Fructose at the center of necroinflammation and fibrosis in nonalcoholic steatohepatitis1
Potential conflict of interest: Nothing to report.
We read with great interest a recent article in Hepatology by Kohli et al.1 on the role of fructose and transfats in mimicking nonalcoholic steatohepatitis (NASH)–associated liver damage.
The mechanisms leading to NASH are likely to be multiple and involve a baseline steatosis that may cause the second hits. Liver steatosis is closely linked to westernized dies, characterized by the consumption of high-fructose corn syrup (a common sweetener used in the food industry) and/or the consumption of certain types of lipids, whereas second hits may be represented by oxidative stressors and proinflammatory cytokines.2
Kohli and colleagues1 found that the free consumption of high-fructose water in combination with a hypercaloric diet with medium-chain transfatty acids caused steatosis, necroinflammation, and fibrosis in C57Bl/6 male mice within 16 weeks. This study fashionably suggested that the excess of fructose combined with the excess of fats was able to induce significant increases in several markers of oxidative stress, including intrahepatic superoxide expression, 4-hydroxynonenal, and plasma levels of the respiratory chain component oxidized coenzyme Q9. The authors highlighted that these oxidative stress parameters (particularly oxidized coenzyme Q9) correlated with fibrogenesis in mice fed a high-fat/high-fructose diet. Furthermore, the group that was fed the combination of transfats and high fructose and developed fibrosis also had necroinflammation, which was indicated by the increased levels of intrahepatic inflammatory cells.
In contrast to these findings, Tetri et al.3 found that transfats played a major role in promoting liver steatosis and injury, including necroinflammation and fibrosis, whereas the addition of a high-fructose corn syrup equivalent induced major food consumption with resultant obesity and impaired insulin sensitivity.
We recently examined Sprague-Dawley rats that were fed a standard diet, a high-fat diet, or a high-fructose/high-fat diet for 14 weeks. Even though our data are still preliminary, on the basis of the data presented by Kohli et al.,1 we suggest that the excess of fat alone may contribute to the development of mild steatosis, whereas the addition of elevated fructose levels could contribute to the development of hepatic oxidative stress, which would predispose rats to necroinflammation and fibrogenesis (Fig. 1). In particular, we found that excessive fructose intake in combination with a high-fat diet (i.e., the high-fructose/high-fat diet) caused greater liver damage than the high-fat diet alone, as indicated by increased intrahepatic collagen VI staining (Fig. 1F), augmented CD163-positive Kupffer cell activation (Fig. 1I), and increased free and protein-bound oxidized glutathione (Fig. 1J).
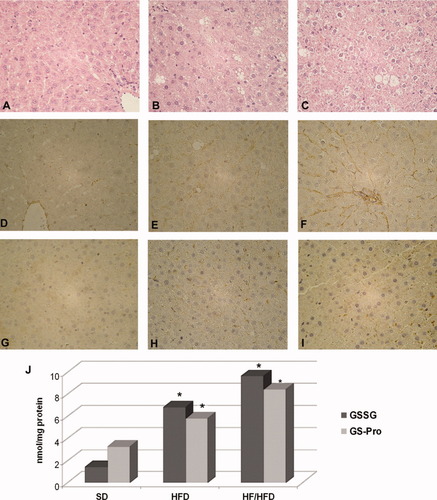
Liver damage in Sprague-Dawley rats after different diet regimens. (A-C) Hematoxylin and eosin, (D-F) collagen VI, and (G-I) CD163 staining of the livers of rats fed a standard diet (SD), a high-fat diet (HFD), or a high-fructose/high-fat diet (HF/HFD). (J) Intrahepatic levels of free oxidized glutathione (GSSG) and protein-bound oxidized glutathione (GS-Pro) by high-performance liquid chromatography.
Several rodent models have been used to study the pathogenesis of NASH, but often they may not reflect the real metabolic context of developing human disease and may fail to reproduce the whole spectrum of liver pathology that characterizes human nonalcoholic fatty liver disease.4 Kohli et al.1 has newly reinforced that, because of the multifactorial etiology of this disease, models of chronic overnutrition (especially fructose-enriched diets) with spontaneous progression of steatosis to steatohepatitis may be the most valid and practical means for understanding the pathophysiology of human NASH and associated fibrosis. In fact, a recent work has demonstrated that daily fructose ingestion is associated with reduced hepatic steatosis and increased fibrosis in patients with nonalcoholic fatty liver disease.5
All these findings should discourage studies on animal models of NASH that omit sugar use. Moreover, although we believe that the exact role of sugars (particularly fructose) in human NASH pathogenesis needs further investigation, the study by Kohli et al.1 provides a new model for testing the ability of potential pharmaceuticals agents (i.e., antioxidants) to counteract progressive liver scarring and damage.
References
Anna Alisi Ph.D.*, Melania Manco M.D., Ph.D.*, Marco Pezzullo Ph.D. , Valerio Nobili M.D.*, * Unit of Metabolic and Autoimmune Liver Diseases, Bambino Gesù Children's Hospital and Research Institute, Rome, Italy, Core Facilities, Bambino Gesù Children's Hospital and Research Institute, Rome, Italy.




