Conditional disruption of mouse HFE2 gene: Maintenance of systemic iron homeostasis requires hepatic but not skeletal muscle hemojuvelin†‡
Potential conflict of interest: Nothing to report.
Supported by a grant from the Canadian Institutes for Health Research (CIHR; MOP-86515).
Abstract
Mutations of the HFE2 gene are linked to juvenile hemochromatosis, a severe hereditary iron overload disease caused by chronic hyperabsorption of dietary iron. HFE2 encodes hemojuvelin (Hjv), a membrane-associated bone morphogenetic protein (BMP) coreceptor that enhances expression of the liver-derived iron regulatory hormone hepcidin. Hjv is primarily expressed in skeletal muscles and at lower levels in the heart and the liver. Moreover, a soluble Hjv form circulates in plasma and is thought to act as a decoy receptor, attenuating BMP signaling to hepcidin. To better understand the regulatory function of Hjv, we generated mice with tissue-specific disruption of this protein in hepatocytes or in muscle cells. The hepatic ablation of Hjv resulted in iron overload, quantitatively comparable to that observed in ubiquitous Hjv−/− mice. Serum iron and ferritin levels, transferrin saturation, and liver iron content were significantly (P < 0.001) elevated in liver-specific Hjv−/− mice. Hepatic Hjv mRNA was undetectable, whereas hepcidin expression was markedly suppressed (12.6-fold; P < 0.001) and hepatic BMP6 mRNA up-regulated (2.4-fold; P < 0.01), as in ubiquitous Hjv−/− counterparts. By contrast, the muscle-specific disruption of Hjv was not associated with iron overload or altered hepcidin expression, suggesting that muscle Hjv mRNA is dispensable for iron metabolism. Our data do not support any significant iron-regulatory function of putative muscle-derived soluble Hjv in mice, at least under physiological conditions. Conclusion: The hemochromatotic phenotype of liver-specific Hjv−/− mice suggests that hepatic Hjv is necessary and sufficient to regulate hepcidin expression and control systemic iron homeostasis. (HEPATOLOGY 2011;)
Body iron homeostasis is regulated by hepcidin, a liver-derived peptide hormone that binds to the iron exporter ferroportin and promotes its phosphorylation, internalization, and lysosomal degradation.1, 2 Thereby, hepcidin limits dietary iron absorption and release of iron from reticuloendothelial macrophages. Hepcidin is transcriptionally activated by iron, inflammatory cytokines, or endoplasmic reticulum stress. The iron-dependent pathway involves bone morphogenetic protein 6 (BMP6) signaling, phosphorylation of SMAD1/5/8, and translocation of this protein along with SMAD4 to the nucleus, for binding to proximal and distal sites on the hepcidin promoter.
Further cofactors include the hemochromatosis protein HFE, transferrin receptor 2 (TfR2), and hemojuvelin (Hjv), as mutations in their genes are associated with blunted hepcidin responses, which eventually leads to various forms of hereditary iron overload (hemochromatosis).3, 4 Among them, early onset juvenile hemochromatosis is caused by mutations in the HFE2 or HAMP genes, encoding Hjv or hepcidin, respectively. Patients with Hjv mutations,5 as well as Hjv−/− mice6, 7 exhibit diminished hepcidin expression despite excessive tissue iron overload, consistent with the function of Hjv as a BMP coreceptor8 that enhances BMP6 signaling to hepcidin.9, 10 Disease-associated Hjv mutants fail to promote hepcidin activation.8, 11
Hjv is identical to repulsive guidance molecule c (RGMc). In contrast to other family members (RGMa and RGMb) that are expressed in neuronal cells,12 Hjv mRNA has been detected predominantly in skeletal muscles and, at lower levels, in the heart and the liver.5 Likewise, immunohistological staining of Hjv was more prominent in skeletal muscles, and negligible in heart and liver.13 The protein is expressed on the cell surface and in perinuclear compartments and associates with membranes by way of a glycosylphosphatidylinositol (GPI) anchor.14, 15 It is glycosylated at Asn residues and undergoes processing by complex mechanisms yielding either a single ≈50 kDa polypeptide or a cleaved derivative consisting of a ≈30 and a ≈20 kDa disulfide bridged subunits.15, 16 Differentiating muscle cells15, 17 and transfected HeLa,16 HEK293,17-20 Cos-7,21 Hep3B,21, 22 and HepG216, 23, 24 cells release soluble ≈50 and ≈40 kDa isoforms (sHjv) in extracellular media; sHjv has also been detected in human,22, 25 rat,17 and mouse21 serum. Hjv interacts with neogenin, which may modulate the secretion of sHjv in a positive24 or a negative20 manner.
