Ablation of the tumor suppressor histidine triad nucleotide binding protein 1 is protective against hepatic ischemia/reperfusion injury†
Potential conflict of interest: Nothing to report.
Abstract
The identification of cellular pathways capable of limiting ischemia/reperfusion (I/R) injury remains a frontier in medicine, and its clinical relevance is urgent. Histidine triad nucleotide binding protein 1 (HINT1) is a tumor suppressor that influences apoptosis. Because apoptotic pathways are a feature of I/R injury, we asked whether Hint1 influences hepatic I/R injury. Hint1−/− and C57BL/6 mice were subjected to 70% liver ischemia followed by reperfusion for 3 or 24 hours or to a sham operation. The serum aminotransferase levels, histological lesions, apoptosis, reactive oxygen species, and expression of B cell lymphoma 2–associated X protein (Bax), heme oxygenase 1 (HO-1), interleukin-6 (IL-6), IL-10, tumor necrosis factor-a, Src, nuclear factor kappa B (p65/RelA), and c-Jun were quantified. The responses to toll-like receptor ligands and nicotinamide adenine dinucleotide phosphate oxidase activity in Kupffer cells were compared in Hint1−/− mice and C57BL/6 mice. After I/R, the levels of serum aminotransferases, parenchymal necrosis, and hepatocellular apoptosis were significantly lower in Hint1−/− mice versus control mice. Furthermore, Bax expression decreased more than 2-fold in Hint1−/− mice, and the increases in reactive oxygen species and HO-1 expression that were evident in wild-type mice after I/R were absent in Hint1−/− mice. The phosphorylation of Src and the nuclear translocation of p65 were increased in Hint1−/− mice, whereas the nuclear expression of phosphorylated c-Jun was decreased. The levels of the protective cytokines IL-6 and IL-10 were increased in Hint1−/− mice. These effects increased survival after I/R in mice lacking Hint1. Hint1−/− Kupffer cells were less activated than control cells after stimulation with lipopolysaccharides. Conclusion: The Hint1 protein influences the course of I/R injury, and its ablation in Kupffer cells may limit the extent of the injury. (HEPATOLOGY 2011)
Ischemia/reperfusion (I/R) injury occurs as a result of multiple conditions, such as stroke, myocardial infarction, shock, and organ transplantation. In hepatic surgery, the blood flow to the liver is interrupted to prevent intraoperative blood loss during parenchymal transection. A normal liver tolerates several minutes of warm ischemia. However, when this interval is prolonged, the flow occlusion provokes ischemic lesions, which are then aggravated by reperfusion. The reperfusion is accompanied by apoptosis and areas of necrosis. Several intersecting signaling pathways have been implicated in the development of I/R injury; tumor necrosis factor α (TNF-α) is a crucial mediator that balances c-Jun N-terminal kinase (JNK) and nuclear factor kappa B (NF-κB) activation.1 Inhibition of TNF-α signaling lessens hepatic reperfusion injury.1, 2 Downstream, the activation of JNK aggravates I/R injury, whereas the activation of NF-κB is protective. JNK2 knockout mice have significantly less hepatic necrosis after I/R injury than control mice.3 Inhibition of NF-κB activation leads to massive hepatic apoptosis in response to I/R injury.4
Histidine triad nucleotide binding protein 1 (Hint1) belongs to the Hint subfamily of the histidine triad protein family, which is characterized by an HxHxHxx motif (x is a hydrophobic amino acid). Hint1 is expressed in all cell types and organs examined thus far. Hint1 is a haplo-insufficient tumor suppressor; mice lacking one or both alleles of the Hint1 gene are prone to developing cancers5, 6 when they are exposed to chemical carcinogens. Experiments with cell lines have suggested that Hint1 influences apoptosis. Overexpression of HINT1 triggers apoptosis in SW480 and MCF7 cancer cells by inducing expression of the proapoptotic factor B cell lymphoma 2–associated X protein (Bax).7 The role of Hint1 in the genesis or modulation of the apoptotic injury that follows reperfusion has never been investigated.
We hypothesized that Hint1 indeed participates in I/R injury, and we investigated the effects of Hint1 ablation on hepatic I/R injury. Mice in which Hint1 was deleted from all cells were subjected to transient warm ischemia followed by reperfusion. Our results show that the absence of Hint1 does affect the outcome of hepatic I/R injury.
Abbreviations
ALT, alanine aminotransferase; AM, attachment medium; AST, aspartate aminotransferase; Bax, B cell lymphoma 2–associated X protein; EDTA, ethylene diamine tetraacetic acid; EGTA, ethylene glycol tetraacetic acid; ELISA, enzyme-linked immunosorbent assay; HEPES, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid; HINT, histidine triad nucleotide binding protein; HO-1, heme oxygenase 1; I/R, ischemia/reperfusion; IL, interleukin; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MOPS, 3-(N-morpholino)propane sulfonic acid; mRNA, messenger RNA; NADPH, nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor kappa B; PMSF, phenylmethylsulfonyl fluoride; P-Src, phosphorylated Src; ROS, reactive oxygen species; TNF-α, tumor necrosis factor α; TNFR, tumor necrosis factor receptor; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
Materials and Methods
Animals and Surgery.
