Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group
Potential conflict of interest: Nothing to report.
C.S. is supported by KFO306 (DFG), the Helmut and Hannelore Greve-Foundation, and the YAEL-Foundation.
Abstract
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder characterized by inflammation and fibrosis of the intra- and/or extrahepatic bile ducts. Magnetic resonance imaging (MRI) is a noninvasive imaging modality that can be used to diagnose PSC and detect disease related complications. Quantitative MRI technologies also have the potential to provide valuable prognostic information. Despite the potential of this imaging technology, the clinical application of MRI in the care of PSC patients and imaging standards vary across institutions. Moreover, a unified position statement about the role of MRI in the care of PSC patients, quality imaging standards, and its potential as a research tool is lacking. Conclusion: Members of the International PSC Study Group and radiologists from North America and Europe have compiled the following position statement to provide guidance regarding the application of MRI in the care of PSC patients, minimum imaging standards, and future areas of research. (Hepatology 2017;66:1675–1688).
Abbreviations
-
- 2D
-
- two-dimensional
-
- 3D
-
- three-dimensional
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- CCA
-
- cholangiocarcinoma
-
- CT
-
- computed tomography
-
- DWI
-
- diffusion weighted imaging
-
- ERCP
-
- endoscopic retrograde cholangiopancreatography
-
- Gd-BOPTA
-
- gadobenate dimeglumine
-
- Gd-EOB-DTPA
-
- gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid
-
- GRADE
-
- Grading of Recommendation Assessment, Development, and Evaluation
-
- IAC
-
- IgG4-associated cholangitis
-
- IBD
-
- inflammatory bowel disease
-
- IgG4
-
- immunoglobulin G4
-
- IPSCSG
-
- International PSC Study Group
-
- kPa
-
- kilopascals
-
- LT
-
- liver transplantation
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- MRE
-
- magnetic resonance elastography
-
- MRI
-
- magnetic resonance imaging
-
- PH
-
- portal hypertension
-
- PSC
-
- primary sclerosing cholangitis
-
- T
-
- Tesla
-
- T1w
-
- T1-weighted
-
- T1wI
-
- T1-weighted image
-
- T2w
-
- T2-weighted
-
- T2wI
-
- T2 weighted image
-
- TE
-
- transient elastography
Primary sclerosing cholangitis (PSC) is a rare and progressive disease in which biliary inflammation and fibrosis lead to bile duct strictures, cirrhosis, death, or liver transplantation (LT) within a median of 15-20 years,1, 2 The disease is frequently associated with inflammatory bowel disease (IBD). PSC is a premalignant condition associated with an increased risk of colorectal and hepatobiliary neoplasia.3
Cholangiography is required to diagnose large-duct PSC.4, 5 Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) has been established as the noninvasive imaging modality of choice when PSC is suspected.4, 5 However, MRI imaging standards and protocols vary across institutions. Furthermore, there is a great unmet need for imaging techniques that enable (1) the early detection of disease, (2) the determination of disease stage, activity, and prognosis, (3) the assessment of treatment response, (4) a clinically meaningful definition of dominant bile duct stenoses, and (5) the early detection of cholangiocarcinoma (CCA). MRI/MRCP offers a noninvasive and rapidly developing technique to potentially address all of these needs. However, up until now there is no well-defined technical standard on how to perform MRI/MRCP in PSC.
Because of these unmet needs, the International PSC Study Group (IPSCSG) created a working group on MRI in PSC that brought together an international team of hepatologists and radiologists with expertise in PSC. This group aimed to assess current practice across different countries with regard to the use of MRI/MRCP in PSC, define a minimum quality standard for MRI /MRCP in PSC, and codify the role of MRI imaging in PSC diagnosis and management. In addition, key research questions were formulated, which need to be answered in order to improve patient care and to avoid unnecessary health-related costs in the future.
