Association of host pharmacodynamic effects with virologic response to pegylated interferon alfa-2a/ribavirin in chronic hepatitis C†
Potential conflict of interest: Dr. Hassanein is on the speakers' bureau of, and receives grants from Roche. Dr. Zhou is employed by RTI Health Solutions and receives funding from Roche and Genentech. Dr. Poordad consults, advises, is on the speakers' bureau of, and receives grants from Roche and Genentech. Drs. Prabhakar and Lentz are employed by Genentech.
Abstract
Patients receiving therapy for chronic hepatitis C virus (HCV) infection frequently experience cytopenias and weight loss. We retrospectively assessed the pharmacodynamic effects of pegylated interferon (PEG-IFN) alfa-2a and ribavirin by evaluating the relationship between changes in hematologic parameters, body weight, and virologic response. Patients with HCV genotypes 1, 4, 5, or 6 receiving 24 or 48 weeks of PEG-IFN alfa-2a and ribavirin therapy were pooled from four phase 3/4 trials. Maximum decreases in hemoglobin level, neutrophil count, platelet count, and weight during therapy were assessed according to virologic response category (sustained virologic response [SVR], relapse, breakthrough, and nonresponder) and race/ethnicity. Of 1,778 patients analyzed, more than half were male, non-Hispanic Caucasian, and infected with HCV genotype 1; had a baseline HCV RNA >800,000; and had alanine aminotransferase levels ≤3 × the upper limit of normal. Virologic responders (SVR, relapse, and breakthrough) experienced greater maximum decreases from baseline in hemoglobin level, neutrophil count, platelet count, and weight compared with nonresponders; however, no clear trend was observed between SVR, relapse, and breakthrough. After adjusting for drug exposure and treatment duration, only decreases in neutrophil count remained associated with virologic response. Significantly greater declines in neutrophil (P < 0.0001) and platelet (P < 0.005) count were observed at weeks 4, 12, and 24 of therapy in virologic responders compared with nonresponders. This difference between responders and nonresponders was also observed among racial/ethnic groups, although statistical significance was not consistent across all groups. Conclusion: This post hoc analysis of HCV patients treated with PEG-IFN alfa-2a and ribavirin shows that maximum decreases from baseline in hematologic parameters and weight loss were associated with virologic response. However, after adjusting for drug exposure and accounting for duration of therapy, only neutropenia was independently associated with virologic response. (HEPATOLOGY 2010;)
Approximately 40%-50% of patients with hepatitis C virus (HCV) genotype 1 treated with pegylated interferon (PEG-IFN) and ribavirin in clinical trials achieve a sustained virologic response (SVR).1-4 Both pretreatment and on-treatment factors can significantly impact response rates (e.g., viral load, age, presence of fibrosis, steatosis, race/ethnicity, presence of insulin resistance, and time to first HCV RNA undetectability).1-9 During therapy, PEG-IFN and ribavirin are known to elicit a pharmacodynamic response in both the virus and the host. The viral response can be measured by the number of patients achieving undetectable HCV RNA levels, whereas the host response commonly manifests as systemic effects such as influenza-like symptoms, weight loss, depression, and myelosuppression (e.g., anemia, neutropenia, and thrombocytopenia).10-13 Both the rapidity of viral clearance (e.g., rapid virologic response and complete early virologic response) and the magnitude of cytopenias and weight loss have been shown to correlate with viral response.4, 14-16
The association of cytopenias and weight loss with viral response raises the potential dilemma of trying to maintain patients on therapy despite the occurrence of adverse events. Anemia is the most significant of the cytopenias, because substantial reductions in hemoglobin can profoundly affect a patient's functional status and quality of life.17 In many cases, the anemia warrants a reduction in the dose of ribavirin.17, 18 However, response rates may be significantly lower among patients who have required ribavirin or PEG-IFN dose reductions,19-21 suggesting that drug exposure is an important predictor of response.
Finding the optimal balance between managing therapy-related adverse effects and optimizing the chance of SVR is more complicated for African Americans and Latinos, because both groups experience significantly lower SVR rates than Caucasians.6-8, 22 African Americans may also have lower baseline leukocyte counts, neutrophil counts, and hemoglobin levels compared with Caucasians,6 potentially decreasing the therapeutic window before dose modification is required. Latinos are more likely to experience significant anemia, neutropenia, and thrombocytopenia during therapy.8 Whether any correlation between viral response and host pharmacodynamic effects holds true for African Americans and Latinos has yet to be clearly demonstrated.
