Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice†
Potential conflict of interest: Nothing to report.
Abstract
The implantation of grafts below 30% of the normal liver volume is associated with a high risk of failure known as small-for-size (SFS) syndrome. Strategies to rescue small grafts may have a dramatic impact on organ shortage. Serotonin is a potent growth factor for the liver. The goal of this study was to determine whether enhanced serotonin signaling could prevent the deleterious effects of SFS syndrome. We performed 30% normal liver volume transplantations in wild-type C57/BL6 and interleukin-6 (IL-6)−/− mice. Some animals received α-methyl-5-HT (DOI), an agonist of serotonin receptor-2 (5-HT2B). Endpoints included long-term survival, serum and hepatic markers of liver injury and regeneration, assessment of hepatic microcirculation by intravital fluorescence microscopy and scanning electron microscopy, and transcript levels of a variety of serotonin receptors, tumor necrosis factor α, and IL-6. All recipients of small grafts (controls) died within 2-4 days of transplantation, whereas half of those receiving DOI survived permanently. Control animals disclosed major liver injury, including diffuse microvesicular steatosis in hepatocytes, impairment of microcirculation, and a failure of regeneration, whereas these parameters were dramatically improved in animals subjected to DOI. Blockage of 5-HT2B blunted the protective effects of DOI. Whereas IL-6 levels were higher in DOI-treated animals, IL-6−/− mice were still protected by DOI, suggesting a protective pathway independent of IL-6. Conclusion: Serotonin through its action on receptor-2B protects SFS liver grafts from injury and prevents microcirculation and regeneration. The mechanism of hepato-protection is independent of IL-6. (Hepatology 2011;)
Orthotopic liver transplantation (OLT) remains the only hope for cure in patients with a variety of end-stage liver diseases. This success has led to a worldwide shortage of available liver grafts, and a significant proportion of patients die waiting for an organ. Partial liver grafts including split cadaveric liver grafts or living donor liver transplantation are established strategies to reduce mortality among those patients on the waiting list.1
However, some major risks are inherent to split cadaveric liver or living donor liver transplantation. In the latter strategy, a healthy donor must be subjected to major surgery. Typically, a hemi-hepatectomy is necessary. The risk of mortality following such surgery is estimated at 0.2%-0.5% with a moderate but significant incidence of serious complications.2-5 Next, the recipient receiving a partial graft from a living donor or split cadaveric organ is subject to the risk of small-for-size (SFS) syndrome.6 It is estimated that at least 35% of the normal liver size (or 0.8% of liver/body ratio) should be implanted to minimize the risk of this syndrome.
A revolutionary strategy would be transplantation of only a small part of the liver (e.g., segments II and III) in a living donor, which can be performed laparoscopically7 and is associated with a low risk of complications similar to the range observed following living kidney donation. Such an operation would likewise be widely accepted and may alleviate the shortage of organs. A similar small graft could also be obtained from a cadaveric organ. To achieve this, however, the issue of SFS liver graft failure must be resolved.
SFS syndrome is morphologically characterized by sinusoidal endothelial cell (SEC) injury and the rapid development of diffuse microsteatosis in hepatocytes. Presumably, due to the limited capacity of a small liver, the graft is unable to meet the functional demand in the recipient6 and fails to regenerate.8-10 Those findings are associated with increased portal flow and hypertension.11
We developed a model of partial liver transplantation in the mouse and showed that full preservation of the arterial supply to the liver is essential.12 We also noted that mice receiving only 30% of the liver displayed histological changes of SFS syndrome with a poor survival rate. In contrast, the use of 50% of the total liver mass triggered a massive regenerative response resulting in a high animal survival after transplantation.10 In subsequent experiments, we demonstrated that SFS syndrome induces Kupffer cell activation with the release of tumor necrosis factor α (TNF-α). Blockade of this pathway by pentoxifylline (PTX) reduces injury and TNF-α production but increases the release of interleukin-6 (IL-6), and animal survival was dramatically improved.8
Concomitantly, we discovered in an in vivo model of major hepatectomy in mice that serotonin secreted by platelets mediates regeneration through its receptor subtypes 5-HT2A and 5-HT2B.13 Serotonin is a ligand for a large family of 5-hydroxytryptamine (5-HT) receptors with a major role in neurotransmission in the central nervous system. Peripherally, serotonin mediates vascular contraction and relaxation, cell proliferation, apoptosis, and platelet aggregation. The family of serotonin receptors is subdivided into seven subgroups. These receptors have been grouped according to their genetic and structural similarities and also according to the intracellular signaling pathways associated with each receptor. Serotonin regulates hepatic function and response to injury, blood flow, and proliferation of hepatocyte.14
In further studies, we could not detect any negative impact of serotonin in a model of ischemia/reperfusion injury. In contrast, we identified a new role for serotonin in tissue repair following ischemic injury.15 We therefore hypothesize that serotonin rescues liver regeneration after implantation of a small graft without enhancing the inherent ischemic damage, and thereby prevents SFS syndrome.
