Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology
Potential conflict of interest: Nothing to report.
Supported by Fondation ARC no. PDF2014601431.
Abstract
The ErbB/HER family comprises four distinct tyrosine kinase receptors, EGFR/ErbB1/HER1, ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4, which trigger intracellular signals at the origin of essential cellular functions, including differentiation, proliferation, survival, and migration. Epithelial cells, named cholangiocytes, that line intrahepatic and extrahepatic bile ducts, contribute substantially to biliary secretory functions and bile transport. Although ErbB receptors have been widely studied in cholangiocarcinoma (CCA), a malignancy of the biliary tract, knowledge of these receptors in biliary epithelium physiology and in non-malignant cholangiopathies is far from complete. Current knowledge suggests a role for epidermal growth factor receptor (EGFR) in cholangiocyte specification and proliferation, and in hepatocyte transdifferentiation into cholangiocytes during liver regeneration to restore biliary epithelium integrity. High expression and activation of EGFR and/or ErbB2 were recently demonstrated in biliary lithiasis and primary sclerosing cholangitis, two cholangiopathies regarded as risk factors for CCA. In CCA, ErbB receptors are frequently overexpressed, leading to tumor progression and low prognosis. Anti-ErbB therapies were efficient only in preclinical trials and have suggested the existence of resistance mechanisms with the need to identify predictive factors of therapy response. This review aims to compile the current knowledge on the functions of ErbB receptors in physiology and physiopathology of the biliary epithelium. (Hepatology 2018;67:762-773).
Abbreviations
-
- AKT
-
- kinase B protein
-
- Arf6
-
- ADP-ribosylation factor 6
-
- AV
-
- ampulla of Vater
-
- BAs
-
- bile acids
-
- BDL
-
- bile duct-ligated
-
- BPK
-
- Balb/c-bpk/bpk
-
- CAF
-
- cancer-associated fibroblasts
-
- CCA
-
- cholangiocarcinoma
-
- COX-2: cyclooxygenase-2; HCC
-
- hepatocellular carcinoma
-
- iCCA
-
- intrahepatic CCA
-
- eCCA
-
- extrahepatic CCA
-
- EGF
-
- epidermal growth factor
-
- EGFR
-
- EGF receptor
-
- EMT
-
- epithelial-mesenchymal transition
-
- EP1
-
- prostaglandin E2 receptor 1
-
- ERK1/2
-
- extracellular signal-regulated kinases 1 and 2
-
- GB
-
- gallbladder
-
- HB-EGF
-
- heparin-binding EGF-like growth factor
-
- HGF
-
- hepatocyte growth factor
-
- HPC
-
- hepatic progenitor cell
-
- iCCA
-
- intrahepatic CCA
-
- IHC
-
- immunohistochemistry
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- Mcl-1
-
- myeloid cell leukemia sequence 1
-
- MEK
-
- mitogen-activated protein kinase kinase
-
- MMP
-
- metalloproteinase
-
- mTOR
-
- mammalian target of rapamycin
-
- MUC5AC
-
- mucin 5AC
-
- NASH
-
- nonalcoholic steato hepatitis
-
- PI3K
-
- phosphoinositide 3 kinase
-
- PBC
-
- primary biliary cholangitis
-
- PCK
-
- polycystic kidney
-
- PKD
-
- polycystic kidney disease
-
- PLD
-
- polycystic liver disease
-
- PSC
-
- primary sclerosing cholangitis
-
- PG
-
- prostaglandins
-
- PGE2
-
- prostaglandin E2
-
- ROS
-
- reactive oxygen species
-
- S1PR2
-
- shingosine 1-phosphate receptor 2
-
- TACE
-
- TNF-α converting enzyme
-
- TNFα, tumor necrosis factor α; TGFα, transforming growth factor α; TGR5
-
- G-protein-coupled bile acid receptor 5
-
- TK
-
- tyrosine kinase
-
- TLR4
-
- Toll-like receptor 4
-
- TKI
-
- tyrosine kinase inhibitor
-
- TME
-
- tumor microenvironment
-
- VEGFR
-
- vascular endothelial growth factor receptor
ErbB/HER Family
The ErbB family of receptor tyrosine kinases comprises epidermal growth factor (EGF) receptor (EGFR; ErbB1/HER1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4; Fig. 1). These plasma membrane receptors are composed of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with a conserved tyrosine kinase (TK) domain, with the exception of ErbB3 which holds an inactive TK domain. They bind specific ligands belonging to the EGF family, with the exception of ErbB2 which has no known ligand. Thus, ErbB2 and ErbB3 are activated through heterodimerization with other family members. ErbB ligands are produced as transmembrane precursors and processed by enzymes of the ADAM family, disintegrins, and metalloproteinases (MMPs), leading to the shedding of proligands and soluble growth factors release.1 Each ErbB receptor binds to specific ligands (Fig. 1). Upon ligand binding, homodimerization or heterodimerization of the receptor and subsequent phosphorylation of the TK domain occurs, allowing activation of intracellular signaling pathways1 (Fig. 1). These cytoplasmic pathways transmit the signal to the nucleus where many transcription factors undergo activity changes, initiating waves of transcription programs.
