Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice†‡
E.C. was supported in part by a Tosteson Postdoctoral Fellowship Awards from the Massachusetts Biomedical Research Corporation (MBRC) at Massachusetts General Hospital. P.V. and A.P. were supported in part by PRIN '08 grant and Telethon GGP10233 grant. J.L.B. was supported in part by NIH grants K08 DK075846 and RO1 DK087727, and by a Claflin Distinguished Scholar Award from the Massachusetts General Hospital.
See Editorial on Page 16
Abstract
The bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway is a central regulator of hepcidin expression and systemic iron balance. However, the molecular mechanisms by which iron is sensed to regulate BMP6-SMAD signaling and hepcidin expression are unknown. Here we examined the effects of circulating and tissue iron on Bmp6-Smad pathway activation and hepcidin expression in vivo after acute and chronic enteral iron administration in mice. We demonstrated that both transferrin saturation and liver iron content independently influence hepcidin expression. Although liver iron content is independently positively correlated with hepatic Bmp6 messenger RNA (mRNA) expression and overall activation of the Smad1/5/8 signaling pathway, transferrin saturation activates the downstream Smad1/5/8 signaling cascade, but does not induce Bmp6 mRNA expression in the liver. Hepatic inhibitory Smad7 mRNA expression is increased by both acute and chronic iron administration and mirrors overall activation of the Smad1/5/8 signaling cascade. In contrast to the Smad pathway, the extracellular signal-regulated kinase 1 and 2 (Erk1/2) mitogen-activated protein kinase (Mapk) signaling pathway in the liver is not activated by acute or chronic iron administration in mice. Conclusion: Our data demonstrate that the hepatic Bmp6-Smad signaling pathway is differentially activated by circulating and tissue iron to induce hepcidin expression, whereas the hepatic Erk1/2 signaling pathway is not activated by iron in vivo. (HEPATOLOGY 2011;)
The liver hormone hepcidin is a main regulator of systemic iron homeostasis (reviewed in1). Hepcidin binds and induces degradation of ferroportin, an iron exporter expressed on the surface of duodenal enterocytes, reticuloendothelial macrophages, and hepatocytes.2 Hepcidin-mediated ferroportin degradation limits iron release from these cells to the bloodstream, thereby reducing iron absorption from the diet and iron mobilization from body stores. Hepcidin deficiency and unregulated ferroportin activity are the common pathogenic mechanism for the iron overload disorder hereditary hemochromatosis due to mutations in the genes encoding hepcidin itself (HAMP), hemojuvelin (HFE2), the hemochromatosis protein HFE, and transferrin receptor 2 (TFR2), whereas hepcidin excess contributes to the anemia of chronic disease (reviewed1).
A number of stimuli modulate hepcidin expression to influence systemic iron balance. Erythropoietic demand and hypoxia down-regulate hepcidin transcription to increase iron availability, whereas iron, inflammatory cytokines, and endoplasmic reticulum stress up-regulate hepcidin transcription to decrease iron availability.1, 3-7 The specific signaling pathways mediating hepcidin transcription in response to these stimuli are increasingly being elucidated. Inflammatory cytokines stimulate hepcidin transcription by way of the signal transducer and activator of transcription 3 (STAT3) signaling pathway, whereas iron stimulates hepcidin transcription by way of the bone morphogenetic protein (BMP)-SMAD signaling pathway (reviewed1).
BMPs belong to the transforming growth factor-beta (TGF-β) superfamily of ligands and are involved in a myriad of cellular and systemic functions during embryonic and adult life (reviewed8). BMPs form a signaling complex with type I and type II serine threonine kinase receptors, leading to phosphorylation of intracellular SMAD1, SMAD5, and SMAD8 proteins. These P-SMAD1/5/8 proteins form a complex with SMAD4 and translocate to the nucleus to modulate transcription of target genes such as ID1 and SMAD7.9, 10 Hepcidin is also a target transcript directly up-regulated by the BMP signaling pathway (reviewed in1). The central importance of the BMP-SMAD signaling pathway in hepcidin regulation and iron metabolism in vivo is demonstrated by the fact that mutations in the genes encoding the BMP coreceptor hemojuvelin,11, 12 the intracellular signaling molecule SMAD4,13 and the ligand BMP69, 14 each result in decreased hepcidin expression and iron overload. Furthermore, pharmacologic modulators of the BMP-SMAD signaling pathway regulate hepcidin expression and systemic iron balance in mice.9, 15, 16
Notably, the molecular mechanisms by which iron is sensed to induce the BMP-SMAD pathway are not fully understood. Both circulating and tissue iron have been suggested to regulate hepcidin expression. Whether they exert independent effects through the BMP-SMAD pathway and/or involve additional pathways is unclear. For example, although liver iron content (LIC) is correlated with hepatic Bmp6 messenger RNA (mRNA) levels in mice,17-19 suggesting that tissue iron levels regulate BMP-SMAD pathway activity by regulating ligand expression, it is unknown whether circulating iron levels also regulate hepatic BMP6 mRNA, or whether circulating iron sensitizes hepatocytes to increase SMAD1/5/8 phosphorylation in response to tonic BMP6 levels. Additionally, although recent studies suggest that HFE and possibly TFR2 may regulate hepcidin expression through an interaction with the BMP6-SMAD signaling pathway,18, 20-24 several studies provide indirect evidence suggesting that hepatic MAPK signaling, more specifically the ERK1/2 kinases, may also be involved in hepcidin regulation by iron mediated by TFR2 and/or HFE.21, 25-27 Notably, crosstalk between the canonical SMAD signaling pathway and the MAPK pathway is well described (reviewed28). However, the physiologic relevance of the ERK/MAPK signaling pathway in iron homeostasis in vivo is still unknown.
