High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis†
Potential conflicts of interest: none.
Abstract
Previous controlled trials are inconclusive regarding the efficacy of ursodeoxycholic acid (UDCA) for treating primary sclerosing cholangitis (PSC). One hundred fifty adult patients with PSC were enrolled in a long-term, randomized, double-blind controlled trial of high-dose UDCA (28-30 mg/kg/day) versus placebo. Liver biopsy and cholangiography were performed before randomization and after 5 years. The primary outcome measures were development of cirrhosis, varices, cholangiocarcinoma, liver transplantation, or death. The study was terminated after 6 years due to futility. At enrollment, the UDCA (n = 76) and placebo (n = 74) groups were similar with respect to sex, age, duration of disease, serum aspartate aminotransferase and alkaline phosphatase levels, liver histology, and Mayo risk score. During therapy, aspartate aminotransferase and alkaline phosphatase levels decreased more in the UDCA group than the placebo group (P < 0.01), but improvements in liver tests were not associated with decreased endpoints. By the end of the study, 30 patients in the UDCA group (39%) versus 19 patients in the placebo group (26%) had reached one of the pre-established clinical endpoints. After adjustment for baseline stratification characteristics, the risk of a primary endpoint was 2.3 times greater for patients on UDCA than for those on placebo (P < 0.01) and 2.1 times greater for death, transplantation, or minimal listing criteria (P = 0.038). Serious adverse events were more common in the UDCA group than the placebo group (63% versus 37% [P < 0.01]). Conclusion: Long-term, high-dose UDCA therapy is associated with improvement in serum liver tests in PSC but does not improve survival and was associated with higher rates of serious adverse events. (HEPATOLOGY 2009.)
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology characterized by fibrosing inflammation and destruction of the extrahepatic and/or intrahepatic bile ducts.1 The disease is slowly progressive, usually leading to biliary cirrhosis, portal hypertension, and liver failure over a 10- to 15-year period.2 PSC is one of the most common adult cholestatic liver diseases and is an important indication for liver transplantation in adults in the United States. At least 70% of cases of PSC are associated with chronic inflammatory bowel disease, usually ulcerative colitis.1
There are no reports of effective medical therapy