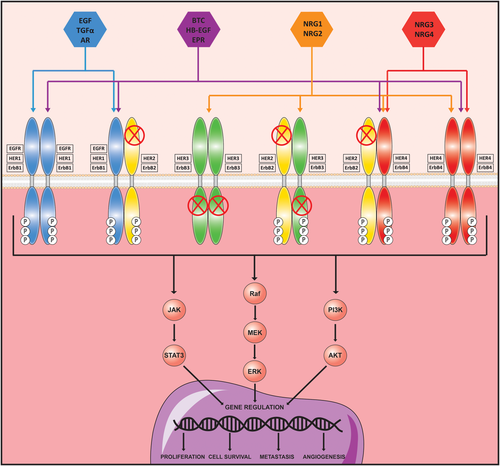
ErbB family of receptor tyrosine kinases. The ErbB family includes four members, EGFR/ErbB1/HER1, ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4. All ErbB receptors have in common an extracellular ligand-binding domain, a single membrane-spanning region, and a cytoplasmic protein TK domain. However, ErbB2 does not have any known ligand and naturally exists in a heterodimer form. Note also that ErbB3 lacks kinase activity, but can form heterodimers, especially with ErbB2, acting as an activating receptor. Their affinity for their ligands varies among the different ErbB members. Once activated and phosphorylated, they relay their signal through the main intracellular signaling pathways: JAK/STAT3, Raf/MEK/ERK, and PI3K/AKT. Abbreviations: AR, amphiregulin; BTC, betacellulin; EPR, epiregulin; JAK, janus kinase; NRG1-4, neurigulin 1-4; STAT3, signal transducer and activator of transcription 3.
EGFR plays a fundamental role in normal physiology of epithelial cells.(1) Other ErbB receptors are involved in cardiac and nervous development.(1) Multiple studies have shown that ErbB receptors are also involved in numerous pathophysiological functions, including cell proliferation, differentiation, survival, adhesion, and migration.1, 2
This review aims to give an overview of the current knowledge on the role of ErbB receptors in cholangiocyte biology during physiological events and pathological conditions. Finally, therapeutic options using anti-ErbB drugs will be discussed.
ErbB Family in the Liver
ErbB receptors have generated great interest since the discovery of the liver's involvement in EGF clearance. Early in the 1970s, it was shown that rat hepatocytes express EGFR and that the liver has the capacity to sequester EGF and secrete it into the bile.3 By immunohistochemistry (IHC), EGFR is not detected or is barely detected in human hepatocytes,4 while it is detected in rodent hepatocytes at their sinusoidal and lateral surfaces, along with ErbB3.5, 6 In cholangiocytes, EGFR is present at the basal membrane of rat7 and human cholangiocytes.8, 9 On the contrary, ErbB2 expression is detected in normal bile ducts only in one study, in large bile ducts.10 Both ErbB3 and ErbB48 are barely or not detected in normal cholangiocytes of portal areas in human liver. Among ErbB ligands, transforming growth factor α (TGFα) is expressed by cholangiocytes.9
ErbB Receptors in Cholangiocyte Physiology
Cholangiocytes are a heterogeneous dynamic population of epithelial cells that line the bile ducts, known as the biliary tree. Their major physiological function lies in the modification of primary hepatic canalicular bile through both secretion and absorption processes. So far, no ErbB receptor has been involved in the regulation of cholangiocyte transport functions.