Recent studies suggest a role for inhibitory SMAD7 in hepcidin regulation and iron homeostasis.10, 17, 23, 24 Inhibitory SMADs function as feedback inhibitors of the BMP/TGF-β pathway by interacting with type I receptors to block their phosphorylation or to promote receptor dephosphorylation or degradation.8 Hepatic Smad7 mRNA is induced by chronic dietary iron loading in mice concordantly with hepcidin and Id1 mRNA,17 and SMAD7 was recently shown to be a specific inhibitor of hepcidin transcription in vitro.10 Alterations in hepatic SMAD7 mRNA expression have also been found in hemochromatosis patients.23, 24 However, the physiologic significance and timing of SMAD7 activation upon iron administration in vivo need further evaluation.
Here we investigated the molecular mechanisms by which iron is sensed to regulate BMP6-SMAD signaling and hepcidin expression. We performed a detailed time course of both acute and chronic enteral iron administration in mice to obtain different conditions of body iron perturbation including isolated increases of either transferrin saturation (Tf sat) or LIC. Then we dissected the BMP6-SMAD signaling pathway from the induction of tissue-specific Bmp6 ligand mRNA expression, to the activation of intracellular signal mediators including P-Smad1/5/8 and Erk1/2 proteins, to the modulation of target transcript expression including hepcidin (Hamp, also known as Hamp1), Id1, and Smad7. Our aim was to determine how tissue and circulating iron stimulate the Bmp6-Smad signaling pathway to regulate hepcidin expression, and whether the Erk1/2 pathway is stimulated by iron.
Abbreviations
BMP, bone morphogenetic protein; CBC, complete blood count; ERK1/2, extracellular signal-regulated kinases 1 and 2; HAMP, hepcidin; HFE, hemochromatosis protein; HFE2, hemojuvelin; LIC, liver iron content; MAPK, mitogen activated protein kinase; P-ERK1/2, phosphorylated ERK1/2 protein; P-SMAD1/5/8, phosphorylated SMAD1, SMAD5, and SMAD8 protein; Tf sat, transferrin saturation; TFR2, transferrin receptor 2.
Materials and Methods
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital and used C57Bl/6 male mice. For chronic iron administration experiments, 7-week-old mice were sacrificed at time zero (Baseline) or received a high iron diet (2% carbonyl iron, TD.08496, Harlan Teklad) for 24 hours to 3 weeks prior to sacrifice (n = 6 per group). A separate cohort of 7-week-old mice were sacrificed at time zero (Baseline), received a high iron diet for 1 week, or received a high iron diet for 1 week followed by a low iron diet (2-6 ppm iron, TD.80396, Harlan Teklad) for 24 hours to 8 days prior to sacrifice (n = 4 per group). For acute iron administration experiments, 9-week-old mice were sacrificed at time zero (Baseline) or received 2 mg of elemental iron per kg mouse weight as iron sulfate (Elixir, CVS) in 100 μL distilled water (Iron groups) or 100 μL distilled water alone (Mock groups) by oral gavage 1 to 24 hours prior to sacrifice (n = 6 per group). To better detect the effects of iron administration for both acute and chronic experiments, mice received a low iron diet for 12-14 days prior to iron administration because this regimen has been reported previously to circumvent the hepcidin stimulation induced by the high iron content of usual rodent diets without inducing hypoferremia6 (Supporting Fig. 1, Supporting Table 1).
Hematologic and Iron Analysis.
Complete blood count (CBC), serum iron, Tf sat, and liver and spleen nonheme iron concentrations were measured as previously described.15, 22
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR).
Total RNA was isolated from liver and Bmp6, Hamp, Id1, and Smad7 relative to Rpl19 mRNA levels were measured using two-step quantitative real-time RT-PCR as described.9, 10, 18
Western Blot.
Liver lysates were generated and western blot for P-Smad1/5/8 relative to Smad1 was performed essentially as described.18, 22 Western blot for phosphorylated Erk1/2 (P-Erk1/2) relative to total Erk1/2 was performed according to the manufacturer's instructions using phospho-p44/42 MAPK (P-Erk1/2, diluted 1:1,000) and p44/42 MAPK (Erk1/2, diluted 1:5,000) rabbit polyclonal antibodies (Cell Signaling Technology). Chemiluminescence was quantified as described.15
Statistics.