Abbreviations
5-HT, 5-hydroxytryptamine; 5-HT2B, serotonin receptor-2B; AST, aspartate aminotransferase; DOI, α-methyl-5-HT; IL-6, interleukin-6; OLT, orthotopic liver transplantation; PCNA, proliferating cell nuclear antigen; PTX, pentoxifylline; SEC, sinusoidal endothelial cell; SFS, small-for-size; TNF-α, tumor necrosis factor α.
Materials and Methods
Animals.
Male inbred C57BL/6 mice were purchased from Harlan, Netherlands, IL-6−/− mice with C57BL/6 background were obtained from Jackson Laboratory and used as syngeneic transplant donors and recipients. Animals were kept in accordance with the guidelines of the University of Zurich Animal Care Committee. The protocol of the study was approved by the Cantonal Veterinary office of Zurich. All mice were kept in a temperature-controlled environment with a 12-hour light/dark cycle and with free access to food and tap water.
Surgical Procedures and Animal Experiments.
We performed 30% partial OLTs in mice using techniques described previously.10 Some mice received a 25% OLT graft consisting of the right liver lobe.
The recipient mice were divided into two groups: (1) α-methyl-5-HT (DOI, an agonist of the serotonin receptor and (2) a control group. DOI (1 mg/kg dissolved in saline) was given intravenously immediately following reperfusion of the partial liver graft. Subsequently, recipients were injected subcutaneously twice a day for 2 days postoperatively. In control recipients, the same amount of vehicle solution was administered. Recipients were sacrificed at 1 hour, 3 hours, 2 days, or 7 days postoperatively. Hepatic regeneration, aspartate aminotransferase (AST) levels in serum, transcript levels of 5-HT2 receptors, IL-6, TNF-α in liver grafts, histology, scanning electron microscopy, and intravital microscopy were evaluated. In separate series of experiments, the recipient survival rates of 7 days after transplantation were tested. Some animals were treated with an antagonist of the 5-HT2B receptor: SB206553 (3 mg/kg) was injected subcutaneously into the donor and recipient before surgery and twice a day for 2 days after transplantation.
Histology, Immunohistochemistry, and Markers of Liver Injury.
Tissues were immersion-fixed in 4% buffered formalin and embedded in paraffin wax, then sectioned, and stained with hematoxylin-eosin. Ki-67 and proliferating cell nuclear antigen (PCNA) staining were used to quantify hepatic regeneration 2 days after transplantation. Serum levels of AST as markers of hepatocyte injury were measured 2 days after transplantation.
Intravital Fluorescence Microscopy.
Mice were prepared 1 hour after transplantation for intravital fluorescence microscopy as described16 on a Leica CLS 150× microscope. Microscopy sequences were captured by a camera and recorded by a video system for offline evaluation.
Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was extracted from liver tissue using TRIzol reagent. Quantitative real-time polymerase chain reaction amplification and data analysis were performed using an ABI Prism 7000 Sequence Detection System. Results were quantified as fold induction comparison with baseline after normalization to 18S RNA.
Scanning Electron Microscopy.
Liver tissue was prepared for scanning electron microscopy 3 hours after transplantation: the graft was flushed with 3% polyvinylpyrrolidone in Hank's balanced salt solution. The fixed liver tissues were cut into small pieces. The specimen were then washed with phosphate-buffered saline and stored at 4°C until further processing for scanning electron microscopy.
Statistical Analysis.
Values are expressed as the mean ± SD. The data were analyzed using GraphPad Prism version 5 software. Differences between groups were evaluated using an unpaired t test. Differences were considered statistically significant at P < 0.05.
Results
Does Serotonin Enhance Hepatocyte Proliferation in an SFS Liver Graft?
