A protective role for CD154 in hepatic steatosis in mice†
Potential conflict of interest: Nothing to report.
Abstract
Inflammation and lipid metabolism pathways are linked, and deregulation of this interface may be critical in hepatic steatosis. The importance of the dialog between inflammatory signaling pathways and the unfolded protein response (UPR) in metabolism has been underlined. Herein, we studied the role of CD154, a key mediator of inflammation, in hepatic steatosis. To this end, Balb/c mice, wild-type or deficient in CD154 (CD154KO), were fed a diet rich in olive oil. In vitro, the effect of CD154 was studied on primary hepatocyte cultures and hepatocyte-derived cell lines. Results showed that CD154KO mice fed a diet rich in olive oil developed hepatic steatosis associated with reduced apolipoprotein B100 (apoB100) expression and decreased secretion of very low-density lipoproteins. This phenotype correlated with an altered UPR as assessed by reduced X-Box binding protein-1 (XBP1) messenger RNA (mRNA) splicing and reduced phosphorylation of eukaryotic initiation factor 2α. Altered UPR signaling in livers of CD154KO mice was confirmed in tunicamycin (TM) challenge experiments. Treatment of primary hepatocyte cultures and hepatocyte-derived cell lines with soluble CD154 increased XBP1 mRNA splicing in cells subjected to either oleic acid (OA) or TM treatment. Moreover, CD154 reduced the inhibition of apoB100 secretion by HepG2 cells grown in the presence of high concentrations of OA, an effect suppressed by XBP1 mRNA silencing and in HepG2 cells expressing a dominant negative form of inositol requiring ER-to-nucleus signaling protein-1. The control of the UPR by CD154 may represent one of the mechanisms involved in the pathophysiology of hepatic steatosis. Conclusion: Our study identifies CD154 as a new mediator of hepatic steatosis. (HEPATOLOGY 2010)
The accumulation of triglycerides (TG) in hepatocytes is a common phenomenon in liver disease. Several mechanisms can account for hepatic steatosis, including increased free fatty acid flux to the liver through diet or peripheral TG lipolysis, defective fat oxidation, increased lipogenesis or decreased very low-density lipoprotein (VLDL) export. These mechanisms have been proposed as causal explanations for hepatic steatosis associated with nonalcoholic fatty liver disease.1-4 The endoplasmic reticulum (ER) is an essential organelle in lipid metabolism. First, VLDL generation depends on a functional ER.5-9 Second, excessive lipid input in hepatocytes, as observed in nonalcoholic fatty liver disease patients, in animal models of fatty livers or in cultured cells is associated with ER stress, though the underlying mechanisms are poorly understood.10-16 In these contexts, ER stress contributes to steatosis through various mechanisms, including increased degradation of apolipoprotein B100 (apoB100) and hepatic lipogenesis through insulin-independent activation of the transcription factor sterol regulatory element binding protein-1c (SREBP-1c).14, 17-20
ER stress signaling pathways, collectively named the unfolded protein response (UPR), regulate ER homeostasis.21-24 The UPR is important for hepatocyte ER adaptation to excessive lipid input in conditions associated with ER stress.21-23 Indeed, the genetic ablation of either branch of the UPR leads to hepatic steatosis in acute ER stress conditions,25, 26 whereas enforced maintenance of ER homeostasis increases apoB100 secretion, prevents SREBP-1c activation, and reduces hepatic steatosis in mice or cell culture models.9, 14, 18-20 Beyond their conventional role in monitoring ER homeostasis in acute ER stress, UPR effectors are activated under physiological conditions and regulate glucose and lipid metabolic pathways, thus contributing to basal cellular homeostasis.27, 28 Importantly, UPR signaling intersects with other signaling cascades, rendering the former amenable to regulation by signals other than directly resulting from ER stress. Among them, inflammatory signals may have a specific importance.27 Inflammation is a key parameter in the progression of hepatic steatosis29-31 and the deregulation of the interface between the UPR and inflammation signaling pathways is likely to be of importance in metabolic disorders.30, 32-34
Here, we study CD154, a member of the tumor necrosis factor (TNF) superfamily, and a critical mediator of inflammation.35 The main reservoir of CD154 in the organism is the blood platelet.36, 37 CD154 expression is inducible by proinflammatory cytokines, and a soluble form (sCD154) retaining biological activity is released from cell surface by a poorly defined mechanism.38 sCD154 levels are increased in the metabolic syndrome.39, 40 Similar to TNF-α receptor activation, CD154 binding to its receptor, CD40, leads to the formation of a trimeric complex which triggers an intricate signaling cascade ending in a pleiotropic range of consequences in inflammation, immunity, or cell survival.35, 38
Here, we tested whether CD154 was involved in the regulation of lipid processing in the liver. We used CD154-deficient (CD154KO) mice and cell culture models. Our results indicate a protective role for CD154 in hepatic steatosis.
Materials and Methods
Mice.