Statistical significance was determined by one-way or two-way analysis of variance (ANOVA) with the Holm-Sidak or the Dunnett's post-hoc tests for pairwise multiple comparisons as indicated. For small sample sizes, we used the Spearman rho test to assess the correlations between continuous variables. Simple and multivariate linear regression analysis was performed to identify the best explanatory variables for Hamp and Bmp6 mRNA levels. Statistical analyses were conducted using SPSS v. 18.0 (Chicago, IL) and SigmaStat v. 3.5 (Systat Software, Richmond, CA) statistical software, and P < 0.05 was considered significant.
Results
Hepcidin Is Independently Positively Correlated with Both Tf sat and LIC.
To further dissect how iron is sensed to modulate hepcidin expression, we treated mice with a single dose of iron by oral gavage (acute iron treatment), with a high iron diet (chronic iron treatment), or with a high iron diet followed by a low iron diet in order to achieve different conditions of body iron perturbation, including isolated increases of either Tf sat or LIC.
In the chronic iron treatment experiment, serum iron (Fig. 1A), Tf sat (Fig. 1B), and LIC (Fig. 1C) all significantly increased by 24 hours. However, whereas serum iron and Tf sat plateaued for the subsequent 3 weeks, LIC continued to progressively increase. Thus, we achieved a condition of increasing LIC in the face of stable (albeit high) circulating iron levels. In the chronic iron treatment setting, hepatic Hamp mRNA expression significantly and progressively increased between baseline and 48 hours, and then plateaued for the remaining 3 weeks (Fig. 1D). Although both LIC and Tf sat positively correlated with Hamp mRNA levels (r = 0.456, P = 0.002; r = 0.658, P < 0.001, respectively) and significantly influenced Hamp mRNA expression by simple linear regression analysis (R2 = 0.21, β = 0.456, P = 0.002; R2 = 0.43, β = 0.658, P < 0.001, respectively) by multivariate analysis, Tf sat was the only independent predictor of hepatic Hamp mRNA levels (R2 = 0.46, β = 0.57, P < 0.001) in this setting.
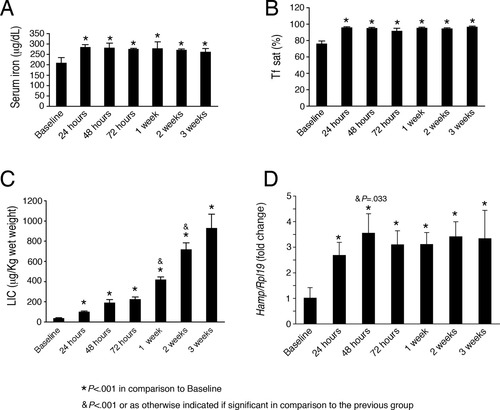
Chronic dietary iron administration increases serum iron, serum transferrin saturation, liver iron content, and hepatic hepcidin mRNA in mice. Seven-week-old male C57BL/6 mice were placed on a high iron diet (2% carbonyl iron) and were sacrificed at time zero (Baseline), 24 hours, 48 hours, 72 hours, 1 week, 2 weeks, or 3 weeks after initiation of the high iron diet (n = 6 per group). Animals were analyzed for serum iron (A), Tf sat (B), LIC (C), and hepcidin (Hamp, also known as Hamp1) relative to Rpl19 mRNA expression by quantitative real-time RT-PCR (D). Results are expressed as the mean ± SD for serum and tissue iron parameters, and as the mean ± SD for the fold change compared to the baseline for hepcidin expression. Statistical significance was determined by one-way ANOVA with Holm-Sidak or the Dunnett's post-hoc tests for pairwise multiple comparisons. A high iron diet significantly increased serum iron (A, F = 10.16, P < 0.001), Tf sat (B, F = 65.79, P < 0.001), LIC (C, F = 172.32, P < 0.001), and Hamp relative to Rpl19 mRNA (D, F = 99.40, P < 0.001). For each group significant changes are shown as (*) P < 0.001 in comparison with the baseline, and (&) for P < 0.001 or as otherwise indicated if significant in comparison with the previous group.
Although the influence of LIC on Hamp mRNA levels was difficult to detect in the chronic iron treatment setting where both LIC and Tf sat were elevated, mice switched to a low iron diet after receiving a high iron diet for 1 week maintained a high LIC for up 8 days (Fig. 2C), whereas serum iron and Tf sat decreased back to baseline levels by 24-48 hours (Fig. 2A,B), allowing us to examine the effects of an isolated elevated LIC with normal circulating iron levels. The low iron diet significantly decreased hepatic Hamp mRNA levels from those achieved by 1 week of a high iron diet within 24 hours and for up to 8 days (Fig. 2D), reflecting the decrease in serum iron and Tf sat, and consistent with a role for circulating iron in regulating hepcidin expression. Notably, Hamp mRNA levels remained significantly elevated above baseline for up to 8 days in these mice, suggesting an independent role for LIC in inducing hepcidin expression. Indeed, by multivariate analysis, both Tf sat and LIC were independent predictors of hepatic Hamp mRNA levels in this model (R2 = 0.856; β = 0.004, P < 0.001 for Tf sat; β = 0.0004, P < 0.001 for LIC).
