Lipid rafts are essential for peroxisome biogenesis in HepG2 cells†
Potential conflict of interest: Nothing to report.
Abstract
Peroxisomes are particularly abundant in the liver and are involved in bile salt synthesis and fatty acid metabolism. Peroxisomal membrane proteins (PMPs) are required for peroxisome biogenesis [e.g., the interacting peroxisomal biogenesis factors Pex13p and Pex14p] and its metabolic function [e.g., the adenosine triphosphate–binding cassette transporters adrenoleukodystrophy protein (ALDP) and PMP70]. Impaired function of PMPs is the underlying cause of Zellweger syndrome and X-linked adrenoleukodystrophy. Here we studied for the first time the putative association of PMPs with cholesterol-enriched lipid rafts and their function in peroxisome biogenesis. Lipid rafts were isolated from Triton X-100–lysed or Lubrol WX–lysed HepG2 cells and analyzed for the presence of various PMPs by western blotting. Lovastatin and methyl-β-cyclodextrin were used to deplete cholesterol and disrupt lipid rafts in HepG2 cells, and this was followed by immunofluorescence microscopy to determine the subcellular location of catalase and PMPs. Cycloheximide was used to inhibit protein synthesis. Green fluorescent protein–tagged fragments of PMP70 and ALDP were analyzed for their lipid raft association. PMP70 and Pex14p were associated with Triton X-100–resistant rafts, ALDP was associated with Lubrol WX–resistant rafts, and Pex13p was not lipid raft–associated in HepG2 cells. The minimal peroxisomal targeting signals in ALDP and PMP70 were not sufficient for lipid raft association. Cholesterol depletion led to dissociation of PMPs from lipid rafts and impaired sorting of newly synthesized catalase and ALDP but not Pex14p and PMP70. Repletion of cholesterol to these cells efficiently reestablished the peroxisomal sorting of catalase but not ALDP. Conclusion: Human PMPs are differentially associated with lipid rafts independently of the protein homology and/or their functional interaction. Cholesterol is required for peroxisomal lipid raft assembly and peroxisome biogenesis. HEPATOLOGY 2010
Peroxisomes are single membrane-bound organelles that are especially abundant in the human liver. They are involved in a wide range of metabolic processes, including β-oxidation of fatty acids, bile acid biosynthesis, plasmalogen biosynthesis, and the removal of reactive oxygen species that are generated as a result of the high peroxisomal metabolic activity.1, 2 Malfunctioning of peroxisomes is associated with life-threatening diseases such as Zellweger syndrome (ZS) and X-linked adrenoleukodystrophy (X-ALD). ZS is caused by mutations in peroxisomal biogenesis factor (PEX) genes and is characterized by impaired bile salt synthesis leading to the accumulation of bile salt intermediates.3 PEX genes encode proteins called peroxins, which are required for normal biogenesis of peroxisomes. Sixteen different human peroxins have been identified to date. Most of these proteins are associated with the peroxisomal membrane and function in the import of peroxisomal matrix proteins, such as catalase and bile acid–coenzyme A:amino acid N-acyltransferase. Pex13p and Pex14p (Fig. 1A) are crucial components for the import of peroxisomal matrix proteins. They physically interact with each other, suggesting that they act together in the process of peroxisomal matrix protein import.4, 5
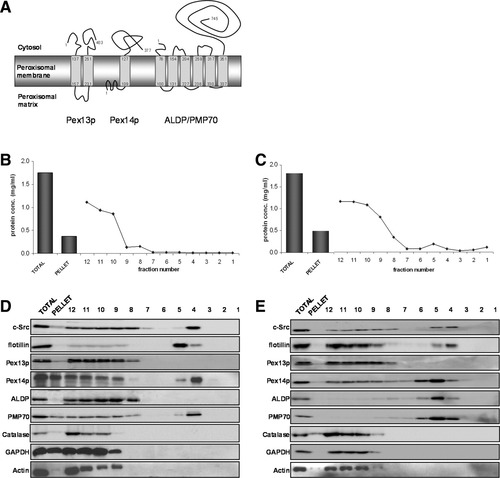
Membrane topology of the human PMPs analyzed in this study and their association with Triton X-100–resistant and Lubrol WX–resistant lipid rafts in HepG2 cells. (A) Schematic representation of the most accepted models for the membrane topology of human Pex13p, Pex14p, ALDP, and PMP70. Numbers indicate amino acid positions. ALDP and PMP70 are highly homologous proteins with comparable membrane topology. The amino acid positions for ALDP are indicated. Human HepG2 cells were lysed in the presence of (B,D) 1% Triton X-100 or (C,D) 1% Lubrol WX, and this was followed by flotation gradient centrifugation. One-milliliter fractions (12 in all) were collected from the top and analyzed (B,C) for the protein concentration and (D,E) for western blot analysis. (D,E) Equal volumes from the gradient fractions were analyzed with specific antibodies against lipid raft markers c-Src and flotillin, non–lipid raft markers β-actin and GAPDH, peroxisomal matrix protein catalase, and peroxisomal membrane proteins Pex13p, Pex14p, ALDP, and PMP70. Equal volumes of the unfractionated total protein lysate and the pellet fraction after flotation gradient centrifugation were also analyzed. Quantification of three independent extraction experiments is shown in Supporting Fig. 1.