sHjv can be generated by proteolytic cleavage with either furin and other pro-protein convertases,19, 21, 26 or matriptase-2.27 The latter, a protease encoded by the TMPRSS6 gene,28 inactivates cellular Hjv27 and inhibits hepcidin expression by attenuating BMP signaling.29 Furin cleaves cellular Hjv at R332 and matriptase-2 at R288.30 Cleavage of the GPI anchor, possibly by phospholipase A, may yield full-length sHjv, consisting of the entire ectodomain.19 In vitro experiments with purified recombinant sHjv and with conditioned media from Hjv-transfected cells suggested that this protein functions as a decoy receptor and inhibits BMP signaling to hepcidin.19, 20, 22 Moreover, a sHjv.Fc fusion construct was shown to directly interact with BMP610 and to decrease hepcidin expression in vivo.31 Nevertheless, sHjv generated by matriptase cleavage did not compete BMP signaling to hepcidin.30
To elucidate the tissue-specific function of Hjv in the control of body iron homeostasis, we generated mice with targeted disruption of the Hfe2 gene in liver hepatocytes or in muscles. We show that only hepatic Hjv is essential for an appropriate hepcidin response, whereas muscle Hjv does not exert any apparent iron-regulatory function.
Abbreviations
BMP, bone morphogenetic protein; GPI, glycosylphosphatidylinositol; Hjv, hemojuvelin; RGM, repulsive guidance molecule; sHjv, soluble hemojuvelin; TfR2, transferrin receptor 2; TIBC, total iron binding capacity.
Materials and Methods
Targeting of the Mouse Hfe2 Gene.
For conditional disruption of Hfe2, a targeting vector32 (kindly provided by Dr. Stephane Richard, McGill University) was employed to “flox” a 3.4 kb region spanning exons 2-4, with loxP sites. A neo-PGK cassette was also introduced for antibiotic selection of homologous recombination events. The targeting construct was transfected into 129S6/SvEvTac embryonic stem cells and two (out of 500) positive clones were identified. The clones were expanded and microinjected into C57BL/6J blastocysts; this procedure was performed at the McIntyre Cancer Centre Transgenic Core Facility of McGill University. The resulting chimeras were crossed with female C57/BL6 mice to produce agouti mice, which were polymerase chain reaction (PCR)-genotyped for germline transmission of the floxed Hfe2 allele. Heterozygotes (Hfe2wt/f) were crossed to obtain Hfe2f/f mice. PCR genotyping from tail genomic DNA was performed by using the RedExtract kit (Sigma) with primers shown in Table 1A.
| Gene | GenBank Accession | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Hfe2 (floxed) | NM_027126.4 | GCCTACCATGTTACCCTTTGTGC | CCTGACTACCAAATAGCAGATGTG |
| Cre recombinase | X03453.1 | GCGGTCTGGCAGTAAAAACTATC | GTGAAACAGCATTGCTGTCACTT |
Animals.