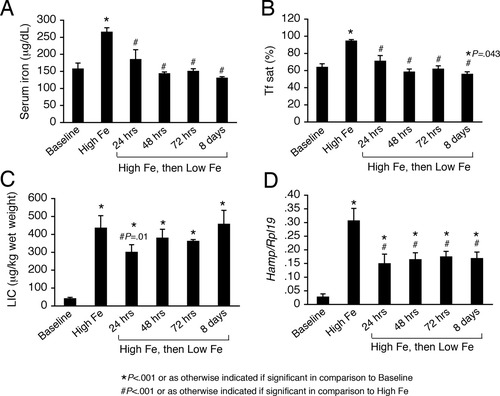
Hamp mRNA levels are independently influenced by both LIC and Tf sat. Seven-week-old male C57BL/6 mice received a standard rodent diet (Baseline), a high iron diet for 1 week (High Fe), or a high iron diet for 1 week followed by a low iron diet (2-6 ppm iron; High Fe then Low Fe) for 24 hours to 8 days as indicated (n = 4 per group). Tissues were analyzed for serum iron (A), Tf sat (B), LIC (C), and Hamp relative to Rpl19 mRNA by quantitative real-time RT-PCR (D). Results are expressed as the mean ± SD. Statistical significance was determined by one-way ANOVA with Holm-Sidak or the Dunnett's post-hoc tests for pairwise multiple comparisons. For each group, *P < 0.001 or as otherwise indicated if significant in comparison to Baseline, #P < 0.001 or as otherwise indicated if significant in comparison with the High Fe group. A high iron diet significantly increased serum iron, Tf sat, LIC, and Hamp relative to Rpl19 mRNA relative to baseline (A-D). After switching to a low iron diet for 24 hours to 8 days, serum iron and Tf sat were significantly decreased back to baseline levels for all timepoints, except 8 days, where there was a small but significant reduction in Tf sat from baseline (A,B). After switching to a low iron diet for 24 hours to 8 days, a significantly increased LIC was maintained compared with the baseline group, and the LIC was not significantly decreased from the High Fe group, except for a small but significant decrease at 24 hours (C). After switching to a low iron diet for 24 hours to 8 days, Hamp relative to Rpl19 mRNA levels were decreased to an intermediate level between those achieved by the high iron diet and baseline (D).
In the acute iron treatment experiment, both serum iron (Fig. 3A) and Tf sat (Fig. 3B) were significantly increased by a single dose of 2 mg/kg iron at 1 and 4 hours after oral gavage (black bars) compared with untreated animals (Baseline) or with mock gavage (gray bars), with a return to baseline by 8-24 hours. In contrast, LIC was unchanged at all timepoints in comparison to baseline and the respective mock groups (Fig. 3C).
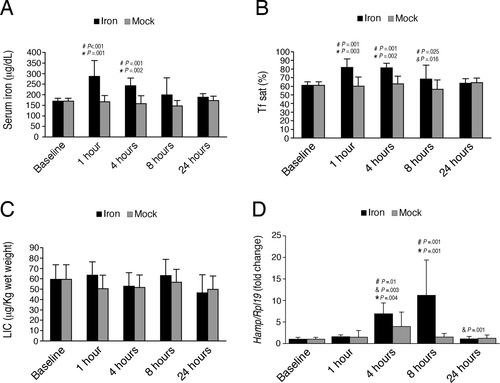
Acute enteral iron administration increases serum iron and Tf sat, but not LIC, and induces hepatic Hamp mRNA expression in mice. Nine-week-old male C57BL/6 mice received a single dose of 2 mg elemental iron per kg animal weight (Iron, black bars) or the same volume of water alone by oral gavage (Mock, gray bars). Mice were sacrificed at time 0 (without any treatment, Baseline), and at 1, 4, 8, and 24 hours after either iron or water gavage (n = 6 per group). Animals were analyzed for serum iron (A), Tf sat (B), LIC (C), and Hamp relative to Rpl19 mRNA expression by quantitative real-time RT-PCR (D). Results are expressed as the mean ± SD for serum and tissue iron parameters, and as the mean ± SD for the fold change compared to the baseline for hepcidin expression. Statistical significance was determined by two-way ANOVA with Holm-Sidak or Dunnett's post-hoc tests for pairwise multiple comparisons. Compared with baseline or mock gavage, a single dose of iron by oral gavage significantly increased serum iron (A, F = 3.974, P = 0.007 for time, F = 25.002, P < 0.001 for treatment), serum Tf sat (B, F = 3.285, P = 0.018 for time, F = 21.223, P < 0.001 for treatment), and Hamp relative to Rpl19 mRNA (D, F = 8.941, P = 0.001 for time, F = 10.678, P = 0.002 for treatment), but did not affect LIC (C, F = 1.60, P = 0.189 for time, F = 0.936, P = 0.338 for treatment). For each iron treated group, significant changes are shown as exact P values for the comparisons with the baseline (*), with the previous group (&), and with the corresponding mock group (#).