The arrival of HCV protease inhibitors over the next 2 years is anticipated to bring significant improvements in SVR; however, they will likely compound the adverse events and costs that are associated with HCV therapy, because they will be added to an established PEG-IFN and ribavirin treatment. Identifying the host effects that correlate with viral response may be important in order to optimize the risk/benefit ratio of triple combination therapy. We therefore conducted a large, pooled, post hoc analysis of patients with HCV genotypes 1, 4, 5, or 6 from four trials of PEG-IFN alfa-2a and ribavirin therapy to better understand the association between virologic response and pharmacodynamic effects as reflected by changes in hematologic parameters and body weight.
Abbreviations
HCV, hepatitis C virus; IFN, interferon; PEG-IFN, pegylated interferon; SVR, sustained virologic response.
Patients and Methods
Patients.
Patients with HCV genotypes 1, 4, 5, or 6 receiving 24 or 48 weeks of combination therapy with PEG-IFN alfa-2a (Pegasys; Roche, Nutley, NJ; 180 μg/week) and ribavirin (Copegus; Roche, Nutley, NJ; 1,000 or 1,200 mg/day) were pooled from two registration trials1, 2 and two phase 4 trials.7, 8 The registration trials were randomized, multicenter, phase 3 studies in IFN-naïve patients with chronic hepatitis C; the first trial compared the efficacy of PEG-IFN alfa-2a and ribavirin therapy with IFN alfa-2b and ribavirin therapy for 48 weeks,1 and the second trial of PEG-IFN alfa-2a and ribavirin therapy compared different treatment duration and ribavirin dose combinations.2 The phase 4 studies were noncomparative, open-label studies of PEG-IFN alfa-2a and ribavirin for 48 weeks in treatment-naïve patients with HCV genotype 1; the majority (>73%) of patients in the first study were African American patients,7 and the second study was conducted in Latino and non-Latino Caucasian patients (ClinicalTrials.gov Identifier NCT00087607).8 All studies included stopping rules for nonresponse except for the trial in African American patients.7 Patients who received PEG-IFN monotherapy or IFN alfa-2b and ribavirin combination therapy (Rebetron) and patients with HCV/human immunodeficiency coinfection were excluded from the study.
Study Design.
The objectives of this study were: (1) to explore the association between pharmacodynamic parameters and virologic response category (SVR, relapse, breakthrough, and nonresponders); (2) to explore the association between pharmacodynamic parameters and race/ethnicity (African American, Latino Caucasians, non-Latino Caucasians, and other races); and (3) to evaluate the effects of clinically significant hemoglobin decline (>3 g/dL versus ≤3 g/dL) on SVR. The pharmacodynamic effects of interest in this analysis were hematologic parameters (hemoglobin level, neutrophil count, and platelet count) and weight loss.
Maximum decrease (baseline value for the hematologic test minus the lowest value for that test while on therapy) was used to assess the change in hematologic parameters. To better adjust for the impact of baseline difference, percentage of change from baseline was used to analyze racial/ethnic group differences and body weight changes. For patients without the specified hematologic test or body weight measurement during treatment, the corresponding maximum decrease was set as missing.
The four mutually exclusive viral response categories were: (1) SVR, undetectable HCV RNA 24 weeks after end of treatment; (2) relapse, undetectable HCV RNA at end of treatment but detectable or missing test result 24 weeks after end of treatment; (3) breakthrough, undetectable HCV RNA during treatment and detectable or missing test result at end of treatment; and (4) nonresponder, no negative test result during treatment or at end of treatment or no postbaseline HCV RNA test. Due to the sample sizes and similar results found in initial analyses, the SVR, relapse, and breakthrough categories were combined to form the responder group in some analyses. Undetectable HCV RNA levels were defined as HCV RNA <28 IU/mL in one study (Roche High Pure System/COBAS TaqMan HCV Monitor Test)8 and <50 IU/mL in three studies (Roche Amplicor polymerase chain reaction assay).1, 2, 7
Statistical Analysis.