CHOLANGIOCYTE SPECIFICATION
During liver organogenesis, hepatic progenitor cells (HPCs), called hepatoblasts, differentiate into hepatocytes or cholangiocytes.11 Upon EGF treatment, the HPPL cell line, derived from mouse hepatoblasts, formed biliary cysts and developed epithelial polarity in three-dimensional culture with specific biliary markers, suggesting a role for EGF in biliary morphogenesis.12 In fetal liver, the Notch pathway plays a major role in the differentiation of hepatoblasts residing in the portal area into cholangiocytes, whereas in adult liver it controls the specification of HPC differentiation toward cholangiocytes and bile duct morphogenesis.11 Kitade et al., using clonal HPC lines generated from liver-specific EGFR knockout mice on a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet, have shown that EGFR-mediated Notch1 signaling was essential for controlling commitment of HPC to biliary lineage.13 Indeed, deletion of EGFR in mouse HPC cells abolished biliary markers expression and branching morphogenesis and strongly down-regulated Notch1. Reintroduction of EGFR restored the branching phenotype and the expression of Notch1 and its target genes, Hes family BHLH transcription factor 1 (Hes1) and SRY (sex determining region Y)-box 9 (Sox9). The role of the EGFR/Notch1 pathway should be confirmed in human liver as well as its applicability during embryonic liver development. Recently, the EGFR/guanine nucleotide exchange factor 100 (GEF100)/ADP-ribosylation factor 6 (Arf6) signaling pathway has been envisaged as a regulator of intrahepatic biliary morphogenesis in a zebrafish model.14 Arf6 knockdown or pharmacological inhibition of EGFR in zebrafish embryo resulted in poor bile duct development. The deregulation of this pathway could notably be implicated in the pathogenesis of biliary atresia, a disease attributed to a defect in early bile duct development.
In regenerative medicine, cholangiocyte specification requires EGF, among other factors, to generate cholangiocytes from human pluripotent stem cells.15, 16 In Ogawa et al.'s study, Notch signaling is first activated in the hepatoblast population to induce the initial stages of cholangiocyte development. Then, the combination of growth factors, including EGF, allows early bile duct morphogenesis.16 However, EGF's precise role remains unclear, and lineage tracing studies evaluating the role of EGFR in this process are still needed.
LIVER REGENERATION
Liver regeneration is a complex process involving many mechanisms and growth factors that, together with their receptors, regulate proliferation. One property of regenerating liver is the ability of mature adult hepatocytes and cholangiocytes to interchange their phenotypes when needed. If a drastic loss of hepatocytes occurs, cholangiocytes can transdifferentiate into hepatocytes to regenerate the liver parenchyma.17 Conversely, hepatocytes can contribute to biliary regeneration in case of acute hepatobiliary or chronic biliary injuries in order to restore the biliary epithelium's structure and function.18 Although the involvement of an ErbB-dependent signaling has not been reported in the conversion of cholangiocyte into hepatocyte, EGFR may contribute to the conversion of hepatocyte into cholangiocyte-like phenotype.19 In rat organoid cultures, EGF, through kinase B protein (AKT)-independent phosphoinositide 3 kinase (PI3K) activation, was the only growth factor, along with hepatocyte growth factor (HGF), capable of promoting hepatocyte transdifferentiation into cholangiocytes. Gene array analysis identified the biliary markers cytokeratin 19, amphiregulin, and secretin receptors among the up-regulated genes.19 The role of ErbB receptors in cholangiocyte proliferation during liver regeneration is undetermined because models of cholangiocyte-specific EGFR knockout are missing.
ErbB Receptors in Non-malignant Cholangiopathies
Cholangiocytes are targets of a number of chronic biliary diseases also known as cholangiopathies. Main primary cholangiopathies regroup autoimmune cholangitis, primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC; formerly primary biliary cirrhosis), and biliary atresia.20 Rare genetic diseases can also affect the biliary tree, such as cystic fibrosis, polycystic liver disease (PLD), and Caroli's disease.20 Finally, chronic hepatolithiasis is a frequent secondary sclerosing cholangitis.