Male Balb/c CD154KO mice were generated from male Bl6/C CD154KO (B6.129S2-CD40lgtm1Imx/J) mice (Jackson Laboratory, Bar Harbor, ME) by repeated (≥10) backcrossings. Mice were housed in a temperature-controlled specific pathogen free environment (transgenic animal housing of Bordeaux 2 University) with a 12-hour light/dark cycle and given free access to food and water. Additional information concerning CD154KO mice is provided in the Supporting Experimental Procedures. The study followed guidelines of and was approved by the animal research ethical committee of Aquitaine Poitou-Charentes. Ten- to 12-week-old mice were used.
Olive Oil Administration and Tunicamycin Injections.
Olive oil (olive oil for human consumption, Puget, France) was administered by way of gavage, 6.6 mL/kg of body weight, three times a week. Tunicamycin (TM) (Merck Darmstadt, FRG) or vehicle was administered as a single intraperitoneal injection at 0.5 mg/kg of body weight.
Primary cell cultures and cell lines, experimental procedures with cultured cells, histology and immunostaining procedures, liver lipid and plasma metabolic parameter measurements, TG production rate study, real-time quantitative reverse-transcription polymerase chain reaction procedures and primers, RNA interference experiments, preparation of liver extracts, immunoprecipitations, immunoblot procedures and antibodies, flow cytometry, enzyme-linked immunosorbent assay, immunoelectron microscopy and statistics are described in the Supporting Experimental Procedures.
Abbreviations
apoB100, apolipoprotein B100; ATF6, activating transcription factor 6; CD154KO, CD154-deficient; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; eIF2α, eukaryotic initiation factor 2α; GRP78, 78-kDa glucose-regulated/binding immunoglobulin protein; IRE1, inositol requiring ER-to-nucleus signaling protein-1; mRNA, messenger RNA; MTTP, microsomal triglyceride transfer protein; OA, oleic acid; PERK, ER membrane protein PKR-like ER kinase; rsCD154, recombinant soluble CD154; sCD154, soluble CD154; siRNA, small interfering RNA; SREBP-1c, sterol regulatory element binding protein-1c; TM, tunicamycin; TG, triglycerides; TNF, tumor necrosis factor; TRAF2, TNF receptor–associated factor 2; UPR, unfolded protein response; VLDL, very low density lipoprotein; WT, wild-type; XBP1, X-Box binding protein-1; XBP1s/u, spliced/unspliced ratio of XBP1.
Results
Hepatic Steatosis in CD154KO Mice.
CD154KO mice fed with an olive oil–rich diet developed a major hepatic steatosis. Differences between wild-type (WT) and CD154KO mice were macroscopically observable and confirmed by microscopic examination. A marked centrolobular steatosis was noted in CD154KO mice, with neither visible lobular inflammation nor signs of hepatocyte damage, as assessed by morphological examination, (Fig. 1A). The absence of hepatocyte damage was also shown by serum liver enzyme measurements (Fig. 1B). Furthermore, there were no signs of apoptosis as assessed by caspase-3 immunostaining (Supporting Fig. 1) and terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (data not shown). Features were evocative of simple steatosis41 and confirmed by oil red O staining of frozen tissue sections (data not shown) and liver TG measurements (Fig. 1C). These results identified CD154 as a novel factor interfering with fat processing in the liver.
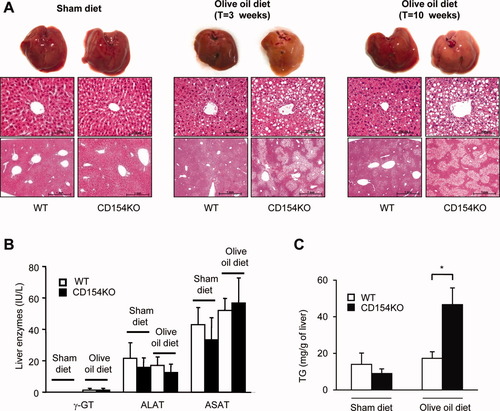
Hepatic steatosis in CD154KO mice. (A) Macroscopic (top) and microscopic (bottom) examination of WT and CD154KO mouse livers. High and low magnifications of hematoxylin-eosin–stained sections of hepatic tissue are shown in the upper and lower rows, respectively, of the bottom panel (n = 8 mice in each group fed for 3 weeks on a sham diet; n = 10 mice in each group fed for 3 weeks on olive oil–rich diet; n = 8 mice in each group fed for 10 weeks on olive oil–rich diet). (B) Serum concentrations of gamma glutamyl transpeptidase (γ-GT), alanine aminotransferase (ALAT), and aspartate aminotransferase (ASAT) in WT and CD154KO mice (n = 8 mice in each group fed for 3 weeks on a sham diet or on an olive oil–rich diet (mean ± SD). (C) TG quantification was performed on liver samples from mice fed for 3 weeks on a sham diet (mean ± SD, n = 3), or olive oil–rich diet (mean ± SD, n = 8; *P < 0.05).
Hepatic VLDL Export Is Impaired in CD154KO Mice.