X-ALD is caused by mutations in the ABCD1 gene encoding the adrenoleukodystrophy protein (ALDP). X-ALD is characterized by elevated levels of very long chain fatty acids (VLCFAs) in body fluids and reduced VLCFA β-oxidation in peroxisomes.6 ALDP is an ATP-binding cassette (ABC-) transporter that, together with highly homologous ALDRP/ABCD2, 70-kDa peroxisomal membrane protein (PMP70)/ABCD3, and PMP70R/ABCD4 (PMP69), forms the ABCD subfamily.2 ALDP expression is detected in most tissues with moderate levels in the liver. PMP70 is highly expressed in the liver.6 ALDP and PMP70 are thought to be involved in the ATP-dependent transport of VLCFAs and long-chain fatty acids across the peroxisomal membrane, respectively. The peroxisomal ABC transporters are so-called half-transporters. They contain six clustered membrane spanning domains and one ATP-binding domain (Fig. 1A). Dimerization leads to the formation of functional substrate pumps resembling the prototypical ABC transporter, P-glycoprotein/multidrug resistance 1 protein. Our knowledge about the functions of peroxins and peroxisomal substrate transporters is steadily increasing, but remarkably little is known about the embedding of these PMPs in the peroxisomal membrane and their association with specific lipids.
Research on protein-lipid interactions has been especially focused on their coexistence in detergent-resistant lipid microdomains, which are routinely called lipid rafts.7, 8 Plasma membrane lipid rafts are enriched in cholesterol and (glyco)sphingolipids as well as specific membrane proteins. Biochemically, lipid rafts are characterized by their insolubility in nonionic detergents (typically Triton X-100) at low temperatures and their subsequent buoyancy in sucrose flotation gradients. They function in protein trafficking, signal transduction, organization of the cytoskeleton, and pathogen internalization.9-11 Moreover, lipid raft association may directly regulate the activity of substrate transporters, including that of ABC-transporters.12-14
In this study, we analyzed the association of the PMPs Pex13p, Pex14p, ALDP, and PMP70 with peroxisomal lipid rafts. Our data reveal that different types of lipid rafts exist in the peroxisomal membrane and that impaired lipid raft assembly leads to peroxisome biogenesis defects.
Abbreviations:
ABC, adenosine triphosphate–binding cassette; ALDP, adrenoleukodystrophy protein; ATP, adenosine triphosphate; DMSO, dimethyl sulfoxide; ECR, ergosterol/ceramide-rich; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; L, lovastatin; m-β-CD, methyl-β-cyclodextrin; mPTS, membrane peroxisomal targeting signal; PEX, peroxisomal biogenesis factor; PMP, peroxisomal membrane protein; TMD, transmembrane domain; VLCFA, very long chain fatty acid; X-ALD, X-linked adrenoleukodystrophy; YFP, yellow fluorescent protein; ZS, Zellweger syndrome.
Materials and Methods
Pre-established cell lines and plasmids15-17 as well as standard culture conditions and procedures for transient transfection, RNA isolation, quantitative polymerase chain reaction,18 sodium dodecyl sulfate–polyacrylamide gel electrophoresis, western blotting19 and immunofluorescence microscopy19 are described in the supporting information.
Isolation of Detergent-Resistant Lipid Microdomains (Lipid Rafts).