Methods and Consensus Process
The IPSCSG introduced a working group on MRI in PSC in 2015. Over 30 experts in the field of hepatology and radiology from 10 different countries met for a 1-day workshop in October 2015 in Hamburg in order to assess current practices across different countries with regard to the use of MRI in PSC and to define a minimum quality standard for MRI and MRCP in PSC. In addition, research questions were formulated, which need to be answered in order to improve patient care and to avoid unnecessary health-related costs in the future. Because information from clinical trials was found to be insufficient to give strong evidence-based recommendations on the use of MRI in PSC, the working group decided to formulate a position statement, reviewing current literature and recommending on evidence, if available, and expert opinion on the use of MRI in PSC. The writing committee consisted of the two working group leads (C.S. and J.E., both hepatologists) and three radiologists highly experienced in the field of MRI (K.I.R., S.V., and J.Y.). The recommendations were discussed at a second working group meeting held in Hamburg in September 2016. Consensus was reached on the recommendations and the revised position statement was sent for review to all members of the IPSCSG. Changes were incorporated and the statement was approved at the IPSCSG meeting during the American Association for the Study of Liver Diseases (AASLD) Boston meeting in 2016. The revised version was approved by the IPSCSG steering committee and the working group members in April 2017.
These recommendations provide a data-supported approach when possible. They are based on the following: (1) formal review and analysis of the recently published literature and (2) the experience of the working group members in the specified topic. Intended for use by physicians, these recommendations suggest preferred approaches to the diagnostic, therapeutic, and preventive aspects of care. They are intended to be flexible, in contrast to standards of care, which are inflexible policies to be followed in every case. Specific recommendations are based on relevant published information and expert opinion. To characterize the available evidence supporting the recommendations, the grade of evidence and strength of recommendation were given according to the modified classification by the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) work group, with minor modifications, as suggested in the AASLD practice guideline recommendation (Table 1).6 Grade and strength of evidence was consented by the members of the working group.
| Strength of Recommendation Criteria | |
| Strong (1) | Factors influencing the strength of the recommendation included the quality of the evidence, presumed patient important outcomes, and cost. |
| Weak (2) | Variability in preferences and values, or more uncertainty. Recommendation is made with less certainty, higher cost, or resource consumption. |
| Quality of Evidence Criteria | |
| High (A) | Further research is unlikely to change confidence in the estimate of the clinical effect. |
| Moderate (B) | Further research may change confidence in the estimate of the clinical effect. |
| Low (C) | Further research is very likely to impact confidence on the estimate of clinical effect. |
MRI/MRCP Overview
TECHNICAL REVIEW
MRCP uses high-strength magnets and takes advantage of the high T2-weighted (T2w) signal intensity of bile relative to surrounding structures to provide detailed images of the biliary tree and the pancreatic duct. Because this imaging modality is dependent on T2w images, contrast agents are not required to obtain a cholangiogram. Patients complete the examination while fasting to reduce fluid in the surrounding enteric structures that can interfere with visualization of the duct anatomy.
MRI contrast agents are used to improve the detection and differentiation of mass lesions and inflammation and assess liver function. Most MRI contrast agents are gadolinium based, an element with strong paramagnetic properties. Based on their biodistribution after intravenous injection, currently available MR contrast agents can be classified as purely extracellular, or extracellular with a hepatocyte-specific component. Analogous to iodine-containing contrast agents used in computed tomography (CT), extracellular contrast agents for MRI (e.g., gadopentetate dimeglumine, Magnevist, gadobutrol, Gadovist, Bayer, Leverkusen, Germany; gadoterate meglumine, Dotarem, Guerbet, Villepinte, France) are well suited for assessment of the vascular system and lesion detection and characterization on the basis of tumor morphology and perfusion, resulting in a nonspecific enhancement behavior.7, 8