ROLE OF ErbB IN CHOLANGIOCYTES
Many studies imply that under the combination of endogenous and exogenous factors, cholangiocytes become reactive with secretion of proinflammatory molecules, leading to chronic inflammation of bile ducts, cholestasis, biliary epithelial cell proliferation, fibrosis, and malignant transformation.20 Therefore, in case of injury, cholangiocyte proliferation is at the crossroads of liver reparation and disease development. Cholangiocytes, virtually quiescent, maintain mitotic capability throughout adult life. This pathological proliferation of cholangiocytes has been studied in order to understand the link between biliary injury, cholangiopathies, and, later, cholangiocarcinoma (CCA). EGFR, ErbB2, and some of their ligands, have been implicated in these processes. EGF is a key mitogenic component for both murine and human cholangiocytes,21, 22 for hyperplastic bile ductular epithelial cells isolated from cholestatic liver (i.e., bile duct-ligated [BDL] mice and BDL/furan rat models), and is involved in ductal morphogenesis,23 and cell-cell junction integrity.24, 25
ErbB EXPRESSION IN CHOLANGIOPATHIES
Hepatolithiasis
EGFR and ErbB2 are frequently expressed in tissue samples with hepatolithiasis.(26-28) Immunohistochemical (IHC) analysis of human gallbladder (GB) tissues with gallstones showed overexpression of mucin 5AC (MUC5AC) associated with neutrophil infiltration and increased expression of EGFR and tumor necrosis factor α (TNFα).26 In vitro, EGF or TGFα, combined with TNFα, induced an overexpression of EGFR resulting in MUC5AC overproduction.26 In addition, bacterial infections and bile flow retardation contribute to stone formation and recurrence in hepatolithiasis.29 Lipopolysaccharide (LPS) increases MUC5AC expression in cholangiocytes by interacting with its receptor Toll-like receptor 4 (TLR4), which increases ADAM17-dependent cleavage of TGFα, promoting activation of EGFR.29 Accordingly, increased expression levels of ErbB ligands have been detected in BDL models.30 Finally, epithelial-mesenchymal transition (EMT), a reversible process by which epithelial cells acquire mesenchymal features, is involved in the development and progression of fibrosis, such as hepatolithiasis-induced biliary fibrosis.31 IHC analysis of human hepatolithiasis showed a strong expression of EGFR in the ductular epithelium that correlated with increased EMT-related protein expression,31 suggesting a link between EGFR and EMT in nontumor cholangiocytes. However, EMT pathophysiological significance in hepatolithiasis remains unclear. Given that cholangiocytes in cholangiopathies do not undergo EMT, but rather acquire some mesenchymal properties as part of a “reactive” phenotype,32 EGFR could take part in the regulation of this reactive phenotype.
PSC
Cholangiocytes from human PSC samples exhibited increased phospho-EGFR compared to normal livers and other liver diseases.33 In vivo, hepatocyte/cholangiocyte-specific ablation of EGFR in Mdr2 knockout mice (Mdr2–/–), led to an aggravation of liver fibrosis, along with a more prominent cholangiocyte proliferation compared to Mdr2–/– control mice, suggesting that cholangiocyte proliferation is independent of EGFR.34 ErbB2 was overexpressed in a small cohort of human samples of PSC, suggesting that it could represent an early dysfunctional event linked to human cholangiocarcinogenesis in this disease.35 Further studies should be pursued to identify whether ErbB members participate in cholangiocyte proliferation in PSC and whether ErbB expression/activation is predictive of CCA occurrence.
PLD
PLD and Caroli's disease are genetic diseases responsible for the formation of cysts along the biliary tract. IHC analysis of human adult biliary cysts failed to show signs of cholangiocyte proliferation, but revealed strong expression of EGFR in cyst epithelia and variable expression of ErbB2. This study suggested that cyst formation happens in a non-proliferative way despite the expression of ErbB receptors.36 On the other hand, in vitro, cholangiocytes isolated from the Balb/c-bpk/bpk (BPK) mice or polycystic kidney (PCK) rat demonstrated an increased sensitivity to the proliferative effect of EGF,22, 37 suggesting a role for EGFR in biliary epithelial hyperplasia and duct ectasia. Therefore, the EGFR tyrosine kinase inhibitors (TKIs), for example, EKI-785 and gefitinib, were expected to reduce biliary cyst formation. They were efficient in reducing biliary ductal ectasia in BPK mice and proliferation of cholangiocytes isolated from the PCK rat.22, 38 However, EKI-785 could not prevent in vivo the development of cystic liver disease in the PCK rat.39 Further studies are needed to conclude definitively on the role of EGFR in cyst formation in PLD.