Lipid rafts were isolated from 1 × 107 HepG2 cells as described by Slimane et al.20 with minor modifications. Cells were washed two times in ice-cold Hank's balanced salt solution (Invitrogen BV, Breda, The Netherlands) and lysed in 2.5 mL of ice-cold TNE buffer (20 mM trishydroxymethylaminomethane–hydrochloric acid, pH 7.4, 150 mM sodium chloride, 1 mM ethylene diamine tetraacetic acid), containing 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) or 1% Lubrol WX (Lubrol 17A17, Serva, Heidelberg, Germany) in the presence of Complete protease inhibitors (Roche, Basel, Switzerland) and 25 U/μL Benzonase (EMD Biosciences, Inc., La Jolla, CA). The cell lysate was passed 10 times through a 22-gauge needle and incubated on ice for 30 minutes. The lysate was mixed 1:1 with 80% (wt/vol) sucrose in TNE buffer, and 4 mL was transferred to the bottom of a 12-mL centrifuge tube and overlaid with 4 mL of 35% (wt/vol) sucrose and 4 mL of 5% (wt/vol) sucrose in TNE buffer. The remainder of the lysate in 40% (wt/vol) sucrose was used as the total protein extract. The gradients were centrifuged for 20 hours at 36,000 rpm and 4°C in a Beckman SW 41 rotor. Fractions (1 mL) were harvested from the top of the gradient. The pellet was resuspended in 1 mL of 35% (wt/vol) sucrose in TNE buffer and saved as the pellet fraction.
Cholesterol Depletion and Repletion of HepG2 Cells and Use of Cycloheximide.
In order to deplete cholesterol from cellular membranes in vitro, the previously described Lubrol WX extraction protocol was performed in the presence of 10 mM methyl-β-cyclodextrin (m-β-CD; Sigma-Aldrich), and this was followed by flotation gradient centrifugation.
Growing HepG2 cells were depleted from cholesterol (in vivo depletion) by incubation for 24 hours at 37°C in Dulbecco's modified Eagle's medium (without fetal bovine serum) in the presence of 10 μM lovastatin (Sigma-Aldrich) and/or 2 mM m-β-CD. In selected experiments, HepG2 cells were cotreated with 0.02 mg/mL cycloheximide to inhibit protein synthesis. In order to restore cellular cholesterol levels, 24-hour lovastatin/m-β-CD–treated cells were subsequently incubated with preformed m-β-CD/cholesterol complexes (400 μg/mL cholesterol) in serum-free Dulbecco's modified Eagle's medium, essentially as described before.20, 21
Cholesterol Isolation and Concentration Measurements.
Cholesterol was isolated from total cell extracts with the Bligh-Dyer method.22 Cholesterol concentrations were determined spectrophotometrically by a cholesterol oxidase/peroxidase assay23 and normalized against protein concentrations.
Results
Pex13p, Pex14p, PMP70, and ALDP Are Differentially Associated with Lipid Rafts.
To determine whether PMPs are associated with detergent-resistant lipid microdomains/lipid rafts, we performed the standard lipid raft extraction protocol on liver-derived human HepG2 cells with Triton X-100 or Lubrol WX, followed by flotation gradient centrifugation. Figure 1D shows that cytosolic proteins [glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and actin] and the peroxisomal matrix protein catalase were solely detected in the bottom fractions 8 to 12 of the gradient after Triton X-100 extraction, whereas significant amounts of lipid raft markers (c-Src and flotillin) were detected in fractions 4 and 5. PMP70 and Pex14p showed a gradient distribution similar to that of c-Src, suggesting that these proteins are at least partly associated with lipid rafts (quantification shown in Supporting Fig. 1A). In contrast, ALDP and Pex13p were detected only in the bottom (solubilized protein) fractions of the gradient. To detect a less stringent association with lipid rafts, HepG2 cells were also extracted with Lubrol WX (Fig. 1C,E). The distribution of marker proteins (GAPDH, actin, catalase, flotillin, and c-Src) showed that the cellular membranes were efficiently disrupted by Lubrol WX and that lipid rafts floated to fractions 4 to 6. The total protein concentrations were slightly higher in fractions 4 to 6 after Lubrol WX extraction (Fig. 1C) versus Triton X-100 (Fig. 1B), confirming the lower stringency of the detergent. As expected, PMP70 and Pex14p were predominantly present in fractions 4 to 6 after Lubrol WX extraction (Fig. 1E). In addition, peak fractions of ALDP were also observed in these fractions, whereas Pex13p was detected solely in the bottom gradient fractions (Fig. 1E and Supporting Fig. 1B).