Plasma TG and TG-containing lipoproteins (VLDL) were decreased in CD154KO mice fed an olive oil–rich diet (Fig. 2A and Supporting Table 1). There was a modest and not significant reduction in WT mice, consistent with the absence of increased plasma VLDL-TG following diets enriched in unsaturated fats.42 We next studied the in vivo plasma TG production rate after VLDL clearance inhibition by Triton WR 1339. As shown in Fig. 2B, CD154KO mice exhibited significantly lower rates of hepatic TG secretion compared with WT mice. These results suggest that the reduced plasma VLDL concentrations in CD154KO mice resulted from an impaired hepatic export of TG-rich lipoproteins.
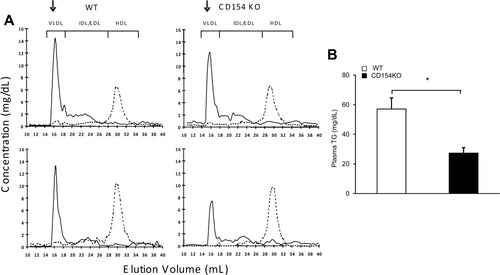
Impaired VLDL hepatic secretion in CD154KO mice. (A) FPLC analysis of plasma lipoproteins in mice. The triglyceride (solid line) and cholesterol (dotted line) distribution in pooled plasma lipoproteins from 4-hour fasted WT and CD154KO mice (n = 7 mice in each group) at time 0 (top panels) and after 3 weeks of an olive oil–rich diet (bottom panels) is shown. The elution positions of VLDL, intermediate-density lipoprotein, low-density lipoprotein, and high-density lipoprotein are indicated. Arrows highlight the VLDL fraction. (B) In vivo TG production rates were estimated after blocking lipase activities and TG-rich lipoprotein clearance with tyloxapol in 4-hour fasted WT and CD154KO mice after 3 weeks of an olive oil–rich diet (n = 5 mice in each group, mean ± SD; *P < 0.05).
apoB100 Expression Is Reduced in CD154KO Mouse Livers Fed an Olive Oil–Rich Diet.
apoB100, a key structural component of VLDL is primarily expressed in the mammalian liver and is essential to VLDL secretion.7, 43 Both WT and CD154KO mice displayed increased liver apoB100 messenger RNA (mRNA) levels when fed an olive oil–rich diet with no significant difference between either mouse strain (Fig. 3A). However, apoB100 protein expression was reduced in CD154KO mice compared with WT mice following the olive oil–rich diet (Fig. 3B). mRNA and protein expression of microsomal triglyceride transfer protein (MTTP), which is required for VLDL assembly and secretion, were not modified for both mouse strains fed the olive oil-rich diet (Fig. 3C,D).
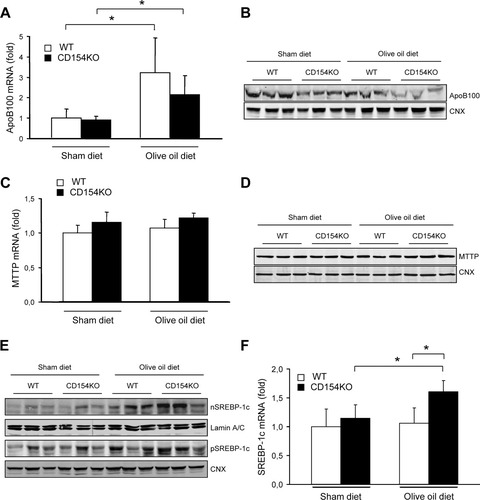
apoB100, MTTP expression, and activation of SREBP-1c in CD154KO mice. (A,C) Fold induction of apoB100 (A) and MTTP (C) mRNAs in WT and CD154KO livers from mice fed for 3 weeks on a sham diet or an olive oil–rich diet (mean ± SD, n = 8 mice in each group; *P < 0.05). Data were normalized to WT mice in sham diet condition. (B,D) apoB100 (B) and MTTP (D) immunoblots from WT and CD154KO liver lysates of mice fed for 3 weeks on a sham diet or an olive oil–rich diet. CNX, calnexin. (E) Immunoblot of the SREBP-1c nuclear form (nSREBP-1c) from nuclear extracts and immunoblot of SREBP-1c precursor form (pSREBP-1c) from microsomal extracts of WT and CD154KO livers from mice fed for 3 weeks on a sham diet or an olive oil–rich diet. (F) Fold induction of SREBP-1c mRNA in WT and CD154KO livers from mice fed for 3 weeks on a sham diet or an olive oil–rich diet (mean ± SD, n = 8 mice in each group; *P < 0.05). Data were normalized to WT mice in sham diet condition. Lamin A/C and calnexin (CNX) were used as loading controls for nuclear and microsomal extract immunoblots, respectively.
Increased Lipogenic Enzyme Gene Expression in CD154KO Mice Fed an Olive Oil–Rich Diet.