ErbB Receptors in CCA
CCA is a heterogeneous group of malignant tumors that emerge along the biliary tree. CCA is often diagnosed at advanced stages and can rarely be treated by surgery, although it is the only curative treatment. For unresectable patients, the treatment is chemotherapy with a combination of gemcitabine and a platinum salt.40
Several studies have addressed the molecular and cellular mechanisms underlying the potential role of ErbB receptors in CCA physiopathology. An integrative genomic analysis on 149 intrahepatic CCA (iCCA) human samples identified two different classes: proliferation and inflammation.41 The proliferation class (62% of samples) was associated with worse outcome and 32% of patients had an EGFR overexpression,41 which suggests that EGFR is a low prognostic factor in CCA.
EGFR
Expression, Mutation and Amplification
Many studies have reported the expression of EGFR by IHC analyses in human CCA samples with a great variability (from 0% to 100%; Supporting Table S1). Some have shown that the expression of EGFR was associated with clinicopathological and poor prognostic features.
EGFR mutations are detected in CCA, in 0%-15% of cases, primarily in exons 18-21 coding for the EGFR TK domain (Supporting Table S2). Among mutations encountered, the T790M mutation in exon 20 was reported in few patients with CCA. This mutation has been involved in acquired resistance to EGFR TKI in other cancers. Furthermore, EGFR mutations were more frequent in CCA developed on chronic advanced liver disease.42 EGFR mutations also turned out to be low prognostic markers in CCA.41 Genetic polymorphisms of EGFR have been reported in CCA with a higher frequency than in hepatolithiasis, suggesting an association between EGFR polymorphisms and susceptibility of CCA and patient survival.43 Finally, EGFR amplification is rarely detected in CCA (Supporting Table S1).
Role of EGFR in CCA Physiopathology
EGFR regulates proliferation,44 migration, and invasion45 of CCA cells. Upon EGF stimulation, CCA cells exhibit sustained EGFR activation attributed to defective receptor internalization, which leads to proliferation.44 Moreover, sustained activation of EGFR, caused by the deregulation of NHERF1/EBP50, led to EMT-associated features, migration, and invasion.45, 46
EGFR signaling is complex because EGFR can be activated indirectly by various compounds known to participate in the pathogenesis of CCA, such as bile acids (BAs). BAs increased cellular myeloid cell leukemia sequence 1 (Mcl-1) protein levels, a potent antiapoptotic protein from the B-cell lymphoma 2 (Bcl-2) family, by inhibiting Mcl-1 degradation through the EGFR/Raf-1 signaling pathway47 and the production of cyclooxygenase-2 (COX-2) through the EGFR/mitogen-activated protein kinase (MAPK) signaling pathway.47 EGFR activation by BAs occurred through a TGFα-dependent mechanism involving MMP activity as a requisite for TGFα membrane release48 (Fig. 2). More recently, it was reported that conjugated BAs promoted the invasive growth of CCA through activation of sphingosine 1-phosphate receptor 2 (S1PR2; Fig. 2) followed by activation of the EGFR/extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling pathway.49 Finally, it was shown, in BDL mice, that BAs trigger cholangiocyte proliferation after binding to their G-protein-coupled bile acid receptor 1 (Gpbar-1 or TGR5; Fig. 2) and activation of the EGFR/ERK1/2 pathway.50 TGR5 was also found overexpressed in human CCA tissue, suggesting that the BAs/TGR5/EGFR pathway identified in nonmalignant cholangiocytes could also play a role in biliary malignancies.50
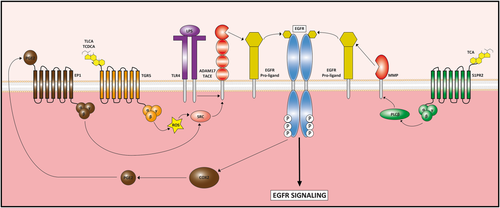
Mechanisms of indirect EGFR activation by bile acids (TLCA, TCDCA, and TCA), LPS, and PGE2. The three compounds are able to stimulate the cleavage of EGFR proligands to produce mature EGFR ligands that, in turn, increase EGFR activation and signaling. Bile acid activation of TGR5 and S1PR2 leads to EGFR proligand cleavage by ADAM17/TACE and MMPs thorough the activation of SRC and PLCβ, respectively. Similarly, TLR4 activation by LPS promotes ADAM17/TACE activity by unknown mechanisms. In addition, EGFR activation induces COX2 signaling, which increases PGE2 production. Then, PGE2 activates EP1 receptor that, in turn, induces SRC signaling to activate EGFR proligand cleavage by ADAM17/TACE. Abbreviations: ADAM17, ADAM metallopeptidase domain 17; PLCβ, phospholipase C-beta; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TLCA, taurolithocholic acid.