These data show that PMPs have differential extractability by Triton X-100 and Lubrol WX, implying a different environment for these proteins in the peroxisomal lipid bilayer.
PMPs Dissociate from Lubrol WX Lipid Rafts After Cholesterol Depletion of HepG2 Membranes In Vitro.
Proteins dissociate from lipid rafts when the membrane cholesterol content is reduced.24 To analyze the cholesterol requirement for PMP lipid raft association, HepG2 cells were extracted with Lubrol WX in the presence of 10 mM m-β-CD, followed by flotation gradient centrifugation. Figure 2 shows that this resulted in a shift of peak fractions of c-Src and all lipid raft–associated PMPs from fractions 4 to 6 to the lower fractions of the gradient. Notably, c-Src was detected in fractions 9 to 12 after m-β-CD treatment, whereas peak levels of ALDP, PMP70, and Pex14p were detected in fractions 8 and 9 at the interface of the 40% and 35% sucrose layers in the gradient. This suggests that the PMPs remained associated with lipids, but with altered flotation behavior in comparison with cholesterol-containing lipid rafts.
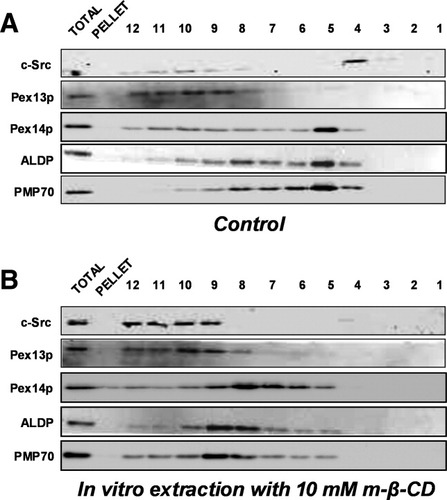
Human PMPs dissociate from Lubrol WX–resistant lipid rafts after in vitro cholesterol depletion of HepG2 membranes. Human HepG2 cells were lysed in the presence of 1% Lubrol WX in (A) the absence and (B) presence of 10 mM m-β-CD, followed by flotation gradient centrifugation. Fractions collected from these gradients were analyzed as described in the legend of Fig. 1.
PMPs Dissociate from Lubrol WX Lipid Rafts After Cholesterol Depletion of HepG2 Membranes In Vivo.
Next, we aimed to analyze the effect of peroxisomal lipid raft depletion in growing HepG2 cells. Standard protocols that depleted the plasma membrane from cholesterol did not disrupt the raft association of PMPs in HepG2 cells (Supporting Fig. 2). Therefore, we optimized the conditions to obtain sufficient depletion of total cellular cholesterol, including that at intracellular sites. Cotreatment of HepG2 cells with lovastatin (10 μM; cholesterol synthesis inhibitor) and m-β-CD (2 mM) led to a greater than 90% reduction of cellular cholesterol levels (Fig. 3A), which was previously shown to be required to disrupt lipid rafts.25 The larger portion of these cells remained viable as indicated by unchanged transcript and protein expression levels of peroxisomal marker proteins (Fig. 3B,C), no induction of apoptotic markers (caspase-3 activity; Supporting Fig. 3A), minor amounts of necrosis (lactate dehydrogenase leakage; Supporting Fig. 3B), and an active endocytosis pathway (Supporting Fig. 3F-H).
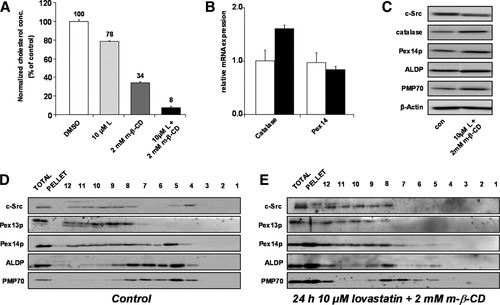
PMPs dissociate from Lubrol WX–resistant lipid rafts after in vivo cholesterol depletion of HepG2 membranes. Human HepG2 cells were cultured for 24 hours in the presence of 10 μM L, 2 mM m-β-CD, or a combination of these two compounds. Treatment with 0.1% DMSO (a solvent for m-β-CD) served as a control. (A) The total cellular cholesterol of the treated cells was determined. Levels of (B) selected messenger RNAs and (C) proteins were determined in HepG2 cells treated with a combination of 10 μM L and 2 mM m-β-CD. (D) Control and (E) L/m-β-CD–treated cells were lysed in the presence of 1% Lubrol WX, followed by flotation gradient centrifugation; gradient fractions were analyzed as described in the legend of Fig. 1. Abbreviations: DMSO, dimethyl sulfoxide; L, lovastatin.