To evaluate whether serotonin had an effect on SFS OLT and could improve liver regeneration, we performed 30% OLT. The recipient was treated with the serotonin agonist DOI or with saline, until grafts were harvested 48 hours after surgery. Ki-67 and PCNA staining were analyzed on liver sections by immunohistochemistry (Fig. 1A,B). Both showed significantly enhanced hepatocyte proliferation in DOI-treated liver grafts when compared with control grafts. Fig. 1C,E (control) and Fig. 1D,F (DOI) demonstrate a strong induction of proliferation markers by DOI.
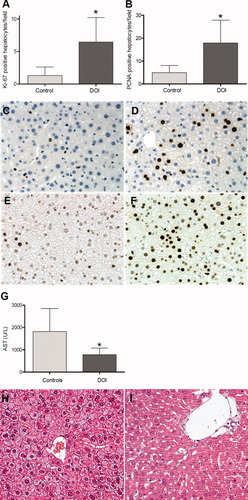
Prevention of SFS syndrome by a serotonin agonist. (A) Ki-67–positive hepatocytes per field in controls and DOI-treated animals (P = 0.017) (n = 5/6). (B) PCNA-positive hepatocytes per field (P = 0.047) (n = 5/6). (C,D) Ki67-stained hepatocytes in control (C) and DOI-treated (D) animals. (E,F) PCNA-stained hepatocytes in control (E) and DOI-treated (F) animals. (G) Liver enzyme levels to demonstrate tissue injury (n = 5/5). (H,I) Representative hematoxylin-eosin–stained sections of untreated (H) and DOI-treated (I) mouse livers. *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
How Does DOI Impact on Ischemia/Reperfusion Injury After 30% Liver Graft Implantation?
Ischemia/reperfusion injury is inevitable in organ transplantation.17, 18 It may be particularly harmful and exacerbate the loss of function in liver grafts contributing to SFS syndrome.19 To test the impact of DOI on ischemia/reperfusion injury of SFS liver grafts, serum AST was tested 2 days after 30% OLT. AST levels were elevated in the recipient control group, whereas application of DOI significantly blunted tissue injury in recipients (Fig. 1G) (P = 0.027). Hematoxylin-eosin staining of embedded liver graft tissue disclosed diffuse microvesicular steatosis in the control (Fig. 1H). Few neutrophils and rare small foci of necrosis were present in control animals.
Does DOI Prevent SEC Injury After Reperfusion?
SECs are highly susceptible to cold ischemic injury.18 Preservation of intact SECs is key for a successful OLT.20 In our model of partial OLT, all grafts are inherently exposed to a short period of cold ischemia. Therefore, we studied SEC on hematoxylin-eosin–stained biopsies and by scanning electron microscopy 3 hours after reperfusion. There was no apparent damage of the SECs, such as necrosis, apoptosis, or detachment from the hepatocyte plate in both groups. However, compared with control mice, we observed an increased number of fenestrae in the endothelial cells of grafts treated with DOI at 3 hours after transplantation (Fig. 2A-C). This suggests that SECs react to serotonin by opening fenestrae that support fluid exchange.
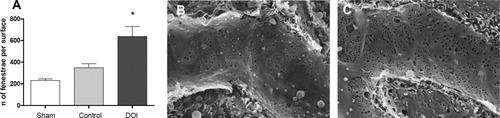
Quantitative analysis of endothelial fenestrae. (A) Determination of fenestrae per surface area (n = 3/3/3). DOI elevated the number of fenestrae significantly. *P < 0.05. (B,C) Fenestration in control (B) and DOI-treated (C) liver endothelia is demonstrated in scanning electron microscopy images of representative sinusoids.
Does DOI Preserve Microcirculation in SFS Liver Graft?
As observed by scanning electron microscopy, DOI appears to affect SECs; therefore, we asked whether this might have functional consequences on microcirculation and perfusion. We tested graft microcirculation by intravital fluorescence microscopy 1 hour after transplantation. The sinusoidal functional density was 480 ± 27.5 (cm/cm2) in DOI-treated mice, which was significantly higher than in controls (346.72 ± 20.9; P = 0.028) (Fig. 3A). Similarly, the sinusoidal red blood cell velocity was higher in DOI-treated animals compared with controls (0.38 ± 0.03 versus 0.25 ± 0.02; P = 0.003) (Fig. 3B). Although there was no obvious cellular damage, the architecture of the sinusoidal network in controls appeared disturbed (Fig. 3C) in contrast to DOI-treated animals, which retained an intact sinusoidal architecture (Fig. 3D).