Because steatosis may result from alterations in uptake, synthesis, storage, and/or oxidation of fatty acids in liver, we studied genes involved in these pathways (Table 1). There was no modification of the fatty acid transporters FABP1 and SLC27A1 expression. However, the lipogenic genes ACC, FAS, and SCD-1 were significantly up-regulated in CD154KO mouse livers for animals fed an olive oil–rich diet. The expression of these latter genes relies on the activation of transcription factors such as SREBP-1c. SREBP-1c was indeed activated in the livers of CD154KO mice receiving olive oil (Fig. 3E). SREBP-1c mRNA was also up-regulated, most likely through self-activation of its promoter20 (Fig. 3F). The hepatocyte major lipid droplet-associated protein, ADFP, was up-regulated by the fat diet in both mouse strains. Fat oxidation gene expression was not altered in CD154KO mice, as exemplified by the lack of change in expression of the main mitochondrial fatty acid transporter CPT-1A, of the acyl coenzyme A dehydrogenases MCAD and LCAD, and of ACOX-1. Transcription factors PPAR α and ChREBP expression was not significantly modified. Finally, there was no difference in plasma insulin levels between both strains (Supporting Table 1). Treatment of HepG2 cells with CD154 did not directly alter the gene expression of ACC, FAS, and SCD-1 (data not shown). Altogether, results showed that CD154 deficiency was associated with hepatic steatosis, decreased plasma VLDL, and apoB100 expression, and increased expression of lipogenic genes in mice fed an olive oil–rich diet. Lipid homeostasis is dependent on an integrated network of signalizations, in which inflammatory and UPR signaling pathways are critical. Because CD154 stimulates the production of proinflammatory cytokines, its absence may lead to a deregulation of this network. In this study, we examined the UPR.
| Gene | Sham Diet | Olive Oil Diet | ||
|---|---|---|---|---|
| WT (n = 8) | CD154KO (n = 8) | WT (n = 8) | CD154KO (n = 8) | |
| Lipid and lipoprotein synthesis | ||||
| ACC | 1.00 ± 0.25 | 1.22 ± 0.23 | 0.86 ± 0.15 | 1.78 ± 0.28*,† |
| FAS | 1.00 ± 0.53 | 1.58 ± 0.93 | 1.00 ± 0.27 | 2.18 ± 0.55† |
| DGAT1 | 1.00 ± 0.16 | 1.48 ± 0.70 | 0.88 ± 0.16 | 1.12 ± 0.20 |
| DGAT2 | 1.00 ± 0.09 | 0.98 ± 0.18 | 0.90 ± 0.14 | 0.89 ± 0.14 |
| SCD-1 | 1.00 ± 0.76 | 1.13 ± 0.37 | 0.67 ± 0.18 | 2.04 ± 1.23† |
| ApoB100 | 1.00 ± 0.44 | 0.91 ± 0.20 | 3.21 ± 1.72* | 2.15 ± 0.92* |
| MTTP | 1.00 ± 0.11 | 1.16 ± 0.15 | 1.07 ± 0.13 | 1.22 ± 0.07 |
| Fatty acid transporters | ||||
| FABP1 | 1.00 ± 0.19 | 0.90 ± 0.33 | 1.02 ± 0.22 | 0.84 ± 0.20 |
| SLC27A1 | 1.00 ± 0.17 | 1.29 ± 0.18 | 0.87 ± 0.24 | 1.18 ± 0.25 |
| CPT-1a | 1.00 ± 0.25 | 0.80 ± 0.31 | 0.84 ± 0.28 | 1.18 ± 0.35 |
| Perilipin, ADFP, Tip-47 proteins | ||||
| ADFP | 1.00 ± 0.08 | 1.30 ± 0.33 | 2.37 ± 0.13* | 2.79 ± 0.95* |
| Lipolytic enzymes | ||||
| ACOX-1 | 1.00 ± 0.12 | 1.18 ± 0.18 | 1.03 ± 0.14 | 1.18 ± 0.27 |
| LCAD | 1.00 ± 0.04 | 1.35 ± 0.29 | 1.14 ± 0.14 | 1.26 ± 0.33 |
| MCAD | 1.00 ± 0.19 | 1.46 ± 0.47 | 1.03 ± 0.19 | 1.49 ± 0.56 |
| Transcription factors | ||||
| SREBP-1c | 1.00 ± 0.30 | 1.15 ± 0.23 | 1.06 ± 0.26 | 1.60 ± 0.20*,† |
| PPARα | 1.00 ± 0.34 | 0.86 ± 0.44 | 0.71 ± 0.29 | 0.88 ± 0.22 |
| ChREBP | 1.00 ± 0.23 | 0.96 ± 0.17 | 1.21 ± 0.22 | 1.13 ± 0.19 |
- Fold Induction of mRNA expression of target genes in livers from mice fed for 3 weeks on sham diet or olive oil-rich diet (mean ± SD). Data were normalized to results from WT mice in sham diet conditions (ACC, Acetyl-Coenzyme A Carboxylase; FAS, Fatty Acid Synthase; DGAT, Diacylglycerol O-AcylTransferase; SCD-1, Stearoyl-Coenzyme A Desaturase 1; ApoB100, Apolipoprotein B100; MTTP, Microsomal Triglyceride Transfer Protein; FABP1, Fatty Acid Binding Protein 1; SLC27A1, Solute Carrier Family 27 A1; CPT-1a, Carnitine PalmitoylTransferase 1A; ADFP, Adipophilin; ACOX-1, Acyl Coenzyme A Oxydase-1; LCAD, Long Chain Acyl-coenzyme A Dehydrogenase; MCAD, Medium Chain Acyl-coenzyme A Dehydrogenase; SREBP-1c, Sterol Regulatory Element Binding Protein-1c; PPARa, Peroxisome Proliferator Activated Receptor alpha; ChREBP, Carbohydrate Response Element Binding Protein).