Lubrol WX extraction of lovastatin/m-β-CD–treated HepG2 cells revealed that c-Src, PMP70, ALDP, and Pex14p were largely absent from the lipid raft-containing fractions 4 and 5 (Fig. 3E; cf. control cells in Fig. 3D). Pex14p and c-Src appeared in fractions 8 to 12, and this indicated that they were solubilized upon Lubrol WX extraction. In contrast, peak levels of PMP70 and ALDP were detected in fractions 7 and 8 at the 35% to 40% sucrose interface; this was similar to the in vitro extraction experiments (Fig. 2). In addition, significant amounts of PMP70, ALDP, and Pex14p were detected in the pellet fraction, which may have resulted from aggregation of these PMPs in cholesterol-depleted HepG2 cells either before or during the Lubrol WX extraction procedure.
Effect of Cellular Cholesterol Depletion on Sorting of PMPs and Catalase.
To determine whether cellular cholesterol depletion affects peroxisome biogenesis, we analyzed the subcellular location of PMP70, ALDP, Pex14p, and the peroxisomal matrix enzyme catalase in lovastatin/m-β-CD–treated HepG2 cells by immunofluorescence microscopy (Figs. 4 and 5 and Supporting Fig. 4). A peroxisomal location for all these proteins was detected in untreated HepG2 cells (panels A-C in Figs. 4 and 5 and Supporting Fig. 4). The 24-hour lovastatin/m-β-CD treatment led to the appearance of a diffuse subcellular location of catalase and ALDP, whereas Pex14p and PMP70 remained localized in peroxisomes (panels D-F in Figs. 4 and 5 and Supporting Fig. 4). The extent of cytosolic staining for catalase was somewhat variable (cf. Figs. 4E and 6E and Supporting Figs. 3F and 4E). The peroxisomal volume in HepG2 cells is approximately 1% of the total cellular volume. As a result, catalase is diluted 100-fold in the cytoplasm in comparison with its peroxisomal location. Any (slight) staining of catalase outside peroxisomes therefore indicated significant missorting of the protein in (cholesterol-depleted) HepG2 cells. Subsequently, the cholesterol-depleted HepG2 cells were resupplemented with cholesterol and analyzed by immunofluorescence microscopy again after 24 hours (panels G-I in Figs. 4 and 5 and Supporting Fig. 4). Catalase staining reappeared in peroxisomes of cholesterol-repleted HepG2 cells and showed clear colocalization with Pex14p (Fig. 4G-I). ALDP staining appeared more heterogeneous after cholesterol repletion. Some dotted colocalization of ALDP with PMP70 and catalase was detected, but mostly a diffuse staining for ALDP was observed (panels G-I in Figs. 5 and Supporting Fig. 4, respectively). Importantly, peroxisomal catalase was detected in cells with diffuse ALDP staining, confirming the recovery of these cells after cholesterol depletion (Supporting Fig. 4, lower panels). To analyze whether the abnormal cellular location of catalase and ALDP in cholesterol-depleted cells is a result of mistargeting of newly synthesized protein or is caused by dissociation of the existing protein from damaged peroxisomes, we performed the lovastatin/m-β-CD treatment in the absence and presence of the protein synthesis inhibitor cycloheximide (Fig. 6). While a diffuse staining for catalase and ALDP became readily detectable in the absence of cycloheximide (Fig. 6E,F), a predominant peroxisomal staining was detected in lovastatin/m-β-CD/cycloheximide–cotreated HepG2 cells (Fig. 6I,J). These data show that cholesterol depletion leads to impaired sorting of newly synthesized catalase and ALDP.