Nine-week-old male Hint1−/− mice and wild-type C57BL/6 mice were fasted for 16 to 18 hours (n = 7 for each group) and then anesthetized under isoflurane/O2 inhalation. Ischemia was induced by the clamping of the median and left lobes for 45 minutes; during this time, the abdomen was closed by a single suture, and the body temperature was monitored. The clamp was removed to initiate reperfusion, and the mice recovered until euthanasia at 3 or 24 hours. The nonischemic lobes were not removed at the end of the ischemia. Sham-operated mice underwent the surgical protocol without clamping (n = 4-5 for each group). The control groups comprised Hint1−/− mice and wild-type mice (n = 8 for each group) without surgical intervention. To evaluate long-term survival, 12 Hint1−/− mice and 12 C57BL/6 mice were subjected to 90 minutes of warm ischemia and reperfusion, and their recovery was monitored for up to 12 days. The protocol was approved by the local animal use committee.
Plasma Aminotransferase Levels.
Plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), markers of liver injury, were quantified with kits for ALT (AXON00024, AxonLab) and AST (AXON00020, AxonLab) modified according to the IFCC and with the Mira Plus biochemical analyzer (ABX Diagnostics).
Measurement of Reactive Oxygen Species (ROS).
Frozen liver (30 mg) was lysed in 300 μL of a buffer [20 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES; pH 7.4), 1.5 mM ethylene diamine tetraacetic acid (EDTA), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), a 1× protease inhibitor mix (Complete minitablets, Roche), and a 1× phosphatase inhibitor (PhosSTOP, Roche)] and homogenized. The homogenate was collected and centrifuged (10,000 rpm for 10 minutes at 4°C). ROS levels were measured in the supernatant by fluorescence after the addition of 2′,7′-dichlorofluorescein (5 μmol/L) for 30 minutes (excitation wavelength = 485 nm, emission wavelength = 530 nm). Values were normalized to the protein concentration, which was determined according to Lowry et al.'s method.8
Histology and Immunohistology.
For histological analysis, liver sections were examined after hematoxylin and eosin staining and peroxidase staining. The tissue was rehydrated, the antigen was retrieved with sodium citrate, endogenous peroxide was inactivated, and nonspecific binding was blocked.9 Liver sections were incubated 4°C overnight with primary antibodies, rabbit polyclonal anti–cleaved lamin A antibody (1:100 dilution; Cell Signaling), rabbit polyclonal anti–heme oxygenase 1 (anti–HO-1) antibody (1:100 dilution; Stressgene), rabbit anti–phospho-c-Jun (serine 63) antibody, and rabbit anti–phospho-p65 NF-κB antibody (serine 276; Cell Signalling). Liver sections were incubated for 1 hour at room temperature with secondary polyclonal goat anti-rabbit biotinylated antibodies (Dako). After incubation with the ABC complex (ABC kit, Vectastain), target expression was determined with 3,3′-diaminobenzidine color development (Liquid DAB+ substrate chromogen system, Dako). The sections were counterstained with a Harris hematoxylin solution. For negative controls, primary antibodies were omitted. The percentage of cells positive for the nuclear expression of cleaved lamin A, phospho-p65 NF-κB, and phospho-c-Jun was calculated as a function of the number of nuclei with MetaMorph software.
Real-Time Quantitative Polymerase Chain Reaction.
Total RNA was extracted from liver tissues with RNeasy columns (Qiagen) with an on-column DNA digestion step as described in the manufacturer's protocol. Total RNA (1 μg) was reverse-transcribed with Superscript III reverse transcriptase (Invitrogen), and complementary DNA, added to a universal polymerase chain reaction master mix, was amplified by an assay-on-demand system (Applied Biosystems) specific for mouse TNF-α, HO-1, and interleukin-6 (IL-6) genes. The data were analyzed according to the 2−ΔΔCt method; the Ct values were normalized to 18s ribosomal RNA, and the calibrators were the values recorded in mice before sham or I/R operation (control, 0 hours). All reactions were performed in triplicate.
Western Blotting.