- * P < 0.05 versus sham diet.
- † P < 0.05 versus WT.
Altered eIF2α Phosphorylation and XBP1 mRNA Splicing in Mice Fed an Olive Oil–Rich Diet.
The UPR is organized in three signaling pathways triggered through the activation of proximal sensors, inositol requiring ER-to-nucleus signaling protein-1 (IRE1), ER membrane protein PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6).23, 44-47 To monitor the UPR, we studied PERK and IRE1α phosphorylation and ATF6 cleavage, together with the expression of phosphorylated eukaryotic initiation factor 2α (eIF2α) and of alternatively spliced X-Box binding protein-1 (XBP1) mRNA, downstream effectors of activated PERK and IRE1, respectively. A moderate induction of IRE1 phosphorylation was observed in WT mice, whereas no obvious induction was observed in CD154KO mice. PERK phosphorylation was not noticeably induced in either strain. There was a moderate decreased expression of the 90-kDa ATF6 precursor band following the olive oil diet, suggesting cleavage-induced activation, with no differences between either mouse strain (Fig. 4A-C). However, whereas eIF2α phosphorylation and XBP1 mRNA splicing were induced in the WT mice, these inductions were not observed in CD154KO mouse livers (Fig. 4E,F). The expression of 78-kDa glucose-regulated/binding immunoglobulin protein (GRP78) did not vary in either mouse strains when fed the olive oil–rich diet (Fig. 4D). Finally, C/EBP homologous protein (CHOP), a key intermediate in ER stress-mediated apoptosis,48 remained undetectable by immunostaining and immunoblot analysis in both WT and CD154KO livers (data not shown), thus correlating with the absence of morphological and biochemical signs of hepatocyte apoptosis. Taken together, these results show that olive oil induced only a low level of induction of ER stress in the liver. However, CD154KO mice subjected to the olive–oil rich diet showed altered XBP1 mRNA splicing and eIF2α phosphorylation.
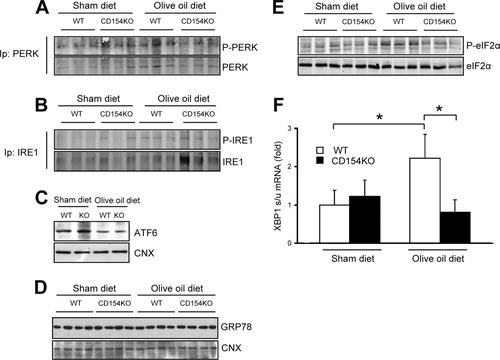
Altered UPR in livers of CD154KO mice given an olive oil–rich diet. Immunoblots of (A) phosphorylated PERK (P-PERK) and total PERK, (B) phosphorylated IRE1 (P-IRE1) and total IRE1, (C) ATF6 (depicting the 90-kDa mouse precursor species), (D) GRP78, and (E) phosphorylated eIF2α (P-eIF2α) and total eIF2α from WT and CD154KO liver lysates from mice fed for 3 weeks on a sham diet or an olive oil–rich diet. CNX, calnexin. (F) Fold induction of the spliced/unspliced ratio of XBP1 (XBP1s/u) mRNA from WT and CD154KO livers from mice fed for 3 weeks on a sham diet or an olive oil–rich diet (mean ± SD, n = 8; *P < 0.05). Data were normalized to results from WT mice in sham diet condition.
Hepatic Steatosis and Altered Unfolded Protein Response in CD154KO Mouse Livers Following TM Treatment.