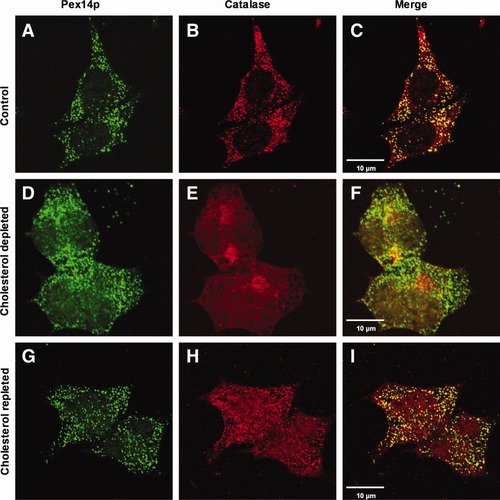
Effect of cholesterol depletion and repletion on the subcellular location of Pex14p and catalase. Human HepG2 cells were cultured for 24 hours in (A-C) the absence or (D-F) presence of 10 μM lovastatin and 2 mM m-β-CD, followed by immunofluorescence microscopy to determine the subcellular location of (A,D) Pex14p and (B,E) catalase (merged images in panels C and F). Cholesterol-depleted HepG2 cells were subsequently cultured for 24 hours in the presence of exogenously added cholesterol (G-I) and the subcellular location of (G) Pex14p and (H) catalase was determined (merged images in panel I).
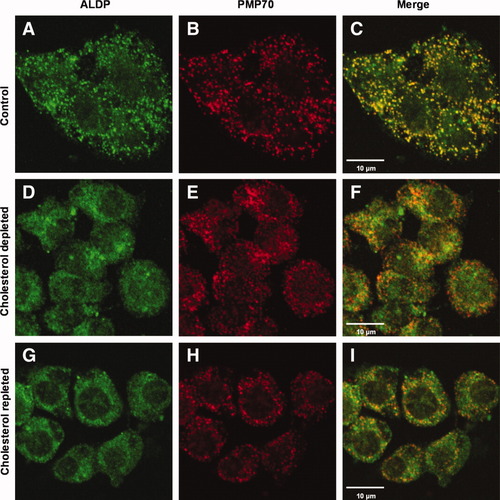
Effect of cholesterol depletion and repletion on the subcellular location of ALDP and PMP70. Human HepG2 cells were cultured for 24 hours in (A-C) the absence or (D-F) presence of 10 μM lovastatin and 2 mM m-β-CD, and this was followed by immunofluorescence microscopy to determine the subcellular location of (A,D) ALDP and (B,E) PMP70 (merged images in panels C and F). Cholesterol-depleted HepG2 cells were subsequently cultured for 24 hours in the presence of exogenously added cholesterol (G-I) and the subcellular location of (G) ALDP and (H) PMP70 was determined (merged images in panel I).
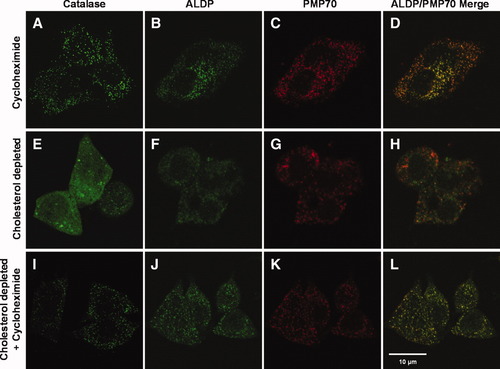
Impaired sorting of newly synthesized catalase and ALDP in cholesterol-depleted HepG2 cells. Human HepG2 cells were cultured for 24 hours in (A-D) the absence or (E-L) presence of 10 μM lovastatin and 2 mM m-β-CD. Protein synthesis was inhibited during the 24-hour period by the addition of cycloheximide to (A-D) control cells or (I-L) lovastatin/m-β-CD–treated cells. Immunofluorescence microscopy was used to determine the subcellular location of (A,E,I) catalase, (B,F,J) ALDP, and (C,G,K) PMP70 (merged images of ALDP and PMP70 in panels D, H, and L).
Protein Sequences That Define the Lipid Raft Association of PMP70 and ALDP.