Liver homogenates prepared for ROS assays were used for immunoblot analyses. Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked for 1 hour with 5% nonfat milk and then were incubated overnight at 4°C with rabbit polyclonal anti-Src (Cell Signalling), rabbit polyclonal anti-phospho Src [pY418] (Invitrogen), rabbit anti-Bax (Cell signalling) antibodies. Membranes were washed and incubated for 1 hour with peroxidase-conjugated secondary antibodies (Pierce). An enhanced chemiluminescence system (PerkinElmer) and exposure with a Fujifilm (Dielsdorf, Switzerland) LAS 100 charge-coupled device camera coupled to a computer led to the detection of the signals on the immunoblots, which were analyzed with AIDA 2.1 software (Raytest, Urdorf, Switzerland). Reincubation with anti–β-actin antibody (Sigma-Aldrich Chemie GmbH, Munich, Germany) allowed the protein levels to be normalized for β-actin expression.
Apoptosis Detection With the Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling (TUNEL) Assay.
Apoptotic cells were stained on paraffin liver sections with a fluorescein in situ cell death detection kit (Roche) according to the manufacturer's instructions. The sections were incubated in a 0.1 M citrate buffer (pH 6.0) and irradiated for 5 minutes in a 350-W microwave oven for antigen retrieval. Liver sections were incubated with a TUNEL reaction mixture for 60 minutes at 37°C. Liver sections were analyzed by fluorescence microscopy (Nikon). The TUNEL reaction mix was omitted from the negative controls.
Analysis of Cytokines Participating in I/R Damage.
Liver homogenates and serum samples were assayed with the Milliplex MAP kit (Millipore) for IL-6, IL-10, interferon-γ, and TNF-α according to the manufacturer's recommendations. A diluted cytokine standard (50 μL), samples, and controls were used for cytokine analysis. Quantification of the reaction was carried out with the Bio-Plex protein array reader, and the data were analyzed with Bio-Plex Manager 4.1 with a standard curve obtained with a recombinant cytokine standard.
Isolation of Hepatocytes and Kupffer Cells.
The mouse liver was perfused in situ with a prewarmed perfusion solution (NaCl, KCl, HEPES, and NaOH) for 5 minutes and then with a dissociation solution [4.76 mM CaCl2 in a perfusion solution with Liberase Blendzyme 3 (Roche)] for 15 minutes. The cells were collected and suspended in an ice-cold preservation buffer (a perfusion buffer completed with 1% bovine serum albumin). The cell suspension was filtered and centrifuged twice at 54g for 2 minutes. The cell pellet was resuspended in a hepatocyte attachment medium (AM; Dulbecco's modified Eagle's medium/nutrient mixture F-12, 0.02% bovine serum albumin, 1 mg/mL d-galactose, 0.03 mg/mL proline, 5 mM sodium pyruvate, 2 mM HEPES, 2 mM glutamine, penicillin/streptomycin, gentamicin, and 10% fetal bovine serum), whereas a supernatant was used for Kupffer cell isolation. After centrifugation at 1350g for 10 minutes at 4°C, the cell pellet was resuspended with 10 mL of a preservation buffer and loaded on top of a two-step Percoll 25%/50% gradient. After centrifugation at 1350g for 30 minutes at 4°C, the interface between the two density cushions was collected. After centrifugation at 1350g for 10 minutes at 4°C, the cells were resuspended with 10 mL of Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco), plated at 2.5 × 106 cells/well in six-well plastic plates, and incubated for 8 minutes at 37°C. The medium was removed, and Kupffer cells were cultured with RPMI1640 medium supplemented with 10% fetal calf serum, 2 mmol/L glutamine, and penicillin/streptomycin. For hepatocyte preparation, cells in AM were loaded on top of a stock isotonic Percoll solution (45 mL of Percoll and 5 mL of 10× Hanks buffer) and centrifuged at 60g for 5 minutes at 4°C. After washing, the hepatocytes were resuspended in 10 mL of AM, counted, and seeded in collagen-coated wells at 105 cells/cm2. After 2 hours, AM was replaced with a hepatocyte feeding medium (Williams' E and GlutaMAX with 0.015 U of insulin, 0.002 mg/mL hydrocortisone, and penicillin/streptomycin).
Mouse IL-6 and TNF-α Levels by Enzyme-Linked Immunosorbent Assay (ELISA).
IL-6 and TNF-α levels in a culture medium were measured with ELISA [Quantikine mouse IL-6 immunoassay (R&D) and mouse TNF-α instant ELISA (Bender MedSystems)] according to the manufacturer's instructions.
Measurement of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase Activity.
The NADPH oxidase activity was measured with lucigenin chemiluminescence. Kupffer cells were homogenized in a buffer containing 20 mM KH2PO4, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM PMSF, and a protease and phosphatase inhibitor. Homogenates were incubated for 20 minutes at room temperature in the dark with 5 μM lucigenin and 100 mM NADPH with or without 100 mM diphenylene iodonium, an inhibitor of NADPH oxidase. Chemiluminescence was measured with an Infinite 200 Tecan microplate reader, and the results were expressed as arbitrary units of chemiluminescence normalized to protein levels determined by the Lowry assay.8
Isolation of Mitochondria.
