1.8 g/kg)28, 35-37 (details of the bioassay are in Supporting Information). We observed a significant decrease of γ-OHPdG in the livers of mice fed the antioxidant diets with different potencies: Theaphenon E > α-lipoic acid > α-tocopherol (Fig. 3). No significant changes of γ-OHPdG levels were found in the lungs, a nontarget organ. We also examined the ratio of reduced to oxidized glutathione, an indicator of oxidative stress. The increases in the ratio of reduced to oxidized glutathione in the liver tissues from the mice fed different antioxidants for 32 weeks are consistent with the decreases of γ-OHPdG (Fig. 3C). We have reported in LEC rats that Theaphenon E, but not α-tocopherol, suppresses γ-OHPdG formation.28
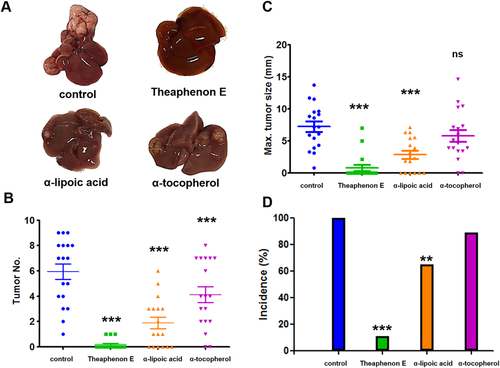
Having demonstrated that antioxidants can suppress γ-OHPdG formation, we examined the relationships of γ-OHPdG with hepatocarcinogenesis in a 72-week lifetime tumor bioassay in Xpa−/− mice. Mice randomized into four groups were fed diets containing antioxidants throughout the bioassay period.28, 35-37 Theaphenon E showed a strong inhibition with a remarkable reduction of tumor incidence to 14% from 100% in mice on the control diet and a significant decrease in tumor numbers and size (Fig. 4B-D). α-Lipoic acid also decreased HCC incidence but less effectively, whereas α-tocopherol had no significant effect. The potency of antioxidants to inhibit HCC showed a close correlation with their effects on γ-OHPdG levels. The protective effects of antioxidants against HCC development were similar in both male and female mice (Supporting Fig. S5), consistent with their suppressing effects on γ-OHPdG in both genders (Supporting Fig. S3). We compared γ-OHPdG adduct levels in nontumorous livers from the control and Theaphenon E groups at 72 weeks (Supporting Fig. S6). Consistent with observations at earlier time points, a significant reduction was observed in the Theaphenon E group. Although a significant decrease in body weight gain was observed in the Theaphenon E group (Supporting Fig. S6A), probably related to thermogenesis and fat oxidation caused by green tea extract,38 no food consumption difference was noted and mice in this group were leaner and healthy without any overt adverse effects (Supporting Fig. S6B).
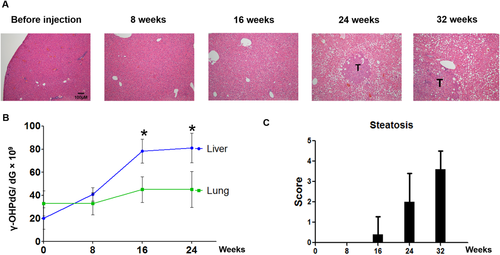
γ-OHPdG LEVELS CORRELATE WITH DEN-INDUCED HEPATOCARCINOGENESIS IN MICE
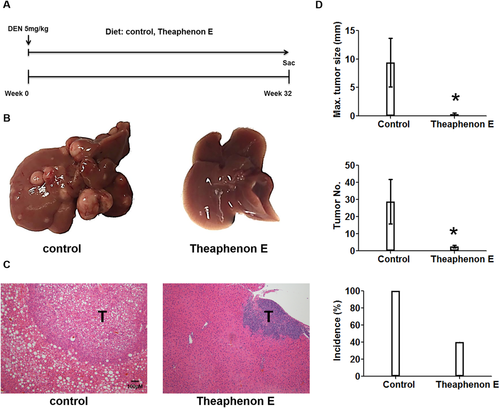
The relationship of γ-OHPdG in HCC was further examined in C57/B6 mice involving a single injection of procarcinogen DEN. Mice develop poorly differentiated HCC nodules within 32 weeks after DEN exposure.39 A time-dependent development of steatosis was observed in DEN-injected mice fed the control diet. Malignant liver nodules were observed after 8 months (Fig. 5). To determine whether γ-OHPdG levels are associated with hepatocarcinogenesis, we measured γ-OHPdG in the livers obtained at four time intervals, shortly before and 8, 16, and 24 weeks after DEN injection, by LC-MS/MS. Similar to Xpa−/− mice, an age-dependent increase of γ-OHPdG was seen in the livers of these mice but not in the lungs (a nontarget tissue) (Fig. 5B). These results are consistent with previous reports that DEN causes oxidative stress and induces LPO.40 Similar to the results in Xpa−/− mice, Theaphenon E showed a remarkable suppression of HCC formation in these mice by decreasing tumor size from 10 mm to <1 mm, tumor multiplicity from 30 to 3 nodules per mouse, and incidence from 100% to 40% (Fig. 6).