In the acute iron treatment experiment, hepatic Hamp mRNA showed a progressive temporal increase, and was significantly increased at 4 and 8 hours after iron gavage in comparison to baseline and the corresponding mock groups, with a return to baseline levels by 24 hours (Fig. 3D, black bars). The mock group did not manifest significant differences in Hamp mRNA compared to baseline, although there was a trend toward a higher value at 4 hours after mock gavage, suggesting a possible effect of the gavage procedure itself in a few animals (Fig. 3D, gray bars). In the iron group, hepatic Hamp mRNA was correlated with Tf sat (r = 0.455, P = 0.012), but not LIC (r = 0.193, P = 0.307), which was in fact not changed. By multivariate analysis, Tf sat was the only independent predictor of Hamp mRNA levels (R2 = 0.23, β = 0.444, and P < 0.001). These data provide further evidence that Tf sat independently regulates hepatic Hamp mRNA expression.
Hepatic Bmp6 mRNA Is Independently Positively Correlated with LIC, but Not Tf sat.
Next, we investigated the role of the BMP6-SMAD signaling pathway in hepcidin induction by acute and chronic iron administration. Similar to prior studies,17, 18 chronic iron treatment significantly increased hepatic Bmp6 mRNA levels in comparison to the baseline group, with a temporal progressive increase similar to LIC (compare Figs. 4A and 1C). Although one prior study suggested that the small intestine may also be a source of BMP6 in response to iron,29 we did not see any effects of chronic iron treatment on Bmp6 mRNA expression in either the duodenum (Supporting Fig. 2), in agreement with other studies,19, 30 or the spleen, another key iron homeostasis organ (Supporting Fig. 3). The Spearman's rho test confirmed a strong correlation between hepatic Bmp6 mRNA levels and LIC (r = 0.902, P < 0.001), and multivariate regression analysis demonstrated that LIC was the only factor associated with hepatic Bmp6 mRNA levels, independent of Tf sat, serum iron, hemoglobin, and mean cellular hemoglobin concentration (R2 = 0.846, β = 1.032, P < 0.001). These data suggest that LIC may have a role in hepatic Bmp6 induction by iron.
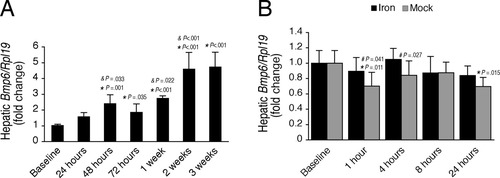
Chronic iron administration stimulates hepatic Bmp6 mRNA expression but acute iron administration does not. The animals that underwent chronic iron administration from Fig. 1 (A) and acute iron administration from Fig. 3 (B) were analyzed for hepatic Bmp6 relative to Rpl19 mRNA expression by quantitative real-time RT-PCR (n = 6 per group). Results are expressed as the mean ± SD for the fold change compared to the baseline. Statistical significance was determined by one-way ANOVA (chronic iron administration, A) or two-way ANOVA (acute iron administration, B) with Holm-Sidak or Dunnett's post-hoc tests for pairwise multiple comparisons. For each group, significant changes are shown as exact P values for the comparisons with the baseline (*), with the previous group (&), and with the corresponding iron group (#). Hepatic Bmp6 relative to Rpl19 mRNA was significantly increased from baseline by chronic iron administration (A, F = 30.20, P < 0.001), but not by acute iron administration (B), although there was a slight decrease in hepatic Bmp6 relative to Rpl19 mRNA in mock gavage groups compared to the baseline group and the corresponding iron timepoints (B, F = 4.509, P = 0.003 for time, F = 6.99, P = 0.011 for treatment).
In contrast, hepatic Bmp6 mRNA was not significantly increased by acute iron administration (Fig. 4B, black bars), where Tf sat increased but LIC did not change. The mock groups (Fig. 4B, gray bars) did show a small but significantly lower Bmp6 expression at 1 and 24 hours after gavage in comparison to the baseline group, and at 1 and 4 hours after gavage in comparison to the corresponding iron timepoints; however, the overall trend of both iron and mock groups were similar, possibly reflecting an effect from the gavage itself or circadian fluctuations of hepatic Bmp6 mRNA. Importantly, we did not find any correlation between Tf sat and hepatic Bmp6 mRNA levels (r = 0.237, P = 0.068). Additionally, we did not see a corresponding decrease in hepatic Smad1/5/8 phosphorylation or Bmp6-Smad target gene expression by mock gavage (see Figs. 5B, 6C,D, gray bars), suggesting that this small decrease in Bmp6 expression in the mock group was not functionally relevant. We also did not find significant increases in duodenal or splenic Bmp6 mRNA in response to acute iron administration (Supporting Figs. 2, 3). Together, these data suggest that Tf sat does not induce hepcidin expression by stimulating Bmp6 mRNA expression.