Isolated hepatocytes grown in a hepatocyte feeding medium for 24 hours were detached with trypsin and centrifuged at 250g for 4 minutes. The cell pellet was resuspended in a chilled buffer [225 mM mannitol, 70 mM sucrose, and 5 mM 3-(N-morpholino)propane sulfonic acid (MOPS)] with 100 mM Na2EDTA, incubated for 15 minutes on ice, homogenized through a G23 needle, and centrifuged at 500g at 4°C for 5 minutes. The supernatant was stored on ice, and the resuspension, homogenization, and centrifugation were repeated with the remaining pellet. The supernatants were pooled and centrifuged at 660g at 4°C for 10 minutes, and the pellet was discarded. After centrifugation at 7000g at 4°C for 15 minutes, the pellet was resuspended in a chilled mannitol buffer without EDTA and used directly for the measurement of O2 consumption. The enrichment of citrate synthase activity in the pellet was comparable among all preparations (10- to 12-fold).
Oxygen Consumption in Isolated Mitochondria.
Oxygen consumption was measured with a model 5300 biological O2 monitor (YSI, Inc.) and a Clark-type O2 electrode in a respiration chamber at 30°C with constant stirring. Mitochondria (1 mg) were added to 1 mL of a respiration buffer [220 mM KCl, 100 mM MOPS, 100 mM KH2PO4, and 100 mM EGTA (pH 7.4)]. Respiration at complex I and complex II was stimulated by the addition of 20 mM glutamate and 10 mM succinate, respectively, in the presence of 0.5 μM rotenone. State 3 respiration was initiated by the addition of 10 mM adenosine diphosphate. State 4 rates of oxygen consumption were measured after complete depletion of adenosine diphosphate. The respiratory control ratio was calculated as the ratio of state 3 respiration to state 4 respiration. Experiments were performed in triplicate.
Statistical Analysis.
Data points are presented as means and standard deviations. Results were compared with the nonparametric Mann-Whitney U test. Survival curves were compared with the log-rank test. A P value less than 0.05 was considered statistically significant.
Results
Deletion of Hint1 Improves Survival After I/R Injury.
After 90 minutes of ischemia followed by reperfusion, a greater proportion of Hint1−/− mice (75%) versus wild-type mice (30%) survived for 12 days (Fig. 1).
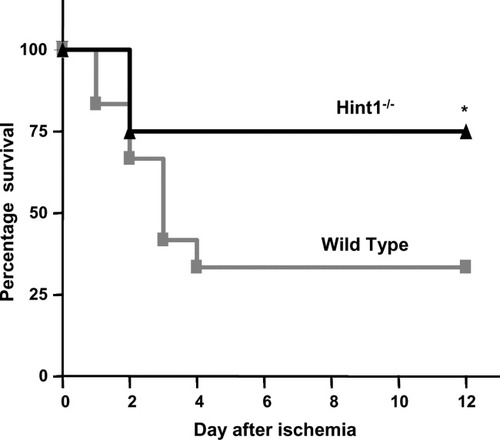
Hint1 deletion improves survival after I/R. Twelve Hint1−/− mice and 12 control mice were subjected to 90 minutes of ischemia followed by reperfusion. Survival was evaluated for 12 days. The Hint1−/− mice survived longer than the wild-type mice (*P = 0.03).
Hint1−/− Mice Show Less Hepatic Injury After I/R.
Three hours after the sham operation, the ALT values were not different between the wild-type mice (186 ± 86 IU/L, n = 5) and Hint1−/− mice (182 ± 69 IU/L, n = 5). After 24 hours, the values decreased similarly in wild-type mice (87 ± 44 IU/L, n = 4) and Hint1−/− mice (75 ± 32 IU/L, n = 4). Three hours after I/R, ALT levels increased more in control mice (1161 ± 325 IU/L, n = 7) than in Hint1−/− mice (534 ± 43 IU/L). Similarly, 24 hours after I/R, the ALT levels remained higher in wild-type mice (465 ± 237 IU/L, n = 7) than in Hint1−/− mice (147 ± 25 IU/L; Fig. 2A). A similar pattern of change was observed for AST (data not shown). According to a histological examination of liver sections, wild-type mice displayed massive areas of hepatic necrosis (Fig. 2B and Supporting Fig. 2) after reperfusion. Necrotic areas were observed in 85% and 100% of C57BL6 mice 3 and 24 hours after injury, respectively (Table 1). Necrosis affected fewer Hint1−/− mice and was less extensive (Fig. 2B). Necrotic areas were observed in only 14% and 71% of the Hint1−/− mice at 3 and 24 hours, respectively (P < 0.05; Table 1).