Alb-Cre mice (B6.Cg-Tg(Alb-cre)21Mgn/J strain) were purchased from the Charles River Laboratories (Cambridge, MA). MCK-Cre mice (FVB-Tg(Ckmm-cre)5Khn/J strain) were acquired from Dr. Mike Rudnicki (University of Ottawa) and ubiquitous Hjv−/− mice (in mixed 129S6/SvEvTac;C57BL/6 background) from Dr. Nancy Andrews (Duke University). Only male animals were used for experiments, housed in macrolone cages (up to 5 mice/cage, 12:12-hour light-dark cycle: 7 AM to 7 PM; 22 ± 1°C, 60 ± 5% humidity) according to standard institutional guidelines. The mice had free access to water and food, a standard diet containing approximately 226 mg of iron per kg (Teklad Global 18% protein rodent diet, TD 2018, Harlan Laboratories, Indianapolis, IN). Experimental procedures were approved by the Animal Care Committee of McGill University (protocol 4966).
Serum Biochemistry.
Transferrin saturation, total iron binding capacity (TIBC), serum iron, and ferritin were measured with a Roche Hitachi 917 Chemistry Analyzer at the Biochemistry Department of the Jewish General Hospital.
Quantification of Nonheme Iron.
Hepatic and splenic nonheme iron was measured by the ferrozine assay, as described.33, 34 Results are expressed as micrograms of iron per gram of dry tissue weight.
Histological Analysis.
Tissue specimens were fixed in 10% buffered formalin and embedded in paraffin. To visualize ferric iron deposits, deparaffinized tissue sections were stained with Perls' Prussian blue using the Accustain Iron Stain kit (Sigma).
Quantitative Real-Time PCR (qPCR).
Total RNA was isolated from frozen tissues using the RNeasy Mini kit (Qiagen) for the liver and the RNeasy Fibrous Tissue Mini kit (Qiagen) for the skeletal muscles and heart; its quality was assessed by determining the 260/280 nm absorbance ratios and by agarose gel electrophoresis. qPCR was performed by using gene-specific primers (Table 1B), as described,35 with β-actin or ribosomal protein S18 (rS18) as housekeeping genes for normalization.
| Gene | GenBank Accession | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Actb | NM_007393.3 | GACGACATGGAGAAGATCTG | GTGAAGCTGTAGCCACGCTC |
| Hamp1 | NM_032541.1 | AAGCAGGGCAGACATTGCGAT | CAGGATGTGGCTCTAGGCTATGT |
| Bmp6 | NM_007556.2 | ACTCGGGATGGACTCCACGTCA | CACCATGAAGGGCTGCTTGTCG |
| Hfe2 | NM_027126.4 | ATCCCCATGTGCGCAGTTT | GCTGGTGGCCTGGACAAA |
Western Blotting.
Duodenal membrane-enriched protein lysates were prepared as in36 and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel; the samples (30 μg of protein) did not contain β-mercaptoethanol and were not boiled before loading on the gel. Following transfer of the proteins onto nitrocellulose filters (BioRad), the blots were saturated with 10% nonfat milk in phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween-20 (PBS-T) and probed with a 1:1,000 diluted ferroportin antibody.35 After three washes with PBS-T, the blots were incubated with 1:5,000 diluted peroxidase-coupled goat antirabbit IgG (Sigma). The peroxidase signal was detected by enhanced chemiluminescence with the Western Lightning ECL kit (Perkin Elmer).
Statistical Analysis.
Quantitative data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using the two-tailed Student's t test with GraphPad Prism software (v. 5.0d). A probability value P < 0.05 was considered statistically significant.
Results
Generation of Hfe2f/f:Alb-Cre and Hfe2f/f:MCK-Cre mice.