γ-OHPdG AS A PROGNOSTIC BIOMARKER FOR HCC RECURRENCE IN PATIENTS
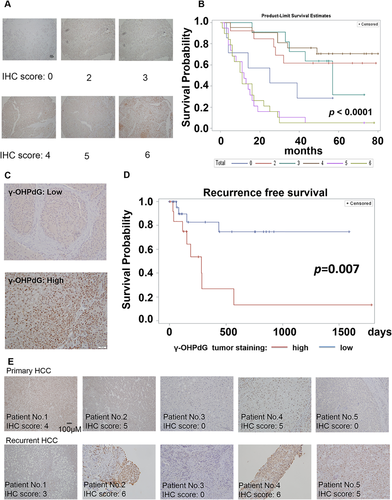
The results described above demonstrated that γ-OHPdG is closely associated with liver carcinogenesis in the animal models. To determine whether γ-OHPdG serves as a prognostic biomarker for the survival of HCC patients, we studied liver samples from a set of 90 HCC patients who underwent surgery without adjuvant therapy between August 2006 and November 2009. These patients were followed up for 4-7 years. We found that the high γ-OHPdG levels in the liver cancerous tissues were strongly correlated (P < 0.0001) with poorer survival in these patients (Fig. 7A,B). We then recruited 45 patients and examined the relationship of γ-OHPdG in the liver cancerous tissues from these patients after surgical resection with their recurrence-free survival. We did not observe a correlation between cancerous tissues and adjacent normal tissue in the levels of γ-OHPdG (Supporting Fig. S8). Patients with low γ-OHPdG tumors (immunohistochemistry score ≤3) experienced a significantly prolonged HCC recurrence-free survival compared to patients with high γ-OHPdG tumors (immunohistochemistry sore >3) (P = 0.007; Fig. 7C,D). After 2 years of follow-up from the date of curative surgical resection, the probability of no cancer recurrence in patients with low γ-OHPdG tumors was 75%, while the probability of no cancer recurrence in patients with high γ-OHPdG tumors was only 13% (P = 0.0002; Supporting Table S2). We also compared the levels of γ-OHPdG in the recurrent tumors to levels in primary HCC and found that 80% showed similar levels of γ-OHPdG compared to their primary HCC tumors (Fig. 7E). The data from the clinical studies strongly support γ-OHPdG as a biomarker for predicting the risk of human HCC recurrence.