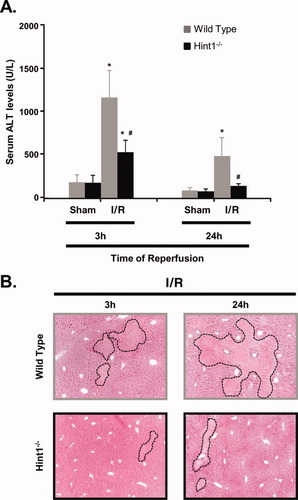
Hint1 deletion protects against hepatic injury after I/R. (A) Serum ALT levels were measured in mice 3 and 24 hours after a sham operation or I/R. ALT levels were significantly higher in the wild-type group versus the Hint1−/− group. The values are means and standard deviations. * and # (P < 0.05) indicate statistical significance versus sham-operated mice and I/R–operated wild-type mice, respectively (at 3 hours, P = 0.0009 for I/R wild-type mice versus sham wild-type mice, P = 0.0012 for I/R Hint1−/− mice versus sham wild-type mice, and P = 0.035 for I/R Hint1−/− mice versus I/R wild-type mice; at 24 hours, P = 0.0012 for I/R wild-type mice versus sham wild-type mice, and P = 0.0007 for I/R Hint1−/− mice versus I/R wild-type mice). (B) Parenchymal hepatic injury was assessed by hematoxylin and eosin staining on liver sections of I/R–operated animals. The necrotic areas were larger in the wild-type group versus the Hint1−/− group after 3 and 24 hours of reperfusion. Dashed lines indicate necrotic areas. The original magnification was ×50.
| Mice | Procedure | Necrosis Grade (n/N) | Necrosis (%)* | Animals Affected by Necrosis (%) | |||
|---|---|---|---|---|---|---|---|
| None or <5% | 5%-20% | 20%-50% | >50% | ||||
| Wild-type | 3-hour sham | 5/5 | 0/5 | 0/5 | 0/5 | 0 ± 0 | 0 |
| 3-hour I/R | 1/7 | 5/7 | 1/7 | 0/7 | 19 ± 10 | 85 | |
| 24-hour sham | 4/4 | 0/4 | 0/4 | 0/4 | 0 ± 0 | 0 | |
| 24-hour I/R | 0/7 | 1/7 | 4/7 | 2/7 | 38 ± 23 | 100 | |
| Hint1−/− | 3-hour sham | 5/5 | 0/5 | 0/5 | 0/5 | 0 ± 0 | 0 |
| 3-hour I/R | 6/7 | 1/7 | 0/7 | 0/7 | 5 ± 6 | 14 | |
| 24-hour sham | 5/5 | 0/5 | 0/5 | 0/5 | 0 ± 0 | 0 | |
| 24-hour I/R | 2/7 | 3/7 | 2/7 | 0/7 | 10 ± 9 | 71 | |
- * Mean and standard deviation.
Deletion of Hint1 Decreases Apoptosis After I/R.
Immunohistochemical staining for cleaved lamin A identified 2- and 10-fold fewer apoptotic cells in Hint1−/− mice versus control mice 3 and 24 hours after I/R injury, respectively (Supporting Fig. 3C). The TUNEL assay detected cell death in situ. Many positive nuclei were present 3 and 24 hours after I/R in the wild-type mice, and very few were present in the Hint1−/− mice (Fig. 3A and Supporting Fig. 3A). This was associated with significantly lower expression of the proapoptotic protein Bax, which was evident in liver homogenate from Hint1−/− mice under all conditions. The sham operation alone triggered an increase in the expression of the Bax protein, but only in the wild-type mice (Fig. 3B and Supporting Fig. 3B).
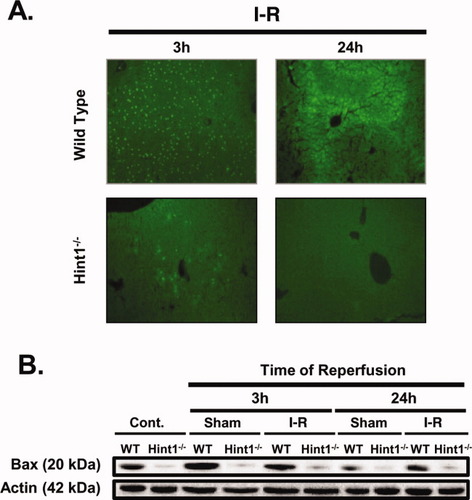
Hint1 deletion reduces apoptosis after I/R. (A) TUNEL assay of liver sections of I/R–operated mice after 3 and 24 hours. Apoptosis was much more abundant after I/R in wild-type mice versus Hint1−/− mice after 3 and 24 hours of reperfusion. (B) Immunoblotting for Bax expression in liver homogenates. The expression of the target Bax protein was normalized to actin expression. Bax expression was decreased in Hint1−/− mice under all conditions.
Deletion of Hint1 Decreases ROS and Reduces the Expression of HO-1.