Analyses were performed using the intent-to-treat population that received at least one dose of study medication. Linear regression analysis was performed to test the null hypothesis that the mean maximum decrease was the same across virologic response categories. The maximum decrease was the dependent variable. Cirrhosis, an independent predictor of non-SVR, was included in the model if it was significant (P < 0.05). To account for the impact of drug exposure, total PEG-IFN received over the whole treatment duration and total ribavirin received per kilogram of baseline weight were included in the model. Per protocol, ribavirin dose was based on baseline weight and was not modified due to changes in weight during treatment. With the virologic response category forced into the model regardless of significance, the backward selection method was used to eliminate the covariates (cirrhosis and PEG-IFN and ribavirin exposures) that were not significant (P > 0.05). Adjusted mean maximum decreases for SVR, relapse, breakthrough, and nonresponder were calculated using the least square means from the final models. A sensitivity analysis including only treatment completers was performed to take into consideration the duration of therapy. In addition, separate models were conducted using the same procedures with changes in pharmacodynamic parameters from baseline to weeks 4, 12, and 24 as dependent variables. In these models, the total dose received up to the corresponding time point was used in the analysis. The same procedures were also used to assess the effects of race/ethnicity on hematologic parameters and weight. The association between hemoglobin decline and SVR was assessed with and without adjustment for drug exposure using logistic regression models with SVR/non-SVR as the dependent variable.
Results
Patient Demographic and Clinical Characteristics at Baseline.
Table 1 presents the baseline demographic and clinical characteristics of 1,778 patients infected with HCV genotypes 1, 4, 5, or 6 from four randomized clinical trials of 24 or 48 weeks of treatment with PEG-IFN alfa-2a and ribavirin. The majority of patients had HCV genotype 1 infection, and more than 70% were assigned to receive PEG-IFN alfa-2a and ribavirin 1,000-1,200 mg/day for 48 weeks.
| Variable | Baseline Value |
|---|---|
| Male sex | 1,183 (66.5) |
| Age (years), mean ± SD (range) | 44.5 ± 9.7 (18.0-76.0) |
| Weight (kg)* | 80.8 ± 17.9 |
| Body mass index (kg/m2)† | 27.5 ± 5.2 |
| <25 kg/m2 | 614 (34.9) |
| 25-<30 kg/m2 | 708 (40.3) |
| ≥30 kg/m2 | 435 (24.8) |
| Race | |
| Non-Latino Caucasian | 1,272 (71.5) |
| Latino Caucasian | 287 (16.1) |
| African American | 131 (7.4) |
| Other | 88 (4.9) |
| ALT quotient ≤3× ULN | 1,328 (74.7) |
| HCV genotype | |
| 1 | 1,711 (96.2) |
| 4, 5, or 6 | 67 (3.8) |
| HCV RNA log10 IU/mL‡ | 6.1 ± 0.7 |
| >800,000 IU/mL | 1,161 (65.3) |
| METAVIR activity score§ | 1.6 ± 0.6 |
| Cirrhosis or transition to cirrhosis | 314 (17.7) |
| Neutrophils, 109/L∥ | 3.6 ± 1.4 |
| Platelets, 109/L∥ | 216.6 ± 64.2 |
| Hemoglobin, g/dL‡ | 15.5 ± 1.2 |
| Treatment arm | |
| 48-week PEG-IFN alfa-2a + 1,000-1,200 mg ribavirin | 1,269 (71.4) |
| 24-week PEG-IFN alfa-2a + 1,000-1,200 mg ribavirin | 136 (7.6) |
| 48-week PEG-IFN alfa-2a + 800 mg ribavirin | 262 (14.7) |
| 24-week PEG-IFN alfa-2a + 800 mg ribavirin | 111 (6.2) |
| Duration of treatment, weeks | 38.9 ± 13.2 |
| 24-week treatment arm (n = 247) | 23.4 (3.5) |
| 48-week treatment arm (n = 1,531) | 41.4 (12.5) |
- Abbreviations: ALT, alanine aminotransferase; ULN, upper limit of normal.
- All data are given as the mean ± SD or n (%) unless noted otherwise.
- * n = 1,769.
- † n = 1,757.
- ‡ n = 1,777.
- § n = 1,495.
- ∥ n = 1,776.