The other major component engaged in EGFR transactivation is the COX-2-derived prostaglandin E2 (PGE2; Fig. 2). In CCA, COX-2 and PGE2 are overexpressed and display mitogenic, antiapoptotic, and angiogenic functions.51 PGE2 released into the extracellular space binds to its receptor, prostaglandin E2 receptor 1 (EP1), a G-protein-coupled receptor, leading to EGFR/AKT axis activation that triggers CCA cell proliferation and invasion.52 To add a higher level of complexity, activation of EGFR by its prototypal ligand, EGF, causes increased production of COX-2 and PGE2, thereby generating a vicious cycle of COX-2/PGE2/EP1/EGFR signaling52 (Fig. 2). LPS, also by interacting with its receptor TLR4, is at the origin of EGFR transactivation (Fig. 2). By activating EGFR through TNF-α converting enzyme (TACE)-dependent TGFα release, LPS increases production of COX-2 and PGE2 that in turn creates a second wave of phosphorylation of EGFR and ERK1/2, triggering a feedback loop.53
Interleukin (IL)-6 is another proinflammatory cytokine that contributes to chronic biliary inflammation and CCA carcinogenesis. Overexpression of IL-6 decreased EGFR promoter methylation, resulting in an increase of EGFR expression in CCA cells.54 Finally, resistance to oxidative stress leads to abnormal proliferation and transformation. H2O2-induced oxidative stress activated the MAPK–activated protein kinase 2 (MK2)–dependent transduction pathway, which itself activated the heparin-binding EGF-like growth factor (HB-EGF)/EGFR axis, therefore allowing cells to survive in this oxidative environment.55
Altogether, EGFR acts as a hub by integrating multiple external information, including its own ligands and other compounds such as BAs, bacterial products, and inflammatory factors, promoting genesis and progression of CCA.
ErbB2
ErbB2 expression levels vary from 0% to 82% in human CCA specimens (Supporting Table S3). Some studies have investigated ErbB2 gene amplification and found results varying from 0% to 100% of samples, depending on methods and localization of the malignancy along the biliary tree. Results on the link between ErbB2 expression and clinicopathological and prognostic factors were also discordant, although no study has shown any impact on survival (Supporting Table S3). In addition, ErbB2 mutation in CCA remains a marginal event (Supporting Table S2).
Transgenic mice specifically overexpressing ErbB2 in epithelial tissues showed development of GB carcinomas and tumors all along the biliary tract.56 Likely, transplantation of BDEneu cells (an immortalized rat cholangiocyte cell line overexpressing ErbB2/neu) in the rat biliary tract resulted in the development of CCA-like tumors.57 The BDEneu cells showed an up-regulation of COX-2.57 and combined therapies with anti-COX-2 and anti-ErbB2 inhibit cell growth.58 A study in rodents also showed up-regulation of COX-2 associated with overexpression of ErbB2/EGFR heterodimers in GB epithelium.56 These results as well as immunostaining observations of COX-2 and ErbB2 in human CCA tissues35 suggest that ErbB2 might play a key role in regulating COX-2 expression in precancerous and neoplastic cholangiocytes. Conjugated BAs increased proliferation of CCA cells through the activation of EGFR/ErbB2 heterodimer.59
ErbB3 AND ErbB4
Expression of ErbB3 was observed in 11.8%-39% of CCA cases in various studies and was associated with poorly differentiated tumors and decreased survival (Supporting Table S4). ErbB3 mutations were identified in 11.8% of GB carcinoma samples, with ErbB3 being the most frequent ErbB mutated receptor (Supporting Table S2). Fluorescent in situ hybridization analysis demonstrated a gene amplification of HER3 in 23% of CCA samples. ErbB4 was expressed in 39.5%-63.1% of CCA samples (Supporting Table S5). Its expression was related directly to lymph node metastasis and to a better survival in EGFR-negative iCCA (Supporting Table S5). So far, one missense mutation in HER4 has been reported in iCCA (Supporting Table S2). Although both receptors are expressed in CCA, the pathophysiological mechanisms underlying their roles in CCA are still unknown.