Three hours after I/R, there was an increase in hepatic ROS in wild-type mice, and this normalized after 24 hours. After I/R in Hint1−/− mice, ROS levels were unchanged (Fig. 4A). Because mitochondria can be a source of ROS, we investigated the hepatocellular respiration. Mitochondria were isolated from Hint1−/− mice and wild-type mice. As shown in Supporting Fig. 4A,B, there was no difference in the state 3 respiration, state 4 respiration, and respiration control ratio between the Hint1−/− mice and the wild-type mice either with glutamate as a substrate or with succinate. As a marker of oxidative stress, the expression of HO-1 was measured at both the messenger RNA (mRNA) and protein levels. The sham operation and I/R injury provoked 5- and 16-fold increases, respectively, in HO-1 mRNA at 3 hours, but only in wild-type mice. After 24 hours, HO-1 mRNA expression was equal in all groups. Similar changes were also observed with immunohistochemistry (Fig. 4B and Supporting Fig. 4C).
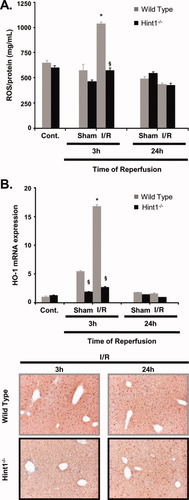
Oxidative stress markers in the livers of control and Hint1−/− mice after reperfusion. (A) The ROS content was measured in liver homogenates that were isolated from sham-operated animals and I/R–operated animals before (control) and 3 and 24 hours after reperfusion. The values were normalized to the protein concentrations. ROS levels were higher in I/R wild-type mice after 3 hours of reperfusion (P = 0.02 for 3-hour I/R wild-type mice versus wild-type control mice and P = 0.0007 for 3-hour I/R Hint1−/− mice versus 3-hour I/R wild-type mice). (B) HO-1 mRNA and protein levels in sham-operated mice and I/R–operated mice. HO-1 mRNA levels were elevated (16-fold) after 3 hours of reperfusion in wild-type mice; they were less elevated (5-fold) after the sham operation. HO-1 mRNA levels increased only 2-fold after I/R in Hint1−/− mice. § and * (P < 0.05) indicate statistical significance versus wild-type mice and sham-operated mice, respectively (P = 0.003 for 3-hour sham Hint1−/− mice versus 3-hour sham wild-type mice, P = 0.00004 for 3-hour I/R Hint1−/− mice versus 3-hour I/R wild-type mice, and P = 0.019 for 3-hour I/R wild-type mice versus 3-hour sham wild-type mice). An immunohistochemistry analysis of liver sections of I/R–operated animals confirmed the induction of HO-1 expression with hepatocytes. The original magnification was ×50.
Deletion of Hint1 Decreases the Activation of Kupffer Cells.
Kupffer cells were isolated from Hint1−/− mice and wild-type mice. The release of TNF-α and IL-6 in response to exposure to lipopolysaccharide (LPS) was assayed by the determination of the concentrations of these cytokines in the medium. There was no difference in the TNF-α release between the Kupffer cells isolated from Hint1−/− mice and those isolated from wild-type mice (Supporting Fig. 1A). In contrast, the release of IL-6 by Kupffer cells isolated from Hint1−/− mice was significantly reduced in comparison with the release by Kupffer cells isolated from wild-type mice (Supporting Fig. 1B). The basal activity of the NADPH oxidase inhibited by diphenylene iodonium was similar between the Kupffer cells isolated from Hint1−/− mice and those isolated from wild-type mice. After stimulation with LPS, the activity increased 5-fold in the Kupffer cells isolated from wild-type animals, whereas it increased only 1.18-fold in Kupffer cells isolated from Hint1−/− mice (Supporting Fig. 1C). The difference in this activity after LPS was significant (7.9 ± 2.6 versus 3.5 ± 0.5, P = 0.04).
Expression of NF-κB, Src, and Phosphorylated c-Jun After I/R in Hint1−/− Mice.
Immunohistochemistry for phosphorylated p65/RelA on serine 276 revealed higher nuclear expression in Hint1−/− mice in comparison with wild-type mice under the basal condition and after surgery, especially at 3 hours (Fig. 5 and Supporting Fig. 5). Src expression was increased in Hint1−/− mice in comparison with wild-type mice 3 and 24 hours after I/R (Fig. 6 and Supporting Fig. 6); Src was also more phosphorylated on tyrosine 418 in Hint1−/− mice in comparison with wild-type mice among control animals, and this difference increased after surgery (Fig. 6 and Supporting Fig. 6). Immunohistochemistry for phosphorylated c-Jun on serine 63 revealed fewer positive nuclei in Hint1−/− mice in comparison with wild-type mice 3 and 24 hours after I/R (Fig. 7 and Supporting Fig. 7).