To test whether such alterations were found in a stronger ER stress induction model, mice were challenged with a single, subtoxic dose of TM, an N-glycosylation inhibitor widely used as an ER stress inducer. TM administration leads to acute ER stress, and, in conditions associated with a defective UPR signaling, to lipid homeostasis disruption and hepatic steatosis.25, 26, 49 As expected,13, 18, 26 TM administration resulted in moderate hepatic steatosis in WT mice. In contrast, a major hepatic steatosis was observed in CD154KO mice (Fig. 5A). There was no detectable apoptosis in WT and CD154KO mouse livers 24 hours after injection as assessed by activated caspase-3 immunostaining (Supporting Fig. 2A) and terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (data not shown), and liver enzyme levels were modestly elevated (Supporting Fig. 2B). Moreover, although CHOP and c-Jun N-terminal kinase inductions at 8 hours were higher in CD154KO mice compared with WT mice, at 24 hours, sustained CHOP expression was not obvious, and c-Jun N-terminal kinase activation was identical in both mouse strains (Fig. 5B and Supporting Fig. 2C). GRP78 expression was increased by TM administration, but no major difference between the strains was observed (Fig. 5C). In WT mice, TM induced PERK and IRE1 phosphorylation, decreased expression of the 90-kDa ATF6 precursor band, suggesting cleavage-induced activation, eIF2α phosphorylation, and XBP1 mRNA splicing. In contrast, in CD154KO livers, PERK and eIF2α phosphorylations as well as XBP1 mRNA splicing were reduced at 24 hours (Fig. 5D,E). Finally, we found an increased lethality in CD154KO mice challenged with TM after 24 hours. This may reflect extrahepatic TM-dependent toxicity, because hepatocyte damage was minimal in these conditions. Taken together, these results show that the main liver phenotype associated with CD154 deficiency in TM-injected mice was hepatic steatosis and suggested compromised eIF2α phosphorylation and XBP1 mRNA splicing. We therefore hypothesized that the CD154 signaling might interfere with the UPR.
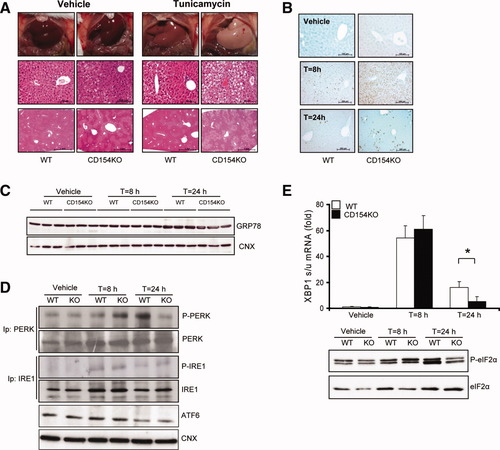
Major hepatic steatosis and altered UPR in livers of CD154KO TM-treated mice. (A) Photographs of representative livers (n = 4 mice in each group) (top) and hematoxylin-eosin–stained liver sections at high (middle) and low (bottom) magnifications of WT and CD154KO mice 24 hours after vehicle or TM injection. (B) CHOP representative immunostaining (n = 3 mice in each group) of liver sections from WT and CD154KO mice 24 hours after vehicle injection and 8 and 24 hours after TM injection. (C,D) Immunoblots of (C) GRP78 from liver lysates of WT and CD154KO mice 24 hours after vehicle injection and 8 and 24 hours after TM injection and (D) phosphorylated PERK (P-PERK) and total PERK, phosphorylated IRE1 (P-IRE1), and total IRE1 and ATF6 from liver lysates of WT and CD154KO mice 24 hours after vehicle injection and 8 and 24 hours after TM injection. Representative of two experiments on pools of three mouse livers. CNX, calnexin. (E) Top: Fold induction of XBP1s/u mRNA from WT and CD154KO mouse livers 24 hours after vehicle injection and 8 (n = 3) and 24 (n = 8) hours after TM injection (mean ± SD; *P < 0.05). Data were normalized to WT mice for vehicle condition. (E) Bottom: Immunoblot (representative of two experiments on pools of three mouse livers) of phosphorylated eIF2α (P-eIF2α) and total eIF2α from liver lysates of WT and CD154KO mice 24 hours after vehicle injection and 8 and 24 hours after TM injection.
CD154 Treatment Increases XBP1 mRNA Splicing in TM- and Oleic Acid–Treated Primary Hepatocyte and Hepatocyte-Derived Cell Line Cultures.
We tested the hypothesis of a connection between CD154 and UPR signaling in cultured cells. CD40 is the canonical CD154 receptor. It was expressed in mouse and human hepatocytes as well as in HepG2, SNU 398, SNU 475, Hep3B, SKHep1 and H2M cells (Supporting Fig. 3A). In mouse livers, electron microscopy confirmed expression of CD40 on hepatocytes and showed expression in Kupffer, hepatic stellate, and endothelial cells (Supporting Fig. 3B). Moreover, CD40 was similarly expressed in CD154KO and WT mouse livers (Supporting Fig. 3C).
In TM-treated HepG2 cells, the UPR was activated, because TM induced a peak of XBP1 mRNA splicing at 12 hours (Fig. 6A), and increased eIF2α phosphorylation (Supporting Fig. 4A). The addition of recombinant soluble CD154 (rsCD154) prolonged XBP1 mRNA splicing (Fig. 6A), an effect that was significantly inhibited by antibody-induced CD40 neutralization (Fig. 6C) or by small interfering RNA (siRNA)-mediated CD40 silencing (Supporting Fig. 5). These results were confirmed in SNU398, SNU475, and SKHEP1 cells (data not shown). The HepG2 cell line was then chosen as an alternative to hepatocytes to investigate the effect of CD154 in HepG2 cells grown in the presence of oleic acid (OA), the major fatty acid in olive oil. Two millimolar OA activated the UPR as a peak of XBP1 mRNA splicing at 4 hours (Fig. 6B), and increased eIF2α phosphorylation (Supporting Fig. 4B) were observed. The addition of rsCD154 resulted in prolonged and amplified splicing of XBP1 mRNA (Fig. 6B), an effect that was suppressed by CD40 neutralization (Fig. 6D) or siRNA-mediated CD40 silencing (Supporting Fig. 5). Thus, in vitro, CD154 increases XBP1 mRNA splicing upon TM or OA treatments, suggesting a regulatory connection between CD154-CD40 signaling and the UPR. Finally, CD154 reduced cell death upon long-term exposure to 2 mM OA, suggesting increased cell adaptation to the OA challenge (Supporting Fig. 6). We then asked whether CD154 could control apoB100 secretion through regulation of XBP1 mRNA splicing.