Discussion
Liver cancer is a type of cancer that evolves over the course of decades and is extremely difficult to treat once diagnosed. Therefore, developing a mechanism-based, biologically relevant biomarker is extremely important for liver cancer intervention. The role of γ-OHPdG, an endogenous promutagenic DNA lesion preferentially formed at p53 mutation hot spots in human cancer,23 has not been investigated. In vitro studies showed that it is repaired by NER pathways and that it causes GC>TA and GC>AT mutations.21, 22 In this work, we provided evidence in vivo that γ-OHPdG is repaired by NER in animals as its levels are significantly higher in Xpa−/− mice compared to WT. We also found that γ-OHPdG levels increase age-dependently in Xpa−/− mice, indicating that the endogenous lesions accumulate over time. A similar trend was observed in LEC rats and DEN-injected mice. DEN-induced reactive oxygen species accumulation, which are known to induce HCC development, may play a role in the increase of γ-OHPdG in DEN-injected mice.41 Such an increase of γ-OHPdG was not observed in the lungs of Xpa−/− mice, suggesting a tissue-specific accumulation in the liver, possibly reflecting its relatively high lipid content in the liver and inhibition of NER by acrolein.42
The potential importance of γ-OHPdG in HCC is also supported by observations that there is an overwhelming representation of GC>TA mutation in Xpa−/− mouse liver tumors. Except for lung cancer, human HCC is the only solid cancer to show a relatively high rate of GC>TA mutation.1 The mutation signature in Xpa−/− mice coincides with the signature of mutational processes of human HCC with a prevalence at 12.1%,2 linking DNA lesions on the G:C pair with HCC. When comparing the mutations in human HCC, we found that a number of genes overlapped; for example, up to 121 mutations per nodule are detected with a high frequency of mutations on the G:C pair.1 This was explained primarily by the methylated cytosine.1 It is known that methylation at CpG sites enhances γ-OHPdG formation at these sites; moreover, CpG methylation greatly increases γ-OHPdG-induced GC>AT and GC>TA mutation frequency.43 Although other DNA lesions associated with oxidative stress may also induce GC>TA, such as 8-oxo-7,8-dihydro-2′-deoxyguanosine, data from these studies together implicate γ-OHPdG as a significant lesion that causes the high-frequency mutations at CpG sites found in human HCC.
A cohort study in Ohsaki, Japan, showed that consumption of green tea is associated with a reduced risk of human liver cancer incidence.44 Other possible explanations for tumor inhibitory effects of Theaphenon E come from evidence showing that tea polyphenols, especially epigallocatechin gallate, can alter DNA methylation patterns of genes and the nuclear factor erythroid 2–related factor 2 pathway.45 Tea polyphenols may possess multiple functional roles; however, we focused in this study on their ability to block the formation of an endogenous DNA adduct as a critical mechanism. Theaphenon E, a green tea extract, is a formulation containing a well-defined composition of polyphenols, mostly epigallocatechin gallate, the most abundant and potent antioxidative catechin in green tea (Supporting Table S5). α-Lipoic acid is an organosulfur antioxidant. Unlike α-lipoic acid and Theaphenon E, α-tocopherol is a lipid-soluble antioxidant. In fact, α-tocopherol has been associated with an increased risk of prostate cancer in healthy men.27 In contrast, the α-tocopherol, β-carotene trial showed that α-tocopherol reduces prostate cancer incidence of heavy smokers. These conflicting clinical outcomes are plausibly due to the lack of valid biomarkers and may highlight the importance of identifying the high-risk population suitable for intervention trials.
Oxidative stress has emerged as a crucial factor in HCC development under various pathological conditions.46, 47 Moreover, it has been shown to be involved in migration, invasion, and metastasis of HCC.48 Serum quantification of derivatives of reactive oxygen metabolite levels, a simple method for measuring hydrogen peroxide, is found to predict the risk of HCC recurrence.49 These findings highlight the potential of developing oxidative stress–related biomarkers as prognostic tools for HCC and its recurrence.
Our studies demonstrated that the LPO-derived γ-OHPdG is closely associated with liver carcinogenesis; it serves as a mechanistically relevant biomarker for HCC. Its levels in HCC, but not in normal adjacent liver, are highly reliable at predicting survival and recurrence-free survival in HCC patients (Supporting Fig. S9). More importantly, γ-OHPdG is independent of other known prognostic biomarkers such as microvascular invasion and tumor differentiation (Supporting Fig. S10).50 The clinical applications of γ-OHPdG as a prognostic biomarker for HCC in guiding future intervention trials warrant study.
Acknowledgment
We thank Drs. Bin Gao and Mingjiang Xu from the National Institute on Alcohol Abuse and Alcoholism, Moon-Shong Tang from New York University School of Medicine, and Xiaolin Wu from the National Cancer Institute for the discussions. We thank Dr. Yukihiko Hara for the generosity of providing Theaphenon E. We thank Maria Cruz, Carlos Benitez, Sanchita Sarangi, Zhuoli Xuan, Yuzana Khine Zaw, and Umar Khan for the animal bioassay and DNA sample preparations.
REFERENCES
Author names in bold designate shared co-first authorship.