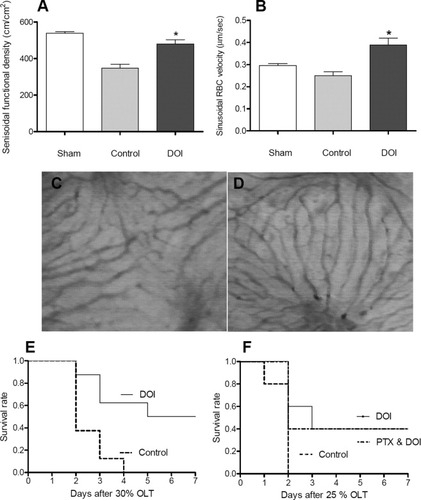
Intravital fluorescence microscopy results 1 hour after reperfusion. Sinusoidal functional density (A, P = 0.028 versus control) and red blood cell (RBC) velocity was determined in hepatic sinusoids (B, P = 0.003 versus control) (n = 3/4/4). Representative pictures of hepatic microcirculation are shown from control (C) and DOI-treated animals (D). (E) Seven-day survival rate of mice after 30% OLT (P = 0.01) in control and DOI-treated animals (n = 8/8). (F) Seven-day survival rate of wild-type mice after 25% OLT in untreated mice compared with DOI treatment in the presence or absence of PTX (n = 5/5/5) (P = 0.4 between DOI and DOI plus PTX).
Does Serotonin Rescue Liver Failure in SFS Transplantation?
We investigated whether serotonin improves survival after 30% OLT. Recipient survival was recorded for 7 days, and is presented as a Kaplan-Meier curve. In the saline-treated control group, all recipients died 2-4 days after surgery. In contrast, application of DOI rescued 50% of the transplanted animals (Fig. 3E) (P = 0.01).
Does the Application of Serotonin and PTX Synergistically Ameliorate SFS Syndrome?
We have shown that PTX reverses SFS syndrome after OLT.8 In those experiments, we observed increased transcript levels of anti-inflammatory cytokines, an improvement of liver regeneration and preservation of microcirculation in the graft. We therefore hypothesized that the combination of DOI with PTX may act synergistically to protect the graft from SFS syndrome.
To clarify the impact of serotonin together with PTX on survival after SFS transplantation, we developed a new model of partial OLT. Because PTX and serotonin treatment alone already improved survival in 30% OLT, we reduced the size of the graft to only 25% of the total liver mass. To achieve this volume, each liver lobe except the right lobe was removed during the donor procedure. We treated the 25% OLT donor and recipients with saline, DOI, and DOI plus PTX and compared 7-day survival among the groups.
As expected, no animals in the control group survived, but approximately half of the animals in the DOI group survived. To our surprise, combination therapy with DOI plus PTX did not further improve survival compared with DOI alone (Fig. 3F).
The lack of additional benefit suggests that the action of serotonin and PTX may share a common pathway. However, we cannot exclude the possibility that toxicity from massive pharmacological interventions may have impeded an improvement of survival by an otherwise beneficial effect of PTX. Furthermore, these results indicate that DOI plays a decisive role in improving the outcome of SFS OLT.
Does DOI Influence the TNF-α and IL-6 Transcript Levels in the Remnant Liver Graft?
TNF- α and IL-6 are established primers and initiators of hepatocyte proliferation after major tissue loss in vivo.21, 22 We previously established that the protective effect of PTX is mediated through IL-6.8 Because the experiment combining serotonin and PTX suggested a common pathway, we tried to establish whether IL-6 was affected by serotonin in SFS grafts. We measured IL-6 transcript levels in SFS liver tissue using real-time polymerase chain reaction. IL-6 was elevated at 1 hour after 30% OLT in the presence or absence of DOI, but there was no difference between controls and DOI-treated recipients. However, 2 and 3 hours postoperatively, there was a significant difference between the two groups (Fig. 4A), suggesting that IL-6 was a target of serotonin action. To verify whether DOI-induced IL-6 was mediated by TNF-α, we also measured TNF-α transcript levels, which were not significantly different between DOI-treated recipient mice and controls at 1 and 3 hours after transplantation (Fig. 4B).