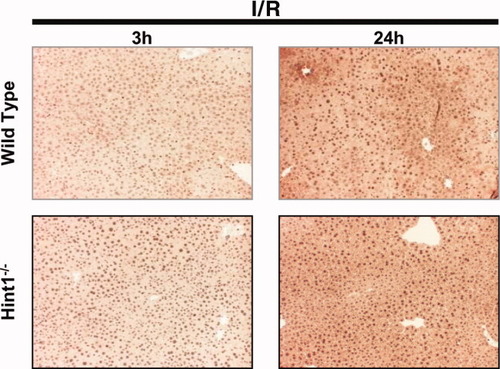
Hint1 deletion enhances the activation of NF-κB: immunostaining of liver sections of I/R–operated animals with antibodies against phosphorylated p65/RelA. Livers from wild-type and Hint1−/− mice were analyzed 3 and 24 hours after reperfusion. Livers of Hint1−/− mice expressed more nuclear phosphorylated p65/RelA than wild-type mice 3 hours after I/R. The original magnification was ×50.
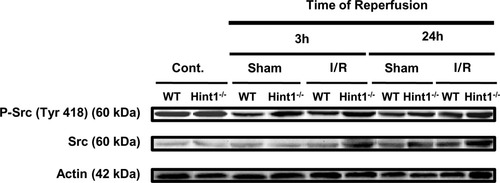
Hint1 deletion increases P-Src and total Src expression after I/R: immunoblotting detection of P-Src and total Src in liver homogenates. Protein expressions were normalized for actin and expressed as fold inductions over wild-type control animals. P-Src expression was higher in Hint1−/− mice versus wild-type mice under control conditions (0 hours) and after 3 and 24 hours of reperfusion, and total Src expression was higher after 3 hours of reperfusion only in Hint1−/− mice. Abbreviation: P-Src, phosphorylated Src.
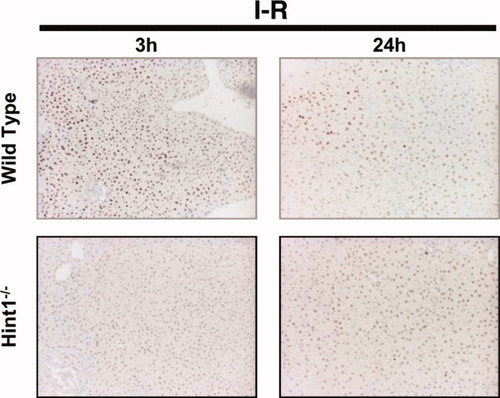
Hint1 deletion blocks the activation of c-Jun after I/R. Immunohistochemical staining was used to analyze the expression of phosphorylated c-Jun in liver sections of I/R–operated animals. The nuclear expression of phosphorylated c-Jun was lower in Hint1−/− mice versus wild-type mice after 3 and 24 hours of reperfusion. The original magnification was ×50.
Deletion of Hint1 Increases the Expression of Protective Cytokines.
The levels of IL-6 and IL-10 in liver homogenates 24 hours after I/R were significantly higher in Hint1−/− mice in comparison with wild-type mice (Table 2). There were no significant differences in the levels of expression of TNF-α and interferon-γ.
| Cytokine | Wild-Type Mice | Hint1−/− Mice |
|---|---|---|
| TNF-α (pg/mg of protein) | ||
| 24-hour sham | 0.420 ± 0.144 | 0.575 ± 0.151 |
| 24-hour I/R | 0.583 ± 0.180 | 0.798 ± 0.222 |
| IL-6 (pg/mg of protein) | ||
| 24-hour sham | 0.083 ± 0.022 | 0.048 ± 0.044 |
| 24-hour I/R | 0.055 ± 0.033 | 0.115 ± 0.059* |
| IL-10 (pg/mg of protein) | ||
| 24-hour sham | 0.018 ± 0.008 | 0.043 ± 0.039 |
| 24-hour I/R | 0.018 ± 0.007 | 0.030 ± 0.018† |
| Interferon-γ (pg/mg of protein) | ||
| 24-hour sham | 0.093 ± 0.045 | 0.070 ± 0.025 |
| 24-hour I/R | 0.094 ± 0.047 | 0.121 ± 0.067 |
- There were no significant differences between the wild-type mice and the Hint1−/− mice after I/R in the TNF-α and interferon-γ levels. The levels of IL-6 and IL-10 were significantly higher in the Hint1−/− mice versus the wild-type mice 24 hours after ischemia.
- * P = 0.04 for the Hint1−/− mice versus the wild-type mice.
- † P = 0.05 for the Hint1−/− mice versus the wild-type mice.
Discussion
Hint1 does indeed influence the development of hepatic I/R injury because the absence of Hint1 from all cell types significantly improves animal survival and lessens hepatic damage. The mechanisms by which Hint1 ablation protects against injury involve a decrease in ROS and an increase in the phosphorylation of Src, which promotes the nuclear translocation of phosphorylated p65/RelA and decreases the nuclear expression of phosphorylated c-Jun. This was associated with higher hepatic levels of protective IL-6 and IL-10.