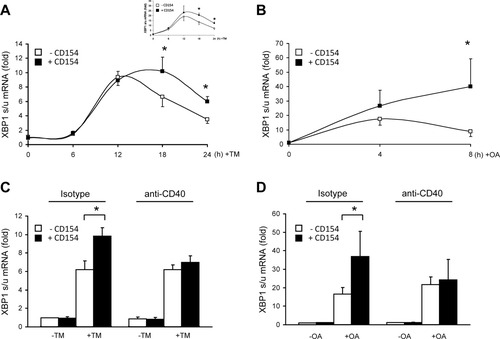
CD154 sustains the splicing of XBP1 mRNA in TM- and oleic acid–induced ER stress in vitro. (A) Fold induction of XBP1s/u mRNA in HepG2 and human hepatocyte (inset) cell lysates following incubation with or without 2.5 μM TM and in the presence or absence of rsCD154 (mean ± SD, n = 5; *P < 0.05). (B) Fold induction of XBP1s/u mRNA in HepG2 cell lysates following incubation with or without 2 mM OA in the presence or absence of rsCD154 (mean ± SD, n = 4; *P < 0.05). (C,D) Fold induction of XBP1s/u mRNA in HepG2 cell lysates following incubation with or without TM for 18 hours (C) or 2 mM OA for 8 hours (D) in the presence or absence of rsCD154 and neutralizing CD40 mAb (anti-CD40) or matched antibody isotype (mean ± SD, n = 3; *P < 0.05). Data were normalized to cells grown in vehicle medium without rsCD154.
CD154 Alleviates the Inhibition of apoB100 Secretion on OA Treatment.
As observed for McA-RH7777 cells,14 high OA concentrations led to an inhibition of apoB100 secretion by HepG2 cells (Supporting Fig. 7). The addition of rsCD154 partially rescued apoB100 secretion, and this was inhibited by the antibody-mediated neutralization of CD40 (Fig. 7A). CD154 treatment did not modify apoB100 mRNA expression and protein secretion in HepG2 cells not exposed to OA (data not shown). Moreover, the effect of CD154 on apoB100 secretion was suppressed in HepG2 cells expressing a dominant negative (DN) form of IRE150 (Fig. 7B) and after siRNA-mediated silencing of XBP1 (Fig. 7C). These results suggested that the IRE1/XBP1 signaling contributed to the CD154-mediated stimulation of apoB100 secretion.
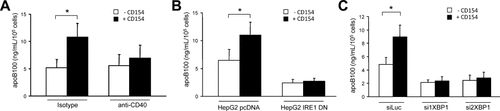
CD154 alleviates the inhibition of apoB100 secretion induced by oleic acid in vitro. (A) apoB100 concentration in the supernatants of HepG2 cells following incubation with 2 mM OA for 8 hours in the presence or absence of rsCD154 and neutralizing CD40 mAb (anti-CD40) or matched antibody isotype (mean ± SD, n = 4; *P < 0.05). (B) apoB100 concentration in the supernatants of HepG2 pcDNA and HepG2 IRE1 DN cells following incubation with 2 mM OA for 8 hours in the presence or absence of rsCD154 (mean ± SD, n = 3; *P < 0.05). (C) apoB100 concentration in the supernatants of HepG2 cells following incubation for 8 hours with 2 mM OA in the presence or absence of rsCD154 and after transfection with luciferase or XBP1 siRNAs (mean ± SD, n = 3; *P < 0.05).
Olive Oil–Rich Diet Increases the Expression of CD154.
A role for CD154 in hepatic steatosis raises the question of its origin in the context of a fat-rich diet. Activated platelets are the primary source of CD154 in the organism.36, 37 Hyperlipidemia has been previously associated with platelet activation and release of sCD154.51, 52 We monitored platelet activation and CD154 expression, both on platelets and in a circulating soluble form, in mice subjected to an olive oil–rich diet or to TM treatment. In both situations, there was increased expression of P-selectin on platelets, suggesting their activation (Supporting Fig. 8A,B). Both circulating sCD154 (Supporting Fig. 8C,D) and platelet-associated CD154 (Supporting Fig. 8E,F) were increased following the olive oil–rich diet and the TM treatment in WT mice. Therefore, the olive oil–rich diet led to platelet activation and to increased circulating sCD154 levels.