In this analysis sample, 42% of patients achieved SVR, 23% relapsed, 10% had breakthrough, and 24% were nonresponders. Approximately one-third of the breakthrough and nonresponder patients completed at least 44 weeks of treatment. Of the four trials included in this analysis, epoetin alfa use was only allowed in the Latino study and was received by 10.4% of patients in the Latino group and 18% of patients in the non-Latino Caucasian group.8 The mean ± SD maximum decrease from baseline was 2.5 ± 1.3 × 109/L for neutrophils, 93.6 ± 44.9 × 109/L for platelets, 3.9 ± 1.5 g/dL for hemoglobin, and 6.3% ± 4.7% for weight.
Change in Pharmacodynamic Parameters by Virologic Response Category.
The mean maximum decreases in hematologic parameters and weight by virologic response category are shown in Fig. 1. Cirrhosis was associated with smaller declines in neutrophils and platelets and was therefore included in the final models. The analysis indicated that there was a correlation between virologic response and maximum decreases from baseline in each parameter. Patients with a virologic response (SVR, relapse, and breakthrough) experienced greater declines in neutrophil count, platelet count, hemoglobin level, and weight than nonresponders (P < 0.01 for all parameters except for the difference in hemoglobin decline between breakthrough and nonresponders, which was not significant).
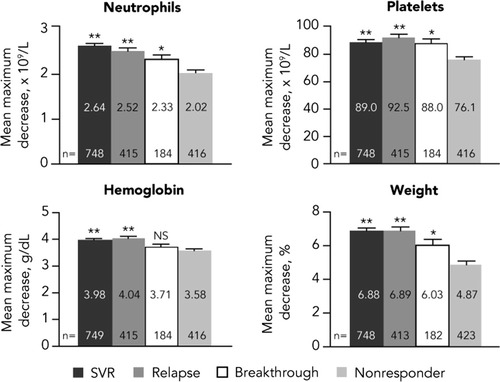
Mean maximum decreases from baseline in pharmacodynamic parameters by virologic response category. Cirrhosis-adjusted least squares means for neutrophil and platelet counts are shown. For hemoglobin and weight, cirrhosis was not significant and adjustment was not required. Error bars represent upper 95% confidence intervals. NS, not significant. *P < 0.01 versus nonresponders. **P < 0.0001 versus nonresponders.
Effects of Drug Exposure and Duration of Therapy on Changes in Pharmacodynamic Parameters.
Among virologic responders, the mean total dose of PEG-IFN alfa-2a was 7,202.8 μg and the mean total dose of ribavirin was 3,803.8 mg/kg. Among nonresponders, the mean total dose of PEG-IFN alfa-2a was 5,074.3 μg and the mean total dose of ribavirin was 2,474.0 mg/kg. After adjusting for total PEG-IFN and ribavirin received (Fig. 2A), the differences between virologic responders and nonresponders in neutrophil and platelet count declines remained statistically significant; however, the decreases in hemoglobin level and weight were no longer consistently associated with virologic response. An additional sensitivity analysis that included only a subset of patients who completed at least 44 weeks of therapy (Fig. 2B) showed that after adjusting for drug exposure and taking into consideration duration of therapy, only neutrophil decline was independently associated with virologic response.
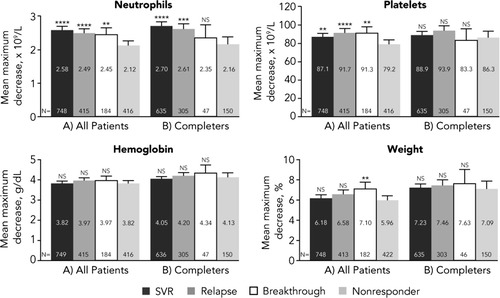
Adjusted mean maximum decreases from baseline in pharmacodynamic parameters by virologic response status. (A) All patients. (B) Treatment completers (patients completing at least 44 weeks of treatment). Least squares means adjusted for cirrhosis and drug exposure (total PEG-IFN and total ribavirin received per kilogram of weight at baseline) are shown if significant (P < 0.05). Error bars represent upper 95% confidence intervals. NS, not significant. *P < 0.05 versus nonresponders. **P < 0.01 versus nonresponders. ***P < 0.001 versus nonresponders. ****P < 0.0001 versus nonresponders.
Change in Pharmacodynamic Parameters from Baseline to Weeks 4, 12, and 24.