Hepatocyte-specific (hepatobiliary) contrast agents are also referred to as combined or bimodal agents, because they offer imaging properties of conventional extracellular and liver-specific gadolinium chelates. By chemical modification of the ligands with lipophilic side chains, partial hepatocellular uptake and subsequent biliary excretion is mediated, increasing the signal intensity of the liver, bile ducts, and hepatocyte containing lesions at T1-weighted (T1w) imaging. Lesion characterization thus depends not only on vascularity, but also on hepatocellular function.9, 10 The bimodal properties of hepatocyte-specific contrast agents allow for dynamic contrast-enhanced imaging as well as for image acquisition in the so-called hepatobiliary phase, including the acquisition of a contrast enhanced T1w MRC. Currently, two hepatocyte-specific contrast agents are available: Gd-BOPTA (gadobenate dimeglumine; MultiHance, Bracco Imaging, Milan, Italy) and Gd-EOB-DTPA (gadoxetate disodium; Primovist, Eovist, Bayer, Leverkusen, Germany). Hepatic uptake and biliary contrast elimination of these contrast agents depend on liver function and amount to approximately 50% in patients with normal liver and kidney function for Gd-EOB-DTPA and 3%-5% for Gd-BOPTA, respectively.11
Of note, kidney function and contraindications need to be considered when applying gadolinium-based MRI contrast agents. In addition to nephrogenic systemic fibrosis, it has recently been described that gadolinium deposits can be detected in the brain after repeated contrast-enhanced MRI in patients even with normal kidney function.12, 13 This seems to depend on the stability of the gadolinium chelate with the more unstable linear chelates of gadolinium associated with higher risk than the more stable macrocyclic formulations.14, 15 However, the long-term clinical meaning of these deposits remains unclear to date.14 In this context, risk should be balanced against the potential benefits of contrast-enhanced MRI in patients with PSC and adherence to recently published consensus recommendations is advisable regarding the technical aspects of MRI.8, 16
FUNCTIONAL MRI REVIEW: A NONINVASIVE MEASURE OF LIVER FUNCTION
Dynamic contrast-enhanced MRI for the assessment of hepatic perfusion uses gadolinium diethylene triamine pentaacetic acid (Gd-DTPA) or gadoxetic acid (Gd-EOB-DTPA).17, 18 Around 50% of Gd-EOB-DTPA is excreted by hepatocytes into bile canaliculi and the biliary system, allowing a two-compartment model measurement of liver perfusion and hepatobiliary function.19 Preclinical studies demonstrated the feasibility of dynamic hepatocyte-specific contrast-enhanced MRI to calculate functional parameters, such as hepatocyte extraction fraction and input-relative blood flow.20, 21 Calculating these parameters in patients with PSC, the segmental hepatocyte extraction fraction and input-relative blood flow was heterogeneously distributed throughout the liver and seemed to correlate with segmental biliary obstruction.20 This method is promising, but an external software is needed for the postprocessing and it seems too time-consuming for use in routine clinical practice.
In a recent MRI study, T1 mapping of the liver was shown to be a feasible technique to evaluate liver function on a global level and may be extrapolated on a segmental level in patients with PSC. T1 reduction correlated with liver enzymes, disease stage, and Mayo risk score.22 An emerging alternative method, which may be more applicable in the clinical setting, is the relative liver parenchymal contrast agent enhancement index, delivering quantitative information on contrast agent uptake as a sign of active inflammation or structural changes.23
MAGNETIC RESONANCE ELASTOGRAPHY REVIEW: A NONINVASIVE MEASURE OF LIVER FIBROSIS
Liver stiffness is a surrogate marker for fibrosis and can be measured by elastography. The two principle elastography techniques in clinical practice include magnetic resonance elastography (MRE) and shear-wave–based ultrasound techniques, such as transient elastography (TE). MRE involves delivering a shear wave to the patient through an external driver. The propagation of this shear wave is visualized with a special MRI sequence, and software algorithms use this information to generate an elastogram where regions are selected by a radiologist to determine the average stiffness (kilopascals; kPa).24 TE is an ultrasound shear-wave–based technology that can also measure liver stiffness. Both have been shown to correlate well with the histological stage of fibrosis and outcomes among patients with PSC.25-27 Use of MRE may offer several key advantages when compared to TE. For example, the performance of MRE is not influenced by obesity or anatomical constraints such as narrow intercostal spaces. Second, fibrosis in PSC can be patchy and MRE can assess more than 1,000 times the volume of liver than TE.28, 29 Next, MRE can be performed at the same time as MRCP without adding a significant amount of time or cost to the examination. This approach also allows for the identification of worsening strictures, which is important because the presence of a biliary obstruction may increase liver stiffness irrespective of the degree of fibrosis.30-32 To date, there are no head to head performance comparisons between TE or other shear-wave–based techniques and MRE among PSC patients, and MRE is not widely available in Europe.