Discussion
The natural history of hepatic steatosis results from a complex interplay between metabolic, endocrine, and immune pathways.1, 3, 4, 31, 32, 53 The dialog between inflammatory and metabolic pathways is emerging as being of increasing importance in metabolic diseases. However, mediators involved in these responses remain incompletely defined. The major finding of our study is the identification of CD154 as a new regulatory mediator in the natural history of hepatic steatosis. CD154 deficiency increases the susceptibility of mice to develop hepatic steatosis when fed an olive oil–rich diet. The steatotic phenotype of the CD154KO mice is associated with an impairment of VLDL secretion by the liver and increased expression of lipogenic genes. CD154KO mice do not show signs of hepatocyte damage after 3 weeks of an olive oil–rich diet, making this regimen potentially interesting to study mechanisms leading to simple steatosis, a first step in the progression of nonalcoholic fatty liver disease.54
UPR signaling pathways intersect with lipid metabolic pathways, and impairment of the UPR branches in ER stress conditions is associated with TG accumulation.9, 14, 18-20, 26 Several arguments suggest that one of the mechanisms by which CD154 is protective against steatosis is through regulatory interactions with the UPR. First, we evidenced a defect in UPR signaling in CD154KO mice fed an olive oil–rich diet, as shown by reduced XBP1 mRNA splicing and eIF2α phosphorylation. Such defects were also seen when mice were challenged by the prototypical ER stress inductor, TM. In that case, we could clearly also demonstrate activation of the upstream UPR transducers IRE1 and PERK; this was not the case in olive oil–fed animals, which is likely due to the much lower level of stress in that condition. We cannot, however, exclude that the distinctive decreased eIF2α phosphorylation observed in CD154KO mice may be linked to ER stress–independent regulations, through CD40-connected kinase pathways. Indeed, eIF2α can be the substrate of PERK-independent kinase activation pathways.28, 55 Secondly, we used an in vitro model partly mimicking the in vivo situation, by using HepG2 cells treated with OA, the main component of olive oil, with or without added CD154. High amounts of oleate lead to hepatocyte TG accumulation, linked in part to the ER stress–dependent inhibition of apoB100 secretion.14 In our hands, OA led to UPR activation as shown by increased XBP1 splicing, an effect that was enhanced in the presence of CD154. Importantly, CD154 alleviated the reduction of apoB100 secretion in HepG2 cells grown in the presence of OA, an effect dependent on the activation of the IRE1 pathway, as shown by its abrogation when using HepG2 IRE1 DN or following XBP1 silencing. Our work highlights a possible connection between CD40 and UPR signaling pathways in the liver. Other examples of extracellular signals modulating UPR pathways have been reported. Toll-like receptor signaling interferes with the UPR by regulating the ATF4-CHOP pathway and the insulin-like growth factor-1 also regulates ER stress pathways.56-58 Finally, XBP1 mRNA splicing in spleen B cells during plasma cell differentiation is induced by antibody-mediated CD40 activation.59, 60 The regulation of the UPR by extracellular signals may represent a mechanism by which the environment controls cell adaptation to stress. How the IRE1 and the CD40 signaling pathways interact remains an open question. The recruitment of TNF receptor–associated factor 2 (TRAF2) mediates the proinflammatory consequences of CD154/CD40 interaction.61, 62 As IRE1 recruits TRAF2 upon activation, TRAF2 may represent a potential link between the CD40 and IRE1 signalization pathways.
Our study does not exclude other mechanisms through which CD154 may interfere with the progression of liver steatosis. These may involve deregulation of the cytokine network. Indeed, CD154 induces inflammatory cytokines, some of which play a role in lipid metabolism, such as IL-6. IL-6 alleviates liver steatosis63 and IL-6−/− mice develop mature-onset obesity and are prone to hepatic steatosis and metabolic alterations.64, 65 According to the regulatory role of CD154 on IL-6 expression, we found that CD154KO mice showed impaired induction of IL-6 following the olive oil–rich diet as shown by a reduced induction of plasma IL-6 levels and liver IL-6 mRNA (Supporting Fig. 9A,B). Hence, the down-regulation of IL-6 expression may provide another mechanism to explain the steatotic phenotype of olive oil–fed CD154KO mice. ER stress also leads to IL-6 production through XBP-1 signaling59, 66 and, accordingly, in HepG2 cells expressing a dominant negative form of IRE1, TM-induced expression of IL-6 was impaired. In this context, the CD154-dependent IL-6 induction was preserved (Supporting Fig. 9A,B). Therefore, the control of IL-6 expression is likely to represent another interface linking CD154, the UPR, and hepatic lipid metabolism. This observation suggests that several integrated signaling pathways are likely to account for the contribution of CD154 in hepatic steatosis.
In conclusion, our study shows that CD154 is a mediator involved in the natural history of hepatic steatosis. CD154 appears as a new link between lipid metabolism and inflammation in the liver, supporting the idea of interdependency between inflammation and metabolic disorders.27, 32
Acknowledgements
The authors thank Chantal Combe, Jérôme Gabet, Alexandra Nicou, and Antonio Palos Pinto for technical help.