The mean changes in pharmacodynamic parameters after adjusting for drug exposure from baseline to weeks 4, 12, and 24 for virologic responders and nonresponders are shown in Fig. 3. Following the initiation of combination therapy, rapid decreases in neutrophil count, platelet count, and hemoglobin level were observed, with most of the decline occurring by week 4 for both responders and nonresponders. Significantly greater declines in neutrophil count (P < 0.0001) and platelet count (P < 0.005) were seen at all time points in virologic responders compared with nonresponders. The mean decreases from baseline in hemoglobin level were similar between virologic responders and nonresponders at all time points after adjusting for drug exposure. A gradual decline in weight was observed during treatment, with a trend toward a greater decline in virologic responders compared with nonresponders observed at weeks 12 and 24 (P < 0.05). These results are consistent with those observed in the analysis of maximum decrease.
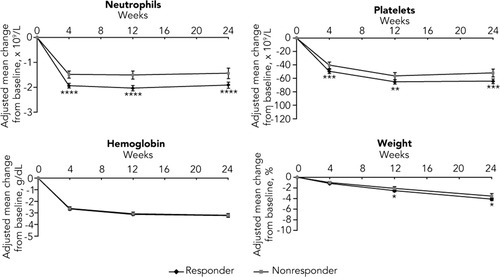
Adjusted mean change in pharmacodynamic parameters from baseline to weeks 4, 12, and 24 for virologic responders and nonresponders. Error bars represent 95% confidence intervals. Least squares means adjusted for cirrhosis and drug exposure (total PEG-IFN and total ribavirin received per kilogram of weight up to the time of measurement) are shown if significant (P < 0.05) at one or more time points. P values were derived from tests comparing responders and nonresponders. NS, not significant; *P < 0.05. **P < 0.005. ***P < 0.001. ****P < 0.0001.
Additional analyses were conducted to assess the effects of baseline creatinine clearance (estimated by Cockcroft and Gault equation) on hemoglobin decline. Patients with low baseline creatinine clearance had greater maximum declines in hemoglobin than those patients with higher creatinine clearance, which is consistent with results from the study by Reau et al.23 However, once drug exposure was adjusted for, adding creatinine clearance to the model did not affect the differences in hemoglobin decline between responders and nonresponders.
Change in Pharmacodynamic Parameters by Race/Ethnicity.
Mean percent decreases from baseline in pharmacodynamic parameters after adjusting for drug exposure by race/ethnicity are presented in Fig. 4. Overall, African Americans, Latinos, and other races (mostly Asian) had significantly smaller declines in neutrophils than non-Latino Caucasians (P < 0.0001). African Americans and other races also had smaller declines in platelets when compared with non-Latino Caucasians. In contrast, Latinos had greater declines in hemoglobin level, and African Americans had greater declines in weight than non-Latino Caucasians (P < 0.01 for both).
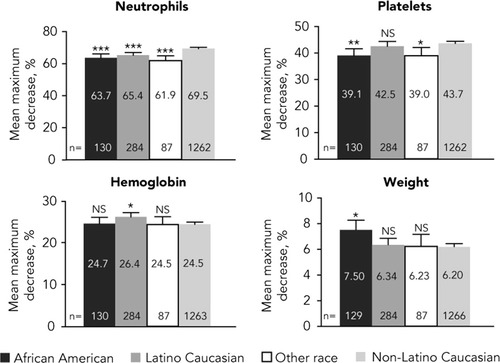
Adjusted mean percent decreases from baseline in pharmacodynamic parameters by race/ethnicity. Least squares means adjusted for cirrhosis and drug exposure (total PEG-IFN and total ribavirin received per kilogram of weight at baseline) are shown if significant (P < 0.05). Error bars represent upper 95% confidence intervals. NS, not significant. *P < 0.01 versus non-Latino Caucasians. **P < 0.001 versus non-Latino Caucasians. ***P < 0.0001 versus non-Latino Caucasians.
The mean maximum decreases in pharmacodynamic parameters after adjusting for drug exposure for virologic responders and nonresponders in each racial/ethnic group are shown in Fig. 5. With the exception of African Americans, a significant difference in pharmacodynamic effects between responders and nonresponders was observed in neutrophil and platelet counts. Although a similar trend was observed in African Americans, the difference was not significant, most likely due to the small sample size of this population. There was no difference between responders and nonresponders in hemoglobin and weight loss.