In addition to MRE, diffusion-weighted imaging (DWI) can be used to quantify liver fibrosis and inflammation and may support the detection of liver tumors. However, it cannot discriminate fibrosis stages as well as MRE or TE and the technique has not been transferred into routine clinical practice.33-35
Establishing a Diagnosis of PSC with MRI/MRCP
CLINICAL NEED AND EVIDENCE SUMMARY
Cholangiography is required to diagnose large-duct PSC.4, 5 Practice guidelines from all major societies support MRCP as the first diagnostic modality in patients with suspected PSC.4, 5, 36 The classic cholangiographic features of large-duct PSC include multifocal strictures and areas of dilatation or ectasia and ductal wall thickening involving the intra- and/or extrahepatic bile ducts (Figs. 1 and 2). T1w, T2w, and contrast-enhanced T1w images may demonstrate thickening of the wall of large bile ducts and signs of periductal inflammation.37 Peripheral intrahepatic duct obliteration (commonly referred to as pruning) can also be observed. Notably, the exclusive involvement of extrahepatic bile ducts is infrequent, and, when encountered, an alternative diagnosis should be considered.38 Retraction of the papilla can also be observed on MRCP.39 The cholangiographic features of PSC are not pathognomonic, and distinguishing between primary versus secondary causes of sclerosing cholangitis, particularly when IBD is absent, is challenging.40 For example, in the absence of pancreatic or other immunoglobulin G4 (IgG4)-related organ involvement, IgG4 associated cholangitis (IAC) can appear very similar to PSC.41, 42
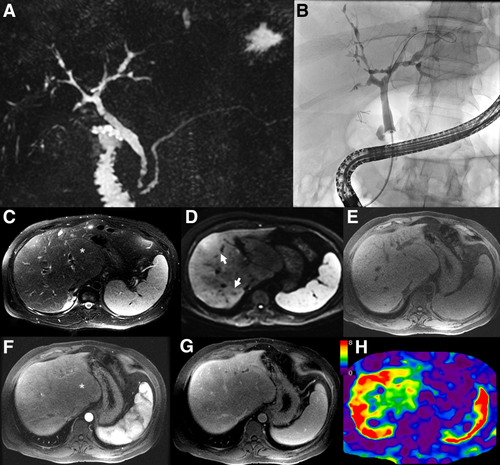
Parenchymal changes may also be observed in PSC. For example, the liver can show segmental or lobular atrophy with compensatory hypertrophy attributed to chronic bile duct obstruction43 (Fig. 2). Patchy areas of peripheral parenchymal enhancement and gallbladder enlargement have been reported in early PSC, but the sensitivity and specificity of these observations in PSC is unknown.44
The diagnostic accuracy of MRCP reaches that of endoscopic retrograde cholangiopancreatography (ERCP; 83% and 85%, respectively).45 A meta-analysis including studies performed between 2000 and 2006 has shown a sensitivity of 0.86 and a specificity of 0.94 for the diagnosis of PSC.46 Furthermore, performing an initial MRCP (rather than ERCP) has shown to be a cost-effective diagnostic approach.47, 48 Moreover, MRI including MRCP is noninvasive, does not expose patients to radiation, and it can assess the liver parenchyma and surrounding structures.
When MRCP was performed in a population of patients with IBD (regardless of symptoms or liver tests), it was useful in detecting subclinical PSC and increased the prevalence of PSC by 3-fold.49 Hence, MRCP has the potential to detect early PSC. In the future, early detection of PSC could aid in prolonging patient transplant-free survival once effective treatment options are available and has the potential to identify patients for clinical studies at a time point when anti-inflammatory or -fibrotic drugs still have a chance of being effective. However, even using modern 3 Tesla (T) scanners, MRI still has limitations in the assessment of the distal part of the common bile duct and also in detecting subtle pathologies of smaller peripheral bile ducts.37, 50, 51 Indeed, approximately 10% of patients with PSC will have a normal cholangiogram (small-duct PSC), and a liver biopsy is required to establish the diagnosis.4, 5, 36, 52
Variations in MRI scanners and imaging protocols may lead to image heterogeneity across institutions. The working group's discussion of a common minimal standard for performing MRI in PSC took into consideration the technical capabilities, cost and duration of the scanning protocol, the increased risk of diagnosing CCA within the first year of diagnosing PSC, and the requirement to compare imaging studies from different centers in future studies. These recommendations are not based on strong evidence, but on multidisciplinary expert discussion, and represent the opinion of the majority of hepatologists and radiologists attending the workshops and participating in the subsequent discussions. For more information on MRI technical aspects, we would like to refer to the recent consensus statement of the European Society of Gastrointestinal and Abdominal Radiology.8
GUIDANCE STATEMENTS
- MRCP should be the first diagnostic imaging modality in patients with suspected PSC. (1A)
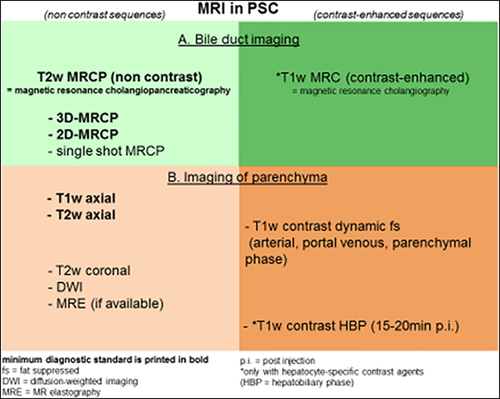