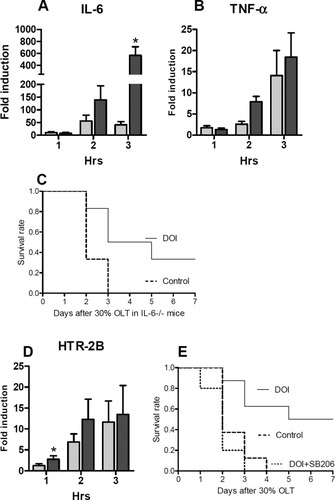
Transcript levels of TNF-α, IL-6, and HTR-2B and 7-day survival rates of SFS OLT in IL-6 −/− mice. (A,B) Transcript levels of IL-6 (A) and TNF-α (B) at 1, 2, and 3 hours after transplantation (n = 5/5). Real-time polymerase chain reaction was performed and normalized to livers of sham control animals. (C) Seven-day survival rate of SFS LT in IL-6−/− mice (P = 0.03) shown as a Kaplan-Meier plot (n = 6/6). (D) Determination of transcript levels of 5-HT2B (P = 0.045, 1 hour) (n = 5/5). (E) application of a 5-HT2B antagonist in combination with DOI. The Kaplan-Meier plot reveals that the antagonist reverses the survival benefit provided by DOI (n = 8/8/5). (A,B,D) the dark color bar is DOI group, and the light color bar is the control group.
To further clarify whether IL-6 is a mediator of hepatoprotection by serotonin, we performed additional 30% OLTs using IL-6−/− mice, as both donor and recipient, treated with saline or DOI, respectively. Recipient survival was monitored for 7 days after transplantaion. A total of 40% of the recipient IL-6−/− mice treated with DOI survived 7 days, whereas all control IL-6−/− animals died within 2-3 days (Fig. 4C). These results provide strong evidence that serotonin mediates hepatoprotection in an IL-6–independent manner.
Which Serotonin Receptor Pathway Is Activated by DOI in SFS Liver Graft?
In earlier studies, we observed that DOI, an agonist of the serotonin receptor-2 family, is very effective in rescuing liver regeneration.13 In a previous study, we demonstrated that the receptor subtypes 5-HT2A and 5-HT2B mediate liver regeneration in vivo13 and therefore determined transcript levels of 5-HT2A, 5-HTB, and 5-HTC in the current experiment. The 5-HT2A receptor transcript levels were similar between controls and the experimental group, whereas 5-HT2C expression was undetectable (data not shown). The 5-HT2B transcript levels increases earlier in DOI-treated livers, at 1 hour after transplantation (Fig. 4D) (P = 0.045), whereas at 2 and 3 hours, the transcripts increased both in treated and untreated animals.
To provide more solid evidence for the role of 5-HT2B, we performed additional experiments wherein we blocked the 5-HT2B receptor in the donor and in the recipient with SB206553, a specific antagonist of 5-HT2B and 5-HT2C. Consistent with our hypothesis, the protective effects of DOI was lost in presence of the antagonist. All recipient mice died within 4 days after transplantation. These results indicated that 5-HT2B is playing a pivotal role in improving the outcome of SFS transplantation in mice (Fig. 4E).
Discussion
In this study, we observed that activation of 5-HT2B significantly improved survival in recipients of a small, otherwise nonviable graft by enhancing liver regeneration and hepatic microcirculation, thereby reducing ischemia/reperfusion injury. We have shown that protection in this model can be achieved by the use of PTX, which also rescues the failure of regeneration, a mechanism involving the IL-6 pathway.8 Serotonin was found to protect the liver in an IL-6–independent manner.
This study is a logical continuation of our previous work demonstrating that platelets containing serotonin mediate liver regeneration in vivo,13 and that the failure of liver function after implanting a small graft is primarily due to a failure of regeneration.8 Another important facet of the hypothesis that serotonin may be beneficial in SFS OLT relies on the absence of a negative impact of serotonin on ischemia/reperfusion injury.15
Although serotonin mediates hepatocyte proliferation through 5-HT2A and 5-HT2B in a hepatectomized mouse model,13 it was unclear whether a similar pathway may be active in an SFS OLT setting. Our investigation indicates that DOI, an agonist of 5-HT2B, significantly enhances hepatocyte proliferation after SFS OLT in mice. This effect occurs exclusively through 5-HT2B activation. The 5-HT2C expression was undetectable within remnant liver grafts of both groups. Further evidence incriminating 5-HT2B was the observation of the loss of the protective effects of DOI in animals exposed to SB206553, a specific antagonist of the 5-HT2B/5-HT2C subtype.