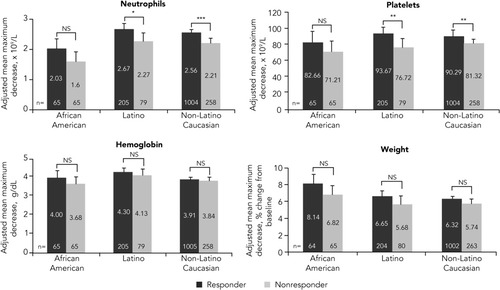
Adjusted mean maximum decrease in pharmacodynamic parameters in virologic responders and nonresponders by race/ethnicity. Error bars represent upper 95% confidence intervals. Least squares means adjusted for cirrhosis and drug exposure (total PEG-IFN and total ribavirin received per kilogram of weight at baseline) are shown if significant (P < 0.05). Patients achieved undetectable HCV RNA levels on treatment. P values were derived from tests comparing responders and nonresponders among racial/ethnic groups. NS, not significant. *P < 0.05. **P < 0.01. ***P < 0.001.
Association Between Maximum Decrease in Hemoglobin Levels and SVR Rates.
Figure 6 shows the predicted percentage of SVR among all patients with a clinically significant hemoglobin level decline >3 g/dL versus a hemoglobin level decline ≤3 g/dL before (Fig. 6A) and after (Fig. 6B) adjusting for total drug exposure. Before adjusting for drug exposure, the rate of SVR was significantly higher among patients who had a decline of >3 g/dL compared with patients who had a decline of ≤3 g/dL (odds ratio, 1.29; P = 0.02). After adjusting for drug exposure, the difference in SVR rates between the two groups was not statistically significant (odds ratio, 0.88; P = 0.30). Similar results were seen when cutoffs of 1, 2, or 4 g/dL decreases in hemoglobin level were used (data not shown).
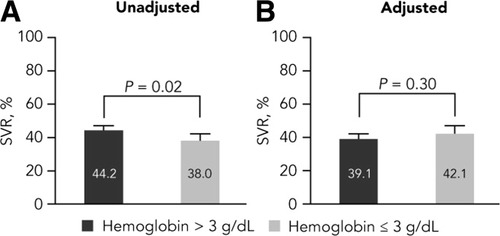
Association between hemoglobin level decline and SVR rates. Predicted percentages of SVR from logistic regression models before and after adjusting for total exposure for PEG-IFN alfa-2a and total ribavirin exposure per kilogram are shown. Cirrhosis was adjusted for in both models. P values were derived from the corresponding tests for odds ratios. Error bars represent upper 95% confidence intervals.
Discussion
Cytopenias and weight loss are commonly observed in patients with chronic hepatitis C treated with PEG-IFN alfa and ribavirin combination therapy. These adverse events are likely reflective of the effect of IFN on the host and specifically on the bone marrow for leukopenia and thrombocytopenia. Anemia is primarily a result of hemolysis caused by ribavirin in addition to a myelosuppressive effect of IFN. These adverse events and their negative effect on quality of life during HCV therapy have been well documented.1-3, 18, 24
Several analyses have demonstrated a relationship between virologic response and the degree of changes in hematologic and weight parameters. In a study of previous HCV nonresponders to prior therapy, patients who achieved at least a 1 log reduction in HCV RNA after 20 weeks of retreatment with PEG-IFN alfa-2a and ribavirin had a greater reduction in body weight, platelets, and white blood cells than null responders (<1 log10 reduction in HCV RNA levels by 20 weeks), possibly reflecting a systemic resistance to IFN in null responders.14 In a another analysis, treatment-naïve HCV genotype 1 patients treated with PEG-IFN alfa-2a and ribavirin who became HCV RNA undetectable at week 12 (i.e., complete early virologic response) experienced greater decreases in hematologic parameters compared with noncomplete early virologic response patients, suggesting that viral response and hematologic changes are pharmacodynamic effects of IFN and ribavirin.15
In this post hoc analysis of treatment-naïve patients infected with HCV genotypes 1, 4, 5, or 6 who were treated with PEG-IFN alfa-2a and ribavirin, four mutually exclusive subgroups of virologic response were compared. Greater declines in neutrophil count, platelet count, hemoglobin level, and weight were demonstrated among patients with responders (SVR, relapse, and breakthrough) compared with nonresponders after adjusting for the presence of cirrhosis, a known predictor of thrombocytopenia and nonresponse.25 However, among responders, no significant differences between patients with SVR, relapse, and breakthrough were observed. This supports the concept that achieving undetectable HCV RNA levels correlates with a similar host pharmacodynamic response to therapy regardless of whether the patient remains HCV RNA undetectable.