Finally, we determined whether the sequences in PMP70 and ALDP that are required for sorting to the peroxisomal membrane [membrane peroxisomal targeting signal (mPTS)] are also sufficient for lipid raft association. Previously, it was shown that the N-terminal regions containing the first two transmembrane domains (TMDs) of PMP70 (aa1-144) or ALDP (aa1-110 and aa67-164) are sufficient for peroxisomal targeting.16, 17 We transiently expressed these and various other fragments of PMP70 and ALDP fused to green fluorescent protein (GFP) or yellow fluorescent protein (YFP) in HepG2 cells, confirmed their peroxisomal targeting in HepG2 cells (data not shown), and performed Lubrol WX extraction to analyze their lipid raft association (Fig. 7). GFP/YFP-tagged full-length PMP70 and ALDP were detected in gradient fractions 4 and 5, indicating that these hybrid proteins at least partly associate with lipid rafts. In contrast, hybrid proteins consisting of GFP and the minimal mPTS-containing regions of PMP70 (aa1-144) or ALDP (aa1-110, aa1-164, and aa67-164) were not detected in the lipid raft–containing fractions 4 and 5. The N-terminal fragments containing the first four TMDs of ALDP (aa1-281) or the six TMDs of PMP70 (aa1-375) were detected in fractions 4 and 5, indicating that sequences outside the minimal mPTS of PMP70 and ALDP are required for lipid raft association. For the PMP70-derived hybrid proteins, similar results were obtained after Triton X-100 extraction (data not shown).
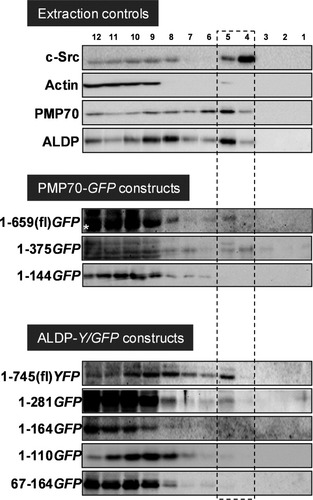
The peroxisomal targeting signals of PMP70 and ALDP do not drive lipid raft association. HepG2 cell were transiently transfected with the indicated YFP/GFP-tagged fragments of PMP70 and ALDP. Forty-eight hours after transfection, cells were lysed in the presence of 1% Lubrol WX, followed by flotation gradient centrifugation. Gradient fractions were analyzed by western blotting as detailed in Fig. 1 with antibodies against GFP and extraction marker proteins c-Src, actin, PMP70, and ALDP. The white asterisk indicates the presence of a nonspecific protein cross-reacting with the α-GFP antibody in fractions 9 to 12.
Discussion
In this study, we have shown that human peroxisomal membrane proteins are differentially associated with lipid rafts and that sequences other than the mPTS are required for lipid raft association. Cholesterol depletion leads to missorting of catalase and ALDP but not of Pex14p and PMP70. Peroxisomal sorting of catalase is effectively restored upon cholesterol repletion. Thus, cholesterol is required for the assembly of peroxisomal lipid rafts and for efficient peroxisome biogenesis.
Lipid rafts have been detected in plasma membranes and various organellar membranes, but this is the first report showing that they also exist in mammalian peroxisomal membranes. A clear hierarchy appears to exist in the lipid raft association of PMPs. The ABC-transporter PMP70 and the peroxin Pex14p show the strongest lipid raft association of the PMPs studied here. In contrast, the PMP70 homologue, ALDP, is effectively extracted by Triton X-100 but resists Lubrol WX solubilization. The significance of the behavior of membrane proteins after Lubrol WX extraction or any other mild detergent is a matter of debate.26-28 However, the fact that the integral PMP Pex13p is completely extracted by both detergents strongly suggests that ALDP is embedded in a lipid environment different from that of PMP70, Pex14p, and Pex13p.
Pex13p and Pex14p physically interact with each other and with Pex5p, the cycling receptor crucial for targeting of peroxisomal matrix proteins.29-32 Studies with the yeast orthologs have shown that the relative amounts of Pex13p and Pex14p must be tightly balanced for normal peroxisome biogenesis.33 In rat livers, however, Pex13p and Pex14p were detected in separate protein complexes in the peroxisomal membrane.34 Our observation that Pex14p and Pex13p show different lipid raft association characteristics is in line with the existence of separate Pex13p-containing and Pex14p-containing protein complexes and suggests that their transient interaction is crucial for peroxisome biogenesis.