We introduced loxP sites flanking exons 2-4 of the Hfe2 gene (encompassing the entire open reading frame of Hjv messenger RNA [mRNA]) and generated a mouse line carrying floxed Hfe2f/f alleles (Fig. 1A). By this approach, we ensured that the remaining exon 1 (encompassing the gene promoter and the 52 untranslated region of Hjv mRNA) could not give rise to a partially functional truncated transcript. The procedure was validated by PCR genotyping (Fig. 1B). For disruption of Hfe2 in hepatocytes, the Hfe2f/f mice were crossed with Alb-Cre transgenic animals, expressing Cre recombinase under the control of the albumin promoter.37 For muscle-specific disruption of Hfe2, the Hfe2f/f mice were crossed with MCK-Cre transgenics, expressing Cre recombinase under the control of the muscle creatinine kinase (MCK) promoter, which is activated in differentiated multinucleated skeletal myotubes and in cardiomyocytes.38 The resulting heterozygous Hfe2wt/f:Alb-Cre and Hfe2wt/f:MCK-Cre animals were crossed with Hfe2f/f mice to obtain Hfe2f/f:Alb-Cre and Hfe2f/f: MCK-Cre progeny, expected to bear liver- and muscle-specific disruption of Hjv, respectively. Ten-week-old male mice were used for phenotypic analysis and further experiments. Quantitative real-time PCR by using primers upstream of the 5′ loxP site and within exon 3 (Fig. 1A) demonstrates the selective ablation of hepatic Hjv mRNA in Hfe2f/f:Alb-Cre animals (Fig. 2A) and of skeletal muscle and heart Hjv mRNA in Hfe2f/f:MCK-Cre counterparts (Fig. 2B,C), respectively. The position of primers indicates that no aberrant Hjv mRNA products could escape detection by this technique; these findings were also validated by northern blotting (data not shown). The unavailability of reliable antibodies did not allow us to confirm the absence of Hjv protein expression in the targeted tissues. All mutant mice were viable and did not exhibit any obvious physical abnormalities or altered behavior.
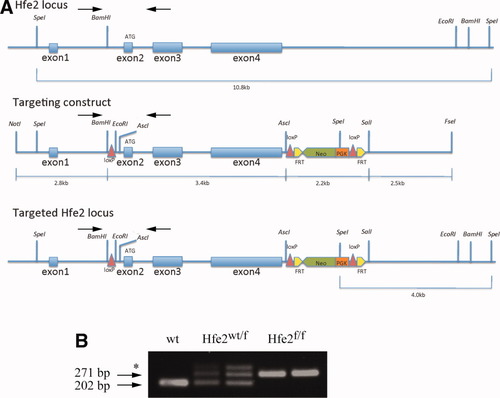
Generation of Hfe2f/f mice. (A) A targeting vector was used to flank exons 2-4 of the mouse Hfe2 gene with loxP sites. The positions of primers used for PCR-genotyping are indicated by arrows. (B) Representative PCR genotyping experiment from tail genomic DNA corresponding to one wildtype, two Hfe2wt/f, and two Hfe2f/f mice. The positions of the 202-bp and 271-bp fragments, corresponding to wildtype and floxed Hfe2 alleles, respectively, are indicated by arrows; the star denotes a nonspecific band.
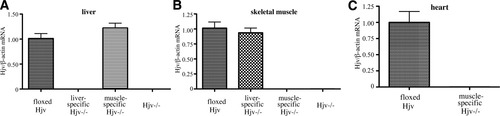
Tissue-specific ablation of Hjv. The expression of Hjv mRNA was assessed by qPCR in the liver (A), skeletal muscles (B), and heart (C) of mice with following genotypes: floxed Hjv (Hfe2f/f; n = 5), liver-specific Hjv−/− (Hfe2f/f:Alb-Cre; n = 6), muscle-specific Hjv−/− (Hfe2f/f:MCK-Cre; n = 6), and Hjv−/− (ubiquitous; n = 5).
Lack of Hepatic Hjv Leads to Hemochromatosis and Inappropriate Hepcidin Expression.