ROLE OF TUMOR MICROENVIRONMENT IN ErbB SIGNALING
Increasing evidence indicates that the tumor microenvironment (TME), with its diversity of cell types and extracellular components, does not simply provide an anatomically supporting tissue, but also contributes to cancer progression and chemoresistance. At the cellular level, the TME in CCA is abundantly composed of cancer-associated fibroblasts (CAFs),40 suggesting a fundamental role for these cells in CCA biology, which has been confirmed by several studies, including ours.40, 60 We showed that CAFs from CCA expressed EGFR ligands, including HB-EGF, which promote CCA cell invasion through activation of the HB-EGF/EGFR axis.60
Anti-ErbB Therapies in Cholangiopathies
There are two major classes of anti-ErbB therapies (Supporting Table S6): monoclonal antibodies, which block ligand binding, and TKI, which target the catalytic domain of the receptor.
IN NON-MALIGNANT CHOLANGIOPATHIES
EGFR TKIs have been tested in models of PKD/PLD. They were efficient in the kidney,61 but results in the liver were contradictory. As mentioned, EGFR TKIs were efficient in reducing biliary ductal ectasia in BPK mice,38 but could not prevent the development of cystic liver disease in the PCK rat.39 Further studies are needed to verify these results.
In a rat model of proliferative cholangitis, treatment with anti-EGFR reduced biliary epithelium hyperplasia, fibrosis, and hyperplasia of peribiliary glands.62 Similarly, in the BDL model, erlotinib significantly reduced fibrosis.63 To date, there are no clinical trials with these therapies.
IN MALIGNANT CHOLANGIOPATHIES
Preclinical Assays
Treatment of CCA cells with anti-EGFR therapies (gefitinib or cetuximab) inhibits cell growth44, 64 and induces G1-phase arrest and apoptosis.64, 65 ErbB2 inhibitors alone were also effective in vitro in CCA cell lines.66 Moreover, EGFR/ErbB2 combined inhibition was more efficient than either anti-EGFR or anti-ErbB2 alone.66 Anti-ErbB therapies have also been tested combined with other types of treatments (chemotherapy or non-ErbB-targeted therapies). Gefitinib/erlotinib and lapatinib (dual EGFR/ErbB2) had antiproliferative effects in CCA cell lines when combined with gemcitabine.67 Combination of anti-EGFR therapies with other targeted therapies, such as mitogen-activated protein kinase kinase (MEK),68 mammalian target of rapamycin (mTOR),69 or vascular endothelial growth factor receptor (VEGFR)70 inhibitors, showed growth inhibition in various CCA cell lines. Besides cell proliferation, EGFR TKIs, such as gefitinib, reduce migratory and invasive properties of CCA cells45, 46 by interfering with EMT.
In vivo, administration of gefitinib was efficient in reducing tumor growth in CCA45 and GB.71 In a mouse CCA xenograft model, EGFR inhibition by gefitinib prevented the ectopic expression of E-cadherin in the cytoplasm and restored its membrane expression in CCA cells,45 implying that gefitinib could reverse EMT in CCA. Similarly, the anti-ErbB2 therapy, pertuzumab, reduced tumor growth in xenografted models of CCA.72 Furthermore, combination of treatments also proved effective in vivo. Association of erlotinib and cetuximab led to tumor growth arrest.73 In mice overexpressing ErbB2, GW2974, a dual EGFR/ErbB2 inhibitor, showed chemopreventive efficiency with a decrease in development of tumors along the biliary tree.71 Other dual anti-EGFR/ErbB2 inhibitors, such as lapatinib66 or NVP-AEE788,74 were more efficient than anti-ErbB therapies alone. Finally, combination of anti-EGFR therapies with other targeted therapies, such as as MEK,68 mTOR,69 or VEGFR70 inhibitors, also showed antitumor effect in vivo.