Hint1, like other proteins with tumor suppressor properties, is associated with proapoptotic properties,10 but the mechanisms have been only partly elucidated. Hint1 modulates the activity of several transcription factors, including activator protein 1.11 Hint1 inhibits activator protein 1 activity by impairing the phosphorylation of c-Jun by JNK2.11 SW480 and MCF7 cells transfected with Hint1 are sensitized to undergo apoptosis because of an up-regulation of the proapoptotic factor Bax.7 Apoptosis and activation of JNK are two important features of I/R injury.12
The mechanisms of hepatic I/R injury are complex and involve the cross-signaling between hepatocytes and Kupffer cells. Activated Kupffer cells generate ROS, which are signals for the release of proinflammatory and proapoptotic mediators.13 We observed that the production of ROS was blunted in Hint1−/− mice 3 hours after reperfusion, whereas wild-type mice presented a significant increase in ROS production; this would be expected to attenuate the I/R injury. The absence of induction of NADPH oxidase activity in Kupffer cells of Hint1−/− mice in response to LPS exposure and the normal mitochondrial respiration of Hint1−/− hepatocytes suggest that Kupffer cells lacking Hint1 are responsible for the absence of ROS production in Hint1−/− mice after reperfusion. LPS are ligands for toll-like receptor 4, and toll-like receptor 4 knockout mice have been reported to be protected against I/R injury.14 In agreement with the reduced levels of ROS, induction of HO-1 gene expression was abrogated in the Hint1−/− group. HO-1 is an enzyme with anti-oxidative and anti-inflammatory functions, is induced by various stimuli such as proinflammatory cytokines and oxidative stress, and has been shown to exert a protective role in cold hepatic I/R injury.15 The most likely explanation for the lack of HO-1 stimulation following I/R injury in our Hint1−/− mice is that the stress stimuli originating in Kupffer cells or hepatocytes and leading to its expression were absent.
I/R provokes the secretion of several cytokines, including IL-6 and IL-10, which are, in this context, protective. IL-6−/− mice are more susceptible to I/R, and the administration of recombinant IL-6 protects rats against I/R.16 Similarly, the administration of recombinant IL-10 reduces I/R injury.17, 18 Moreover, the secretion of IL-10 by conventional dendritic cells has been recently found to reduce I/R injury.19 The higher levels of IL-6 and IL-10 that we measured in the livers of Hint1−/− mice after I/R in comparison with wild-type mice may explain the resistance to hepatic I/R injury by mice constitutively lacking Hint1. The levels of hepatic TNF-α and interferon-γ after I/R were not affected by the absence of Hint1. The proapoptotic protein Bax, which is activated after TNF-α stimulation,20 was also reduced in our studies. Ben-Ari et al.21 reported that mice lacking Bax showed less apoptotic injury after I/R in the liver, and Bailly-Maitre et al.22 found that Bax inhibitor 1 protects against I/R injury. Therefore, the fact that our Hint1−/− mice expressed less Bax than wild-type mice may explain why we observed less apoptosis after I/R in these animals. Bax expression increased in controls after the sham operation to the same extent that it increased after I/R, and this suggests that the surgical stress is enough to stimulate the expression of Bax.
A further mechanism of Hint1−/−-induced I/R injury protection must implicate the NF-κB pathway. Activation of NF-κB can be mediated by a pathway different from the classic I kappa B kinase/proteasome–dependent pathway. NF-κB can be activated by Src-mediated phosphorylation of IκB-α.23, 24 Our results show that Hint1−/− mice express more Src after I/R, and this is in line with the results of Cen et al.,25 who reported that HINT1 is a negative regulator of Src transcription. We also observed more phosphorylation of Src and increased nuclear translocation of p65/RelA after I/R. These observations are compatible with an activation of NF-κB via this noncanonical pathway. NF-κB protects against TNF-α–induced cell death not only by inducing the expression of antiapoptotic protein but also by attenuating TNF-α–induced JNK activation.26, 27 Because NF-κB controls the expression of antioxidant enzymes that can prevent the formation of ROS, NF-κB contributes to the prevention of prolonged activation of JNK by ROS, which in turn are produced in response to TNF-α.28, 29 Moreover, Src interferes directly with the apoptotic process by phosphorylating and inactivating caspase-8.30
In conclusion, our work reveals that the tumor suppressor Hint1 is a heretofore unrecognized molecule involved in the pathogenesis of I/R injury. Kupffer cells lacking Hint1 are less reactive, and the ablation of this tumor suppressor from all cell types protects against hepatic I/R injury. This discovery should lead to the pharmacological inhibition of Hint1 as a new strategy for preventing hepatic I/R injury.
Acknowledgements
The authors are very grateful to the late B. Weinstein, who generously provided Hint1−/− mice. They also thank Monika Ledermann for her expert surgical assistance.