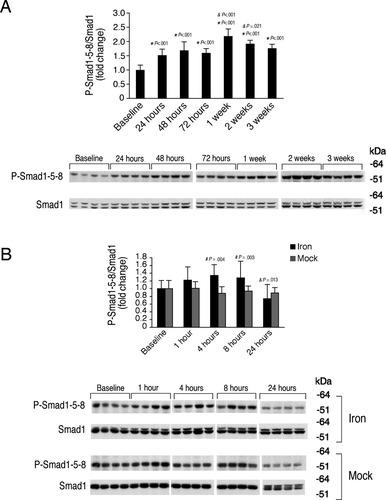
Chronic and acute iron administration both stimulate hepatic Smad1/5/8 signaling. The animals that underwent chronic iron administration from Fig. 1 (A) and acute iron administration from Fig. 3 (B) were analyzed for hepatic phosphorylated Smad1/5/8 (P-Smad 1-5-8) relative to Smad1 protein by western blot followed by chemiluminescence quantitation. Results are expressed and statistics analyzed as in Fig. 4. Chronic iron treatment significantly increased hepatic P-Smad1/5/8 relative to total Smad1 protein (A, F = 21.75, P < 0.001). Similarly, acute iron administration significantly increased hepatic P-Smad1/5/8 relative to total Smad1 protein compared with the corresponding mock treatment groups, and showed a trend toward a temporal progressive increase compared to baseline (B, F = 5.855, P = 0.02 for treatment, F = 2.246, P = 0.073 for time). For each iron treated group, significant changes are shown as exact P values for the comparisons with the baseline (*), with the previous group (&), and with the corresponding mock group (#).
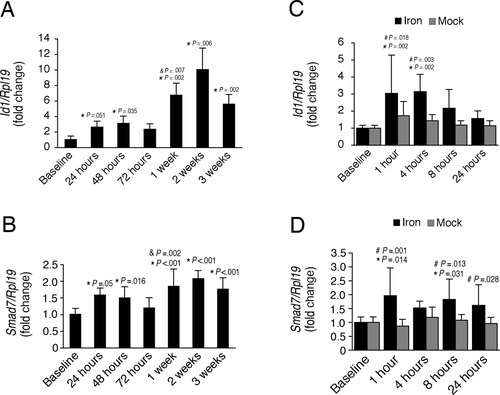
Chronic and acute iron administration both stimulate expression of hepatic Bmp6-Smad1/5/8 target transcripts Id1 and Smad7. The animals that underwent chronic iron administration from Fig. 1 (A,B) and acute iron administration from Fig. 3 (C,D) were analyzed for hepatic Id1 (A,C) and Smad7 (B,D) relative to Rpl19 mRNA by quantitative real-time RT-PCR. Results are expressed and statistics analyzed as in Fig. 4. Chronic iron treatment significantly increased hepatic Id1 (A, F = 27.745, P < 0.001) and Smad7 relative to Rpl19 mRNA (B, F = 7.41, P < 0.001). Acute iron administration also significantly increased hepatic Id1 relative to Rpl19 mRNA compared with the corresponding mock treatment groups and the baseline (C, F = 13.676, P < 0.001 for treatment, F = 4.944, P = 0.002 for time). Acute iron administration also significantly increased hepatic Smad7 relative to Rpl19 mRNA compared with the corresponding mock groups, although there was only an overall trend toward increased expression compared with the baseline group (D, F = 19.208, P < 0.001 for treatment, F = 1.548, P = 0.203 for time). For each iron treated group, significant changes are shown as exact P values for the comparisons with the baseline (*), with the previous group (&), and with the corresponding mock group (#).
Increases in LIC and Tf sat Each Stimulate Hepatic Smad1/5/8 Signaling and Smad7 mRNA Expression.
Next, we analyzed the intracellular signaling mediators and targets of BMP6 signaling (P-Smad1/5/8 protein, Id1 mRNA, and Smad7 mRNA) in the liver after both chronic and acute iron administration. In the chronic iron administration setting, hepatic P-Smad1/5/8 protein (Fig. 5A), Id1 mRNA (Fig. 6A), and Smad7 mRNA (Fig. 6B) exhibited significant increases at nearly all timepoints in comparison to the baseline group. The pattern of increase reflected the trend of both LIC and hepatic Bmp6 mRNA, where there was a relative plateau or decrease in the rate of increase between 48-72 hours and between 2-3 weeks (compare Figs. 5A, 6A,B with Figs. 1C, 4A). These data support the hypothesis that LIC activates the Smad1/5/8 signaling pathway through Bmp6 ligand induction. These data also suggest that hepatic Smad7 mRNA expression follows the overall activation of the Bmp6-Smad1/5/8 pathway.
In the acute setting, mock gavage had no effect on hepatic P-Smad1/5/8 protein, Id1 mRNA, or Smad7 mRNA expression (Figs. 5B, 6C,D, gray bars). After acute iron administration, hepatic P-Smad1/5/8 protein showed a trend toward a temporal progressive increase that reached its peak at 4 hours after gavage and then decreased back to baseline (Fig. 5B). Although the increase in hepatic P-Smad1/5/8 protein did not achieve statistical significance for the time variable, it was significantly increased in the iron group compared with the corresponding mock groups at 4 and 8 hours after gavage (Fig. 5B). Reflecting the increased hepatic P-Smad1/58 protein, hepatic Id1 mRNA expression exhibited significant increases between the iron and the corresponding mock gavage groups as well as the baseline (Fig. 6C). Similarly, hepatic Smad7 mRNA expression was significantly increased in the iron groups compared with the corresponding mock groups, although there was only an overall trend toward increased hepatic Smad7 mRNA expression after iron gavage compared with the baseline group (Fig. 6D). These data are consistent with the hypothesis that increases in Tf sat activate the Smad1/5/8 signaling cascade downstream of BMP6 ligand, and that hepatic Smad7 mRNA expression follows the overall activation of the Bmp6-Smad1/5/8 signaling pathway.