Clinical Trials
Among anti-ErbB therapies in CCA, anti-EGFR therapies have been the most studied. Several clinical trials were conducted with these drugs, alone or in combination with other therapies or chemotherapies (Supporting Table S7). Although they showed efficacy in preclinical studies, they did not show significant improvement of overall survival in phases II and III clinical trials. One recent open-label phase II trial found a higher-than-expected clinical benefit rate with the combination of gemicitabine, cisplatine, and panitimumab in KRAS wild-type CCA patients, suggesting a possible benefit of anti-EGFR therapies in selected patients. The only phase III comparing GEMOX (gemcitabine and oxaliplatine) with erlorinib versus chemotherapy alone did not show any difference in median overall survival. Irreversible blockers of ErbB receptors have been developed and are being tested in clinical trials. Indeed, one clinical trial showed limited, but encouraging, activity for afatinib in some patients with CCA. Only one clinical trial has assessed the efficacy of lapatinib in advanced CCA with a 0% response rate. Finally, a recent phase Ib study showed longer median overall survival in CCA patients treated with pulsatile erlotinib combined with chemotherapy compared to patients treated with standard chemotherapy, suggesting an effect for pulsatile administration of anti-EGFR. Nevertheless, results from clinical trials have been disappointing, suggesting the existence of resistance mechanisms to these therapies in CCA.
RESISTANCE MECHANISMS TO ANTI-ErbB THERAPIES
Two types of resistance (primary or innate, and secondary or acquired) are involved in anti-EGFR treatment failure in cancer.
Primary resistance often occurs as a result of primary mutations. EGFR mutations were first described in lung cancer, where they were responsible for up-regulation of the downstream signaling pathways, conferring higher sensitivity to gefitinib.75 As mentioned before, primary mutations of EGFR TK domain have been found in CCA (Supporting Table S2), but their impact on anti-EGFR sensitivity is unknown. Resistance to anti-EGFR treatment in CCA can also result from primary mutations in downstream signaling proteins (e.g., BRAF or KRAS). Mutations in KRAS and BRAF are found, respectively, in 3%-54% and in 0%-33% of CCA.76 KRAS mutations are known to preclude any therapeutic benefit from anti-EGFR therapies in other cancers, but only two studies have suggested this effect in CCA for erlotinib and panitumumab.77, 78 The recent development of a patient-derived xenograft model of iCCA bearing the most frequent KRAS mutation (G12D) should provide answers on the role of this mutation in anti-EGFR treatment efficacy.68
Secondary resistance appears under long-term anti-EGFR treatment. In lung cancer, the EGFR T790M secondary mutation leads to resistance to anti-EGFR therapies.75 No secondary EGFR mutation is known in CCA. However, under targeted therapy, tumor cells can use alternative signaling pathways through other growth receptors. In CCA, we recently underlined cellular and molecular mechanisms involved in secondary resistance to erlotinib. More specifically, we observed an activation of the insulin-like growth factor signaling axis that regulates an EMT program and stemness in erlotinib-resistant cells.79
Conclusion
ErbB receptors are involved in many physiological and pathological functions. EGFR does not seem crucial for liver development, but may play a role in cholangiocyte specification and bile duct morphogenesis. Although many studies have shown roles for ErbB receptors (especially EGFR) in hepatocytes during liver regeneration and in hepatic stellate cells during biliary fibrogenesis, their specific role in cholangiocytes remains unknown, and only mice models with specific deletion of ErbB receptors in cholangiocyte lineage would help highlight their role in development and diseases. Several signaling pathways leading to cholangiocyte proliferation are involved in different cholangiopathies, which are main risk factors for CCA. Advances in understanding the molecular basis of CCA have been made, but all mechanisms have not yet been clarified. There is an evident role of the ErbB family in CCA with EGFR and ErbB2 at the front line. Indeed, they are often overexpressed and associated with low prognostic factors. Recent data have revealed specific genetic mutations, aberrant signaling pathway activation, and microenvironment interactions, which are responsible for low prognosis and resistance to treatments in CCA. However, further research is necessary in order to decipher the role of this family in cholangiocyte pathophysiology, especially that of ErbB3 and ErbB4, which have been poorly investigated in cholangiocyte pathophysiology. Preclinical evidence of the efficiency of anti-ErbB therapies in CCA is scarce and results in clinical trials are disappointing. Data on the mechanisms involved in the chemoresistance to these molecules are lacking. Altogether, further studies are needed to enhance the molecular understanding of the role of the ErbB receptors in order to develop better therapies targeting major components of the ErbB signaling network.
Acknowledgments
A.P. is a medical intern who has received a scholarship “Year of research” from the French government. J.V. is a recipient of two following postdoctoral fellowships from the Spanish Association for the Study of the Liver (AEEH) and the Fondation ARC No. PDF2014601431. L.F. is supported by grants from Fondation de France (No. 2014 00047502) and La Ligue National contre le Cancer (No. RS14/75-112).
REFERENCES
Author names in bold designate shared co-first authorship.