- The diagnostic workup of patients with suspected PSC can be performed using either the minimum standard alone (A) or a more complete workup that includes use of contrast media (B). (Fig. 3) (2C):
- Suggested minimum standard for the diagnostic workup of patients with suspected PSC:
- MRI scanners with field strength of at least 1.5T should be used. Modern 3T scanners yield higher spatial resolution and are preferred over 1.5T scanners if available.53 (1C)
- Ideally, MRCP should be performed before interventions or stent placement. (1C)
- A fasting period of a minimum of 4 hours is recommended before MRCP. Suppression of stomach and duodenal content signal can be helpful, for example, using diluted intravenous gadolinium contrast (e.g., 1 mL in 200 mL of water) or pineapple juice.54, 55 (1C)
- MRCP: T2w MRCP (better than T1w imaging for the visualization of second and third-order bile ducts)56; three-dimensional (3D)-MRCP (slice thickness of 1 mm is suggested) should be preferred over two-dimensional (2D)-MRCP because of the use of thinner section source images resulting in higher resolution. Consider 2D T2W single-shot sequences if the 3D acquisition has artifacts, the patient cannot hold breath consistently, or respiratory triggering is not feasible, which is required for good 3D-MRCP acquisition.37, 57 (1C)
- Orthogonal coronal plane acquisition covering most of the liver anterior to posterior are preferred for adequate evaluation of peripheral ducts.58 (1C)
- For MRI: T2-weighted image (T2wI) axial sequences should be used. T1wI should be considered, because it adds information on liver parenchyma. Fat-suppressed sequences should be preferred.37 (1C)
- The following complete workup is suggested for performing the first diagnostic MRI/MRCP in patients with suspected PSC. This workup includes the use of MRI contrast media:
- MRCP, see recommendation for minimum standard above (A). If a hepatobiliary contrast agent is used, high-resolution 3D MRCP sequences should be acquired before contrast injection.59-61 (1C)
- There are insufficient data to recommend one MRI contrast medium over another, so both options below could be followed:
- ○ for MRI with extracellular contrast, the following sequences should be applied: T1-weighted image (T1wI) with Gd-based extracellular contrast agent (precontrast, arterial, portal venous, parenchymal phase after 3-5 minutes, fat suppressed). T2wI axial and coronal. (1C)
- ○ for MRI with hepatobiliary contrast, the following sequences should be applied: T1wI with Gd-EOB-DTPA (precontrast, arterial, portal venous, delayed phase, hepatobiliary phase [fat suppressed]), as well as T2wI axial and coronal. (1C)
- DWI should be considered. (2C)
- If available, MRE should be considered. (1C)
- Suggested minimum standard for the diagnostic workup of patients with suspected PSC:
- Patients with suspected PSC should be assessed in experienced centers, which include the performance and interpretation of MRI. Patients unable to travel to specialty centers should have their MRI/MRCP images and clinical course presented to multidisciplinary teams with experience in PSC diagnosis and treatment. (1C)
Use of MRI/MRCP in Symptomatic PSC
CLINICAL NEED AND EVIDENCE SUMMARY
In patients presenting with symptoms of cholestasis or cholangitis, or worsening laboratory tests suggestive of biliary obstruction, endoscopic intervention can improve symptoms and may have a positive impact on disease progression.62 A preprocedural MRI/MRCP can provide valuable information to better guide an endoscopic or percutaneous intervention. This is exemplified by several clinical scenarios commonly encountered during the care of patients with PSC. First, it can provide a roadmap to better facilitate biliary drainage by: identifying the extent and location of strictures without the need to inject contrast in the biliary tree; recognizing the presence and location of atrophic segments so they can be avoided because stenting an atrophic lobe is unlikely to improve jaundice and is associated with infection63; targeting strictures associated with an abscess; assessing biliary anatomic variants (occurs in approximately 40% of the population); and postoperative anatomy (e.g., posttransplant anastomotic strictures).64 Second, T1w images may also aid in the detection of biliary stones, which often show a higher signal intensity on T1w images and may be found at higher frequencies in PSC, especially in prestenotic and dilated bile ducts.65 Third, it can allow more specific targeting of brushings and biopsies when there is concern for malignancy. Last, it can be used as a triage tool to determine whether an ERCP can be avoided.