Increases in LIC and Tf sat Do Not Affect Hepatic Erk Signaling or the Inflammatory Pathway.
Because it has been suggested that Erk1/2 proteins might be involved in hepcidin regulation,21, 25-27 we also measured the phosphorylation levels of these kinases in the liver after both chronic and acute iron administration. In contrast to hepatic P-Smad1/5/8 protein and Id1 mRNA, P-Erk1/2 expression did not significantly increase after either chronic iron administration (Fig. 7A) or acute iron gavage in comparison to the baseline or the corresponding mock groups (Fig. 7B). In fact, there was a temporal progressive decrease in P-Erk1/2 for both the acute iron and mock gavage groups, possibly reflecting a circadian fluctuation or an effect of the gavage itself. For both the chronic and acute iron administration experiments, there was a large variability of hepatic P-Erk1/2 expression within each group, suggesting that other factors might drive phosphorylation of these MAP kinases.
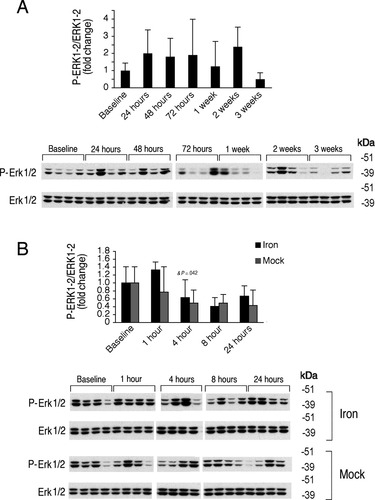
Chronic and acute iron administration have no effect of hepatic Erk1/2 phosphorylation. The animals that underwent chronic iron administration from Fig. 1 (A) and acute iron administration from Fig. 3 (B) were analyzed for hepatic phosphorylated Erk1/2 (P-ERk1/2) relative to total Erk1/2 protein by western blot followed by chemiluminescence quantitation. Results are expressed and statistics analyzed as in Fig. 4. Chronic iron administration had no significant effect on hepatic P-Erk1/2 relative to total Erk1/2 (A, F = 0.761, P = 0.608). Acute iron administration had no significant effect on hepatic P-Erk1/2 relative to total Erk1/2 compared with the corresponding mock gavage groups, although there was a temporal progressive decrease in hepatic P-Erk1/2 relative to Erk1/2 in both the iron and mock gavage groups (B,F = 2.246, P = 0.142 for treatment, F = 5.188, P = 0.002 for time). For each iron-treated group, significant changes are shown as exact P values for the comparisons with the baseline (*), with the previous group (&), and with the corresponding mock group (#).
Because inflammatory cytokines such as IL6 are also potent stimulators of hepcidin expression,1, 3-6 we examined whether chronic or acute iron administration or the gavage procedure affected the inflammatory pathway. There was no significant stimulation of hepatic Il6 mRNA or target transcript Crp expression in the chronic iron treatment setting or in response to acute mock or iron gavage (Supporting Fig. 4).
Discussion
It is well known that iron overload induces hepcidin transcription,3 and it was previously shown that hepcidin correlates with LIC.31, 32 The fact that transferrin-bound iron might induce hepcidin expression has been suggested in humans,6, 33 and demonstrated in vitro.33 To study the separate effects of circulating and tissue iron on hepcidin regulation, we treated animals with acute or chronic iron administration to obtain isolated increases of either Tf sat or LIC. We aimed to make the iron treatments as physiologic as possible by choosing an enteral administration route and a 2 mg/kg iron dose for gavage, the lowest effective dose to significantly increase Tf sat without affecting LIC in preliminary experiments (data not shown), about equivalent to a human patient taking two over-the-counter iron sulfate supplement pills (65 mg elemental iron each). Although the presence of circulating nontransferrin-bound iron (NTBI) and its redox active form (labile iron pool) LPI may not be excluded,34 we targeted and achieved a submaximal Tf sat of up to 82% with acute iron treatment and 95% with chronic iron treatment. In the acute iron administration setting, where Tf sat was increased but LIC was not, Hamp mRNA expression was also significantly increased. Additionally, in the chronic iron administration setting, Tf sat was an independent predictor of Hamp mRNA level by multivariate analysis. Thus, our data clearly demonstrate that Tf sat plays a crucial role in hepcidin regulation in vivo.