Having established the liver-specific disruption of Hjv, we analyzed iron metabolism in Hfe2f/f:Alb-Cre mice. These animals manifested significantly elevated (P < 0.001) transferrin saturation and levels of serum iron and ferritin as compared to age- and sex-matched Hjvf/f controls (Table 2). Moreover, staining with Perls' Prussian blue revealed deposits of nonheme iron in the liver parenchyma, the pancreas, and the heart of Hfe2f/f:Alb-Cre mice, whereas their spleen macrophages were iron-deficient (Fig. 3). Quantitatively, the lack of hepatic Hjv expression caused a 12.9-fold (P < 0.001) increase of nonheme iron levels in the liver (Fig. 4A; Table 2) and a 2.4-fold (P < 0.001) decrease in the spleen (Table 2). Serum iron indices and hepatic and splenic iron content of heterozygous Hfe2wt/f:Alb-Cre mice did not differ substantially from those of Hfe2f/f controls (Table 2); we speculate that the relatively lower ferritin levels in Hfe2wt/f:Alb-Cre mice (and slightly elevated transferrin saturation in Hfe2wt/f:MCK-Cre animals) may be related to genetic background variability. The disruption of hepatic Hjv was associated with a 13.1-fold (P < 0.001) decrease in hepcidin mRNA expression in the liver (Fig. 4B). Hepatic BMP6 mRNA levels were significantly (P < 0.01) increased by 2.4-fold (Fig. 5A), whereas duodenal BMP6 mRNA expression was largely unaffected (Fig. 5B). The expression of ferroportin was markedly up-regulated in the duodenum of mice lacking hepatic Hjv (Fig. 5C). We conclude that hepatic Hjv is essential for preventing iron overload by way of appropriate signaling to hepcidin.
| Genotype | n | Serum Iron (μmol/L) | Tf Saturation (%) | TIBC (μmol/L) | Ferritin | Liver Iron (μg/g) | Spleen Iron (μg/g) |
|---|---|---|---|---|---|---|---|
| Hfe2f/f | 5 | 31.60 ± 2.06 | 50.00 ± 4.91 | 63.80 ± 2.31 | 1318.0 ± 371.2 | 400.4 ± 31.12 | 1509.0 ± 162.5 |
| Hfe2wt/f:Alb-Cre | 6 | 29.00 ± 0.57 | 41.71 ± 3.57 | 68.00 ± 4.00 | 620.0 ± 157.1 | 392.1 ± 24.33 | 1403.0 ± 126.4 |
| Hfe2f/f:Alb-Cre | 6 | 61.50 ± 4.69*** | 93.33 ± 3.073*** | 66.83 ± 7.14 | 5737.0 ± 371.2** | 5187.0 ± 519.2 | 620.2 ± 55.8*** |
| Hfe2wt/f:MCK-Cre | 5 | 39.60 ± 2.249 | 64.60 ± 2.731 | 63.20 ± 2.15 | 1432.0 ± 283.0 | 514.3 ± 46.49 | 1652.0 ± 26.15 |
| Hfe2f/f:MCK-Cre | 6 | 34.60 ± 1.53 | 52.43 ± 2.438 | 67.71 ± 2.12 | 1175.0 ± 421.2 | 415.5 ± 46.36 | 1464.0 ± 205.2 |
- *** P < 0.001 vs Hfef/f (Student's t test)
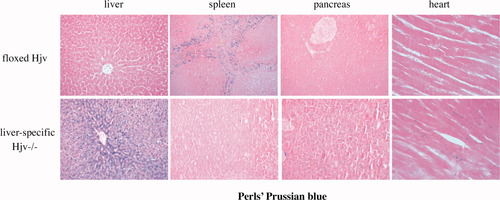
Mice with liver-specific disruption of Hjv manifest iron overload in parenchymal cells of the liver, pancreas, and heart, and iron-deficient splenic macrophages. Perl's staining of tissue sections (magnification for liver, spleen, and pancreas: 20×; for heart: 40×).
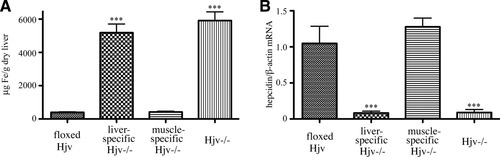
Hepatic iron overload and suppression of hepcidin expression in mice bearing liver- but not muscle-specific disruption of Hjv. (A) Quantification of hepatic nonheme iron by the ferrozine assay. (B) Assessment of hepatic hepcidin mRNA expression by qPCR. Values correspond to animals described in Fig. 2. ***P < 0.001 versus floxed Hjv mice (Student's t test).