MRI/MRCP can also detect disease-related complications. Chief among these is CCA (discussed below). However, MRI/MRCP can detect other malignancies associated with PSC, including hepatocellular carcinoma or gallbladder cancer. MRI/MRCP can distinguish between hepatic abscesses from malignant mimickers with accuracy greater than 95%, particularly when DWI is used.66 Furthermore, it can evaluate for the progression of biliary strictures.67 Endoscopic or percutaneous dilations are frequently performed in the setting of “dominant strictures,” and there is consensus that this should be done in patients with signs of bacterial cholangitis and new or worsening symptoms of cholestasis, such as pruritus or jaundice.4, 5 The term dominant stricture derives from ERCP studies and defines strictures of less than 1.5 mm in diameter in the common bile duct and less than 1 mm in the left or right and also common hepatic duct.68 However, the applicability of this definition and inter-rater agreement of so-called dominant strictures when MRI/MRCP is used is not well understood.
GUIDANCE STATEMENTS
- MRI/MRCP with contrast media should be performed among patients presenting with symptoms of worsening cholestasis, cholangitis, or if laboratory tests (including CA 19-9 levels) suggest worsening biliary obstruction before an ERCP or percutaneous cholangiogram. (1C)
Role of MRI/MRCP in CCA Diagnosis and Screening
CLINICAL NEED AND EVIDENCE SUMMARY
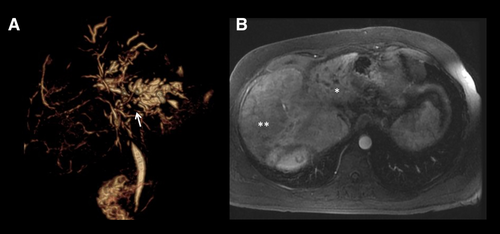
CCA may develop in approximately 10%-20% of individuals with PSC, and it is the leading cause of mortality.52, 69 The annual incidence of CCA is approximately 0.5%-2.0%, and nearly 30% of biliary cancers are detected within the first year of establishing a PSC diagnosis.52, 70 This illustrates that the development of a malignant biliary stricture can be a heralding event that brings patients to the attention of clinicians. When jaundice and other manifestations of a malignant biliary stricture occur, CCA is often detected at a late stage when curative therapy is no longer an option. For example, in a study that examined dominant strictures associated with CCA, median survival was only 6 months.71 Patients diagnosed with PSC late in life have an increased incidence of CCA when compared to their younger counterparts.72, 73 Early detection of CCA in this high-risk population is important. Protocols that utilize neoadjuvant chemoradiation, brachytherapy, and, ultimately, LT offer a potential cure among a select group of PSC patients with early-stage CCA.74 The presence of a mass lesion with delayed venous enhancement is nearly 100% specific for CCA (Fig. 4).36, 75, 76 Vascular encasement may also be present. Cross-sectional imaging is essential for delineating the size and extent of the tumor, which will, in turn, help determine patients' eligibility for curative therapies.74 Indeed, MRI/MRCP is more sensitive (89%) than CT (75%) and ultrasound (57%).75 Prospective studies have also reinforced that the sensitivity/specificity of MRI/MRCP (88%/85%) is better than CT (79%/79%) for CCA detection in PSC.77 The use of contrast media increases the sensitivity for CCA detection by 10% without diminishing the specificity when compared to a noncontrast MRCP.75 Hence, a contrast-enhanced MRI/MRCP is preferred when there is a concern for CCA. A mass lesion is often absent in early-stage CCA, and distinguishing benign from malignant strictures is difficult. Imaging features considered indeterminate for CCA include bile-duct wall thickening, irregularity or enhancement, marked biliary dilation, the presence of a so-called dominant stricture, or focal biliary dilation with ipsilateral lobe atrophy. The development of such features should prompt additional studies including ERCP with biliary brushings/biopsies.78-80
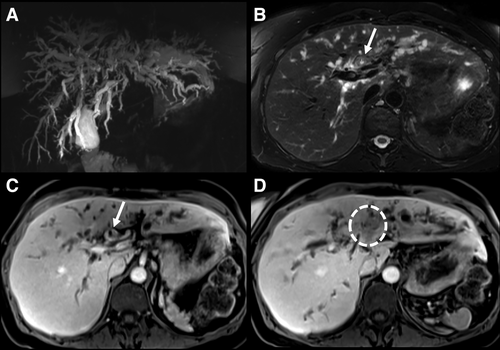