The BMP-SMAD signaling pathway is a main regulator of hepcidin expression and systemic iron homeostasis,1 and Tf sat has been suggested to signal to hepcidin through the BMP-SMAD pathway by indirect proofs and in vitro.33 Here we showed that hepatic P-Smad1/5/8 protein and Id1 mRNA were increased in the acute iron administration setting where Tf sat was increased but LIC and hepatic Bmp6 mRNA were not. Thus, our data demonstrates that Tf sat activates the BMP-SMAD signaling pathway independently of LIC and downstream of hepatic BMP6 mRNA induction. The mechanism by which Tf sat activates SMAD phosphorylation remains uncertain. We did not see a clear effect of acute iron administration on expression of the BMP coreceptor hemojuvelin or the serine protease TMPRSS6, which is reported to cleave hemojuvelin35 (Supporting Fig. 5). Interestingly, SMAD phosphorylation and BMP-SMAD target transcript expression has been demonstrated to be impaired relative to the degree of iron overload and BMP6 expression in Hfe and Tfr2 null mice and human patients with HFE mutations18, 20-24 (Corradini E, Babitt JL, Fleming RE, et al., unpubl. data), suggesting that HFE and TFR2 may be involved. This may be one mechanism accounting for the blunted hepcidin induction in response to an oral iron challenge in human patients with HFE hemochromatosis.36
LIC was also found to be an independent factor predictive of hepatic Hamp mRNA levels by multivariate analysis in the setting of an isolated elevated LIC with normal circulating iron levels. In the chronic iron treatment setting, LIC was strongly and independently associated with increases in hepatic Bmp6 mRNA expression, a main stimulator of hepcidin expression,9, 14 similar to published studies.17-19 These data suggest that the mechanism by which LIC stimulates hepcidin expression is by stimulating Bmp6 mRNA expression, thereby activating the SMAD signaling pathway. Indeed, whereas 1 week of a high iron diet increased Hamp mRNA levels 10-fold above baseline, coadministration of a neutralizing anti-BMP6 antibody (which we have demonstrated specifically blocks BMP6-SMAD signaling9) with the high iron diet blocked the Hamp mRNA increase, resulting in increased liver iron deposition (Supporting Fig. 6). These data are consistent with the phenotype of Bmp6 null mice, which exhibit decreased hepatic nuclear P-Smad1/5/8 expression, hepcidin deficiency, and iron overload.9, 14
Interestingly, we did not detect an independent effect of LIC on Hamp mRNA expression in the chronic iron treatment setting. Indeed, although Hamp mRNA was increased over 24-48 hours on a high iron diet in the setting of increases in both Tf sat and LIC, Hamp subsequently plateaued over the next 3 weeks despite continued increases in LIC. LIC may have more of an apparent effect on hepcidin regulation under conditions where Tf sat is not elevated. It is also possible that this plateau in hepatic Hamp mRNA levels was due to competing influences of stimulation by Bmp6 and feedback inhibition, for example, through inhibitory Smad7 that was also increased in this setting. Indeed, Smad7 has been shown to inhibit hepcidin transcription in vitro.10 Interestingly, in contrast to hepcidin, other Bmp6-Smad pathway target transcripts such as Id1 continued to increase after 48 hours on a high iron diet along with progressive increases in LIC and Bmp6 mRNA. This suggests that Smad7 may have a preferential inhibitory role on hepcidin expression compared with other Bmp6-Smad target genes, or that there are other mechanisms involved in the negative feedback of hepcidin expression that do not involve the BMP-SMAD pathway. Further studies will be needed to more definitively determine the role of inhibitory Smad7 in hepcidin regulation in vivo.
In addition to the BMP-SMAD pathway, the ERK1/2 MAP kinase signaling pathway has also been suggested to be involved in iron homeostasis. In particular, holotransferrin, by way of TFR2 and possibly HFE, induces the ERK1/2 cascade in vitro, and hepatic P-Erk1/2 is reduced in Hfe, Tfr2, and Hfe-Tfr2 null mice.21, 25-27 Here we demonstrated that, whereas both chronic and acute iron treatment in mice induced hepatic P-Smad1/5/8, Bmp-Smad target transcripts, and Hamp mRNA, neither chronic nor acute iron treatment induced a significant increase in hepatic P-Erk1/2, supporting a role for the BMP-SMAD pathway, but not the ERK1/2 pathway, in hepcidin regulation by iron in vivo. Our data also suggest that the inflammatory pathway is not involved in hepcidin regulation by iron.
In summary, our results demonstrate that circulating iron and tissue iron differentially activate the BMP-SMAD signaling pathway to modulate hepcidin expression. The liver is the predominant source of the BMP6 that regulates hepcidin in response to iron in vivo, and increases in LIC induce hepatic expression of BMP6 ligand, whereas increases in Tf sat activate SMAD1/5/8 phosphorylation downstream of BMP6. Inhibitory SMAD7 is significantly modulated by both acute and chronic iron administration, mirroring the overall activation of the SMAD1/5/8 signaling cascade, and may play a role in feedback inhibition of hepcidin expression. Hepatic Erk1/2 phosphorylation is not stimulated by either acute or chronic iron administration in mice, suggesting that these MAP kinases are not involved in hepcidin regulation by iron in vivo. Future studies will be needed to further delineate the precise molecular mechanisms involved in iron sensing and BMP6-SMAD pathway activation in hepcidin regulation and iron homeostasis.