Drug exposure and duration of therapy can affect both virologic response and pharmacodynamic effects. Up to a point, greater cumulative exposure results in greater virologic and pharmacodynamic effects. However, treatment discontinuation for nonresponse may lead to less total exposure to therapy in studies with week 12 or week 24 stopping rules. Three of the four studies1, 2, 8 in our analysis included nonresponse stopping rules, whereas the study comparing African American patients with Caucasian patients7 continued treatment for the full duration regardless of on-treatment response. Our analysis comparing virologic and pharmacodynamic responses of therapy among treatment completers accounts for the effect of drug exposure independent of the variable duration of therapy received. These results suggest that neutropenia may be the best surrogate pharmacodynamic marker for virologic response after adjusting for drug exposure and accounting for duration of therapy. In contrast, the sensitivity analysis confirms that thrombocytopenia, anemia, and weight loss were no longer associated with virologic response after adjusting for drug exposure and duration of therapy. These findings suggest that thrombocytopenia, anemia, and weight loss may be largely affected by the extent of drug exposure that is common to all patients rather than to specific differences in host effects.
Clear differences were observed between African Americans and non–African Americans for declines in neutrophil and platelet counts, but not for hemoglobin levels. This is consistent with evidence demonstrating a blunted systemic response to IFN for pharmacodynamic parameters, including virologic response, in African Americans.14 These observed differences indicate that the blunted responses were more attributable to specific host effects. In contrast, there were no differences between the two groups in hemoglobin level decline, suggesting that although host genetic factors could explain some of the observed between-group differences, genetic markers for anemia are likely different from any markers related to viral response or myelosuppression.26, 27 Similarly, the greater degree of weight loss among African Americans versus non–African Americans and anemia among Latino and non-Latino Caucasians may be related to varying genetic profiles between racial/ethnic groups.
Despite the importance of these findings, our study has several limitations. It would have been of interest to know the underlying host predispositions to IFN responsiveness, such as IL28B genotype, which may have explained some of the differences observed between racial/ethnic groups in this analysis.26, 28 Similarly, genetic variants in the host inosine triphosphatase gene (ITPA) were recently found to be strongly associated with anemia in HCV-infected patients receiving ribavirin. Interestingly, it has been shown that variations that predicted inosine triphosphatase deficiency may protect against treatment-induced anemia.27, 29 However, because DNA samples were not collected during the conduct of these trials, we did not perform any analysis of potential genetic contributions to our findings. In addition, the original trials used in this analysis were not designed to evaluate the pharmacodynamic effect of PEG-IFN and ribavirin, hence serum levels of PEG-IFN or ribavirin were not measured. Therefore, low levels of PEG-IFN or ribavirin in the serum may have contributed to the reduction in decline observed in some pharmacodynamic parameters.
In conclusion, this post hoc analysis in patients infected with HCV genotypes 1, 4, 5, or 6 and treated with PEG-IFN alfa-2a and ribavirin shows that maximum decreases from baseline in hematologic parameters and weight loss were associated with virologic response. The blunted effect observed in nonresponders, as well as African Americans, supports the hypothesis that these patients may have systemic resistance to combination therapy and that the lack of IFN response may have a genetic basis. Measuring the pharmacodynamic response while taking into consideration the effect of cumulative drug exposure and treatment duration in patients receiving combination therapy may provide a better understanding of the mechanisms involved in response and resistance to antiviral therapy. The important implication for clinical practice is that, as markers of therapeutic effectiveness, changes in pharmacodynamic parameters may help guide clinical decisions for individualized treatments involving therapy continuation, extension, or discontinuation, as well as for evaluating the effect that novel HCV therapies will have when added to PEG-IFN and ribavirin therapy.
Acknowledgements
We thank Paul MacCallum for third-party writing assistance (furnished by Genentech, Inc.).