There is neither supporting nor refuting evidence to suggest that CCA screening with any test is associated with improved outcomes or cost-savings. However, MRI/MRCP is the preferred imaging modality to assess for CCA because of its improved ability to define biliary strictures.37, 75 Therefore, many practitioners take a rational approach to biliary tract cancer screening that involves the use of laboratory tests and imaging. Indeed, the majority of large-volume centers perform follow-up MRI/MRCP yearly or every other year.3, 69, 76, 79, 81 The rationale behind this strategy is to monitor for suspicious bile-duct strictures, new mass lesions, or gallbladder polyps to detect early malignancies and capture patients who might be eligible for curative therapy. Follow-up MRI/MRCP for CCA screening should be evaluated in prospective, multicenter studies.
GUIDANCE STATEMENTS
- MRI/MRCP with contrast media should be performed before an ERCP or percutaneous cholangiogram if a concern for CCA develops among patients with PSC. (1C)
- If the initial MRI/MRCP at the time of establishing a PSC diagnosis has been performed without contrast media, a second MRI/MRCP including contrast media should be considered within 6 months of the diagnosis because of the higher risk of prevalent CCA when PSC is detected. (1C)
- The use of MRI/MRCP to screen for biliary cancers among asymptomatic patients with PSC should be an individualized decision. There is no quality evidence supporting or refuting CCA screening. However, many experts in the field of PSC recommend regular CCA screening with MRI/MRCP. (1C)
MRI/MRCP as a Prognostic Marker of Disease Severity
CLINICAL NEED AND EVIDENCE SUMMARY
Biomarkers that reflect PSC disease severity are needed for routine clinical practice and to serve as surrogate endpoints and stratification tools in clinical trials. Although evidence supporting MRI/MRCP as a biomarker in PSC is underdeveloped, this noninvasive tool has the potential to provide both structural and functional information that may hold prognostic relevance.
The radiological progression of PSC has been measured on MRI/MRCP, and nearly 60% of subjects were found to have radiographic disease advancement after 4 years in one study.67 Factors associated with radiographic progression include hepatic dysmorphy (e.g., hepatic atrophy), intrahepatic ductal dilation and the presence of portal hypertension (PH; when contrast was not used), or dysmorphy and parenchymal enhancement heterogeneity (when contrast was used).67 Whether these findings can be reproduced or predict clinical outcomes is unclear. However, the presence of PH can often be identified on cross-sectional imaging and is generally accepted as a marker of disease severity and, if detected, should prompt clinicians to institute screening measures such as assessing for varices.82 The presence and extent of arterial peribiliary hyperenhancement, in contrast to enhancement on other phases, was associated with a higher Mayo PSC risk score and may be a marker of active biliary inflammation.83 While in the early phases of development, several dynamic gadoxetate-enhanced MRI studies have shown that patients with PSC have a heterogeneously distributed liver function (compared to healthy controls) with delayed hepatobiliary excretion of this contrast agent. Delayed excretion correlated with liver tests, Mayo PSC risk score, and downstream biliary obstruction.22, 84-86 Liver stiffness, as measured by MRE, has been shown to predict hepatic decompensation in PSC and the optimal cutoff to predict cirrhosis was 4.9 kPa. This value is nearly identical to the values reported in TE-based PSC studies (recognizing that shear-based MRE measurements can be compared to Young's modulus-based TE measurements by dividing by a conversion factor of 3).25, 27 However, despite the potential of MRI/MRCP as a prognostic biomarker, there is a dearth of published information relating radiographic covariates and clinical outcomes, thereby limiting its contemporary use as a validated surrogate endpoint in clinical trials.