The cholesterol content in the peroxisomal membrane is low.35 Still, it appears to serve a crucial role in peroxisomal lipid raft assembly and sorting of peroxisomal matrix and membrane proteins. In cholesterol-depleted HepG2 cells, Pex14p and PMP70 become Triton X-100–extractable, but sorting to the peroxisomes is not disturbed for these proteins. In contrast, targeting of catalase and ALDP is impaired under these conditions. This implies that cholesterol depletion leads to selective effects on the sorting of peroxisomal proteins, which is most likely a direct effect of the disruption of peroxisomal lipid rafts. If there were a basal requirement for cholesterol for peroxisome biogenesis, then a generalized effect on the sorting of peroxisomal proteins would be expected when cholesterol levels are insufficient. The peroxisomal sorting of catalase is efficiently re-established when cholesterol is supplied to the cholesterol-depleted HepG2 cells. This is most likely the result of the reestablishment of the lipid raft association of PMPs involved in matrix protein import and the ability of the import machinery to transport fully folded and oligomeric proteins.36 Thus, both newly synthesized catalase and cytosol-accumulated catalase are targeted to peroxisomes when the import machinery is restored. Importantly, cholesterol depletion does not induce leakage of resident proteins from peroxisomes as shown by cycloheximide cotreatment. Interestingly, cholesterol repletion did not efficiently restore the peroxisomal location of ALDP. This indicates that missorted ALDP is unable to return to peroxisomes of cholesterol-repleted HepG2 cells and that effective targeting of newly synthesized ALDP is only slowly established in these cells.
To date, the only other report about lipid microdomains in peroxisomal membranes is on ergosterol/ceramide-rich (ECR) domains in peroxisomes of the yeast Yarrowia lipolytica.37 These ECR domains play a role in a complex transition of preperoxisomal structures to mature peroxisomes, but it is unclear whether similar mechanisms are involved in human peroxisome biogenesis. Therefore, it remains to be determined whether a functional relationship exists between the human peroxisomal lipid rafts and the Y. lipolytica ECR domains.
The distinct detergent extractability of ALDP and PMP70 indicates that the lipid environment of these two highly homologous ABC-transporters in the peroxisomal membrane is different. Whether they exist in spatially separated lipid rafts or the different extractability is a result of a heterogeneous lipid distribution within one raft remains to be determined, as for lipid rafts occurring in other cellular membranes.28
Several other ABC transporters (multidrug resistance 1 protein/P-glycoprotein/ABCB1, multidrug resistance–associated protein 1/ABCC1, and breast cancer resistance protein/ABCG2) have been shown to reside in lipid rafts, and their substrate transporting activity is regulated by their lipid environment.13, 14, 38-41 It is therefore likely that the activity of PMP70 and ALDP also is controlled by their association with specific lipids. The protein-lipid interaction is not (solely) determined by the mPTSs of ALDP and PMP70 because correctly sorted GFP-tagged fragments of ALDP and PMP70 appear not to be lipid raft–associated. It is interesting to note that significant amounts of ALDP and PMP70 appear to remain associated with lipids after Lubrol WX extraction of cholesterol-depleted HepG2 cells, as indicated by their accumulation at the 40% to 35% sucrose interphase after flotation gradient centrifugation. Pex14p and c-Src, in contrast, are fully solubilized under these conditions. This may indicate that ALDP and PMP70 are associated with other lipids that reside in the peroxisomal membrane. The slight but highly reproducible difference in peak fractions of raft-associated c-Src versus PMPs after flotation gradient centrifugation of Lubrol WX–extracted HepG2 cells may be a result of this peroxisome-specific lipid environment.
It is relevant to note that many X-ALD patients are treated with cholesterol-lowering drugs such as lovastatin and simvastatin42, 43 aimed at reducing VLCFA levels. In X-ALD patients with residual ALDP protein activity, cholesterol reduction could theoretically affect the subcellular location of ALDP and thereby further reduce its function and aggravate X-ALD disease symptoms. In our HepG2 cells, treatment with lovastatin alone reduced the cellular cholesterol levels maximally to 50% versus control cells. This reduction did not affect the raft association of ALDP, PMP70, or Pex14p (Supporting Fig. 5). It is therefore unlikely that lovastatin treatment alone will be harmful because of effects on peroxisome biogenesis.
In summary, our data show that PMPs are differentially associated with detergent-resistant lipid rafts. Cholesterol depletion leads to disruption of the lipid raft association and peroxisome biogenesis defects. The role of peroxisomal lipid rafts in diseases in which peroxisomes are malfunctioning (e.g., ZS and X-ALD) needs further analysis.
Acknowledgements
Dr. J. W. Kok (Department of Cell Biology, University Medical Center Groningen, Groningen, the Netherlands) is kindly acknowledged for his help and suggestions during this project.