The preserved microcirculation is vital for the success of OLT. The sinusoidal endothelium of an SFS graft is subjected to portal hypertension and increased flow.23 Activation of 5-HT2B may trigger relaxation of the actin in sinusoids, which appear to serve as a mechanical buffer for portal hypertension and increased blood flow in hepatic sinusoids after the implantation of the small graft. This hypothesis could not be fully explored because we could not convincingly measure the portal pressure in a continuous manner due to the presence of adhesion following the OLT procedure. The only surrogate evidence relies on the preserved microcirculation in DOI-treated recipient animals.
Ellis et al.24 showed that targeting 5-HT2B receptors causes endothelium-dependent relaxation of rat jugular vein. Another study showed that serotonin-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT2B.25, 26 Cummings et al.27 documented consistent expression of 5-HT2B on the endothelial cell of hepatic sinusoidal. Serotonin is able to mediate vascular contraction and relaxation peripherally, and is considered the major constrictor of the portal vein,28 probably through 5-HT2A. Hironaka et al.29 showed that specific 5-HT2A receptor blockade with sarpogrelate inhibited monocrotaline-induced pulmonary artery hypertension and prolonged survival in rats. In our study, 5-HT2A was not elevated after DOI treatment. Ishida et al.30 reported that activation of 5-HT2B/5-HT1B receptors produced nitric oxide in human coronary artery endothelial cells. The nitric oxide production is completely inhibited by nonselective 5-HT1/5-HT2 antagonist, but not by the 5-HT2A/5-HT2C antagonist ketanserin. Application of DOI enhances the nitric oxide production. They conclude that 5-HT evoked endothelium-dependent relaxation of human coronary artery in vivo might be triggered by net effects of the activation of the 5-HT2B/5-HT1B receptors. Although there was no significant damage on the sinusoidal endothelium 1 hour after transplantation in controls or DOI-treated recipients, the number of fenestrae in DOI-treated animals increased significantly. This suggests that the perfusion of the parenchyma with macromolecules is improved, which might enhance the functional recovery of the regenerating hepatocytes. Another possibility is that DOI preserves the liver from endothelial injury; however, we were not able to detect significant endothelial damage at the investigated time points of 1 and 3 hours after transplantation.
IL-6 is an important initiator or facilitator of liver regeneration in vivo.21, 22, 31, 32 In a previous study looking at the TNF-α pathway in a similar SFS model,8 we found that PTX was the most effective strategy to restore regeneration and improve animal survival. The effect of PTX was related to an induction of the IL-6 pathway, because mice lacking IL-6 were not protected by PTX.8 In the current study, we documented different IL-6 transcript levels between controls and DOI-treated animals after transplantation. To identify an interaction of IL-6 and 5-HT2B, we performed 30% OLT using IL-6−/− mice as donors and recipients. We found that 40% of recipient of IL-6−/− mice pretreated with DOI survived permanently, whereas none survived over 3 days in the untreated control group. It indicates that the hepatic protective effect of serotonin is IL-6–independent. Whether serotonin and PTX share a common pathway or act independently from each other cannot be answered at this point, but the lack of an additive or synergistic effect of a combined treatment is in favor of the former possibility. Further investigations are obviously needed to investigate the distinct mechanisms of liver regeneration promoted by IL-6 and serotonin.
Ischemia/reperfusion injury is inevitable in organ transplantation, causing hepatocyte and hepatic SEC injury. Our data revealed that AST levels were reduced in DOI-treated recipient mice at 2 days after transplantation compared with controls. We speculate that DOI does not directly protect the liver from cold ischemic injury, but rather preserves microcirculation and accelerates liver regeneration through the activation of the 5-HT2B receptor, thus preventing the liver parenchyma from further injury. This interpretation may be supported by the observation that AST levels were not different between the controls and DOI-treated animals at the time points of 1 and 3 hours after transplantation (data not shown). At this point, further studies are required to dissect cause and effect of improved liver regeneration in DOI-treated recipients of an SFS liver graft.
In conclusion, the data demonstrate that serotonin improves SFS liver graft failure through a 5-HT2B pathway by preservation of hepatic microcirculation, which in turn facilitates liver regeneration. The protective effect of serotonin and activation of 5-HT2B is independent of IL-6. This finding opens new doors for the most limiting factor in clinical practice in using small grafts for OLT.
Acknowledgements
We thank Udo Ungethüm and Martha Bain-Stucki for excellent technical help.