GUIDANCE STATEMENTS
- Presently, there is insufficient evidence to recommend the routine use of MRI/MRCP as a prognostic marker. (1C)
- Individuals who appear to have advanced liver disease on MRI/MRCP (cirrhotic appearing liver, features of PH, or a liver stiffness greater than 4.9 kPa if MRE is performed) should receive preventative health measures, such as an upper endoscopy, to screen for varices. (1C)
Conclusion and Future Areas of Research
In summary, this consensus statement is intended to guide clinicians on the use of MRI/MRCP among patients with PSC. MRI/MRCP plays an essential role in the diagnosis of PSC and detection of disease-related complications and holds some promise to serve as a method of quantifying disease severity. There are a number of unmet needs and areas of uncertainty that our working group has surfaced as important research priorities (Table 2). Heterogeneity in image quality and protocols across institutions is an important limitation. We hope to address this by providing a minimum standard protocol for performing MRI/MRCP in PSC, which is aimed at reducing this heterogeneity. A standardized approach to imaging has the potential to improve patient care and better enable research collaboration across centers.
| PSC Diagnosis |
| • MRI changes associated with early PSC or small-duct PSC, such as parenchymal and periductular changes in diffusion or contrast uptake |
| • Development of a radiological diagnostic score for early PSC (including “patchy” parenchyma and gallbladder volume, which may be increased in PSC, and comparison with other liver diseases as well as other biliary diseases) |
| • Differentiating PSC from IgG4-related disease and other forms of secondary cholangitis |
| Detection of Disease-Related Complications |
| • Use of extracellular and hepatobiliary contrast agents such as Gd-EOB-DTPA for the early diagnosis of CCA |
| • Differentiation of CCA from benign stenoses using MRI |
| • Definition of dominant stenoses on MRI, emphasizing the need to define stenosis, which require endoscopic intervention (combining imaging and clinical findings) |
| • Ability of MRI vs. ultrasound to detect premalignant gallbladder polyps in PSC |
| Prognostic Value of MRI/MRCP |
| • Correlation of MRI findings with liver histology and ERCP in different stages of disease |
| • Development and validation of scores which categorize the severity and distribution of disease and predict disease prognosis and complications |
| • Value of follow-up MRI in PSC for the prediction of disease prognosis |
| • The value of quantitative MRI techniques, such as DWI, MRE, and T1-mapping, for the assessment of liver function, treatment response, and disease course in PSC |
| • Value and safety of extracellular and hepatobiliary contrast agents, such as Gd-EOB-DTPA, and the assessment of disease prognosis |
| • The role of MRE in comparison to other biomarkers of disease stage and fibrosis progression, including TE |
| • The significance of changes in liver stiffness over time as a prognostic marker |
Acknowledgments
We are grateful to Prof. Benjamin Yeh, Department of Radiology, University of California (San Francisco, CA), and Prof. Ann Fulcher, Department of Radiology, Virginia Commonwealth University Medical Center (Richmond, VA), for critical revision of the manuscript.
Appendix
Members of the MRI working group of the IPSCSG who participated in creating this summary: Katherine Arndtz (Birmingham, UK), Lionel Arrive (Paris, France), David Assis (New Haven, CT), Ahmed Ba-Ssalamah (Vienna, Austria), Helen Bungay (Oxford, UK), Vincenzo Cardinale (Rome, Italy), Vanja Cengija (Oslo, Norway), Roger Chapman (Oxford, UK), Olivier Chazouilleres (Paris, France), Peter Eddowes (Birmingham, UK), Martti Farkkila (Helsinki, Finland), Annarosa Floreani (Padova, Italy), Irene Franceschet (Padova, Italy), Emina Halilbasic (Vienna, Austria), Harald Ittrich (Hamburg, Germany), Sarah Keller (Hamburg, Germany), Gunter Kemmerich (Oslo, Norway), Guido Kukuk (Bonn, Germany), Henrike Lenzen (Hannover, Germany), Kati Lind (Helsinki, Finland), Ansgar W. Lohse (Hamburg, Germany), Sarah Pötter-Lang (Vienna, Austria), Jurgen Runge (Amsterdam, Netherlands), Michael Trauner (Vienna, Austria), Mette Vesterhus (Bergen, Norway), Tobias J. Weismüller (Bonn, Germany), Kidist Yimam (San Francisco, CA), Roman Zenouzi (Hamburg, Germany).
REFERENCES
Author names in bold designate shared co-first authorship.