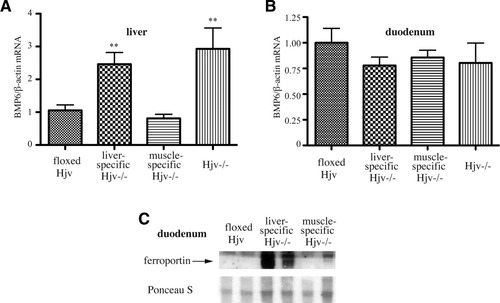
The liver-specific disruption of Hjv leads to increased expression of BMP6 in the liver and ferroportin in the duodenum. BMP6 mRNA was assessed by qPCR in the liver (A) and the duodenum (B). Values correspond to animals described in Fig. 2. Ferroportin expression was evaluated by western blotting in membrane-enriched duodenal extracts (C); two representative samples are shown. The lower panel depicts a section of the nitrocellulose filter with proteins visualized by Ponceau S staining, and serves as loading control. **P < 0.01 versus floxed Hjv mice (Student's t test).
Ablation of Muscle Hjv Does Not Impinge on Systemic Iron Homeostasis.
Hfe2f/f:MCK-Cre mice bearing muscle-specific disruption of Hjv presented with physiological serum iron indices (Table 2) and did not develop iron overload in the liver, pancreas, or, notably, in the heart (Fig. 4A; Supporting Fig. S1). Splenic macrophages contained stainable nonheme iron (Fig. S1), by analogy to Hfe2f/f controls. The expression of hepcidin mRNA (Fig. 4B), hepatic BMP6 mRNA (Fig. 5A), and duodenal BMP6 mRNA (Fig. 5B) did not significantly differ between Hfe2f/f:MCK-Cre and Hfe2f/f mice, whereas expression of duodenal ferroportin was undetectable (Fig. 5C). Thus, the absence of muscle Hjv did not affect iron metabolism in the whole body and, apparently, also in the heart, a tissue that normally expresses Hjv.
Comparison Between Mouse Lines with Altered Hjv Expression.
We compared the hepatic iron content and hepcidin mRNA expression levels among age- and sex-matched Hfe2f/f, Hfe2f/f:Alb-Cre, Hfe2f/f:MCK-Cre, and ubiquitous Hjv-/-7 mice; all lines shared a mixed 129S6/C57 genetic background, albeit with variable genomic ratios. The degree of hepatic iron overload (Fig. 4A), the deregulation of hepcidin expression (Fig. 4B), and the increase of hepatic BMP6 mRNA (Fig. 5B) were quantitatively similar among ubiquitous Hjv−/− and liver-specific Hjv−/− (Hfe2f/f:Alb-Cre) animals. Likewise, control floxed (Hfe2f/f) and muscle-specific Hjv−/− (Hfe2f/f:MCK-Cre) mice were phenotypically indistinguishable. Taken together, these results indicate that the absence of hepatic Hjv suffices to cause full-scale iron overload, whereas the lack of muscle Hjv does not affect iron balance.
Discussion
Genetic studies in humans5 and mice6, 7 uncovered an important role of Hjv in the control of systemic iron homeostasis. Corroborating evidence was provided from biochemical data showing that Hjv activates the iron-dependent pathway for signaling to hepcidin by acting as a BMP coreceptor,8 whereas a circulating sHjv isoform is widely considered to antagonize this response.19, 20, 22, 31 We report here that the targeted disruption of Hjv in liver hepatocytes recapitulates the hemochromatotic phenotype of mice lacking Hjv ubiquitously.6, 7 Thus, the liver-specific ablation of Hjv leads to high transferrin saturation, hyperferremia, hyperferritinemia, hepatic iron overload, macrophage iron deficiency, and inappropriately low hepcidin expression, which are hallmarks of hereditary hemochromatosis. In addition, it is associated with increased hepatic BMP6 mRNA expression, as in ubiquitous Hjv−/− mice.34 We did not observe an up-regulation of duodenal BMP6 mRNA expression, which has been reported to occur in response to increased iron absorption,39 presumably because the ferroportin-rich duodenal enterocytes of liver-specific Hjv−/− animals were iron-deficient. Similar results were recently obtained with ubiquitous Hjv−/− mice.34
These results are consistent with the restoration of hepcidin expression and normalization of iron parameters upon reintroduction of Hjv to hepatocytes of Hjv−/− mice by an adenoviral system,34 and highlight the importance of the liver in body iron metabolism as a site of both hepcidin production and regulation by Hjv. Our data support the hypothesis that Hjv may constitute part of an “iron sensing complex” (possibly together with HFE, TfR2, and further molecules) that responds to alterations in transferrin saturation and/or BMP6 levels, and transmits signals for hepcidin transcriptional activation by way of the BMP/SMAD pathway.40, 41 According to this model, Hjv would be expected to operate in a cell-autonomous fashion at the sites of hepcidin production in the liver. Although hepcidin appears to be predominantly expressed in periportal hepatocytes,20, 42 discordant results have been published about the site of hepatic Hjv expression. By measuring lacZ activity in liver sections of Hjv−/− mice where lacZ was introduced from the targeting vector, Niederkofler et al.6 reported a patterned distribution of Hjv around periportal hepatocytes. However, by in situ hybridization of Hjv mRNA and immunohistochemical staining with an Hjv antibody, Lee at al.20 concluded that Hjv is mostly expressed around the central vein of the liver. Certainly, more work is required to clarify this important issue. Periportal expression of Hjv would support a cell-autonomous activity of this protein on hepcidin regulation. Nevertheless, if the majority of hepatic Hjv is concentrated around perivenous areas, an activity of Hjv in trans would be conceivable.
We also show here that mice with specific ablation of Hjv in skeletal muscles and cardiomyocytes do not develop iron overload and do not exhibit any apparent phenotypic abnormalities. This finding is intriguing, considering that skeletal muscles express substantially higher levels of Hjv compared to the liver,5, 13 and suggests that muscle Hjv is not involved in the regulation of systemic iron homeostasis, at least under standard laboratory conditions. The absence of cardiac iron overload in muscle-specific Hjv−/− mice appears to exclude the possibility for a local iron regulatory function of this protein Nevertheless, it will be interesting to evaluate iron metabolism in Hfe2f/f:MCK-Cre mice subjected to stress, such as strenuous physical exercise or hypoxia. Moreover, it will be important to examine whether these mice exhibit any possible phenotype in muscles, unrelated to iron metabolism.
In light of the high abundance of Hjv in skeletal muscles and the capacity of differentiating muscle cells to release sHjv,15, 17 it is reasonable to speculate that circulating sHjv may primarily derive from muscle tissue. If circulating sHjv had an inhibitory function on BMP signaling in liver hepatocytes, one would expect Hfe2f/f:MCK-Cre mice to express higher hepcidin mRNA levels than Hfe2f/f controls, associated with iron retention in splenic macrophages and possibly also reduced hepatic iron content. Our data show that this is not the case, implying the lack of any major iron regulatory role of putative muscle-derived sHjv under physiological conditions. We do not expect that the small genetic background differences of the mice would substantially affect iron parameters, as between distinct pure inbred strains.43, 44 Nevertheless, direct measurement of sHjv levels in the serum of mice with tissue-specific disruption of Hjv and wildtype controls, as well as assessment of its capacity to inhibit BMP signaling, are required to further validate the origin and the function of circulating sHjv. In conclusion, our overall data demonstrate that hepatic Hjv is necessary and sufficient to prevent iron overload and control hepcidin expression, whereas muscle Hjv is dispensable. Similar conclusions were drawn in a report that was recently published while this article was under review.45
Acknowledgements
We thank Dr. Mike Rudnicki (University of Ottawa) for the MCK-Cre mice and Dr. Nancy Andrews (Duke University) for the Hjv−/− mice. We also thank Dr. Naciba Benlimame for assistance with histology. K.P. holds a Chercheur National career award from the Fonds de la Recherche en Santé du Quebéc (FRSQ). K.G. is supported by doctoral awards from the J. Latsis and A. Onassis Public Benefit Foundations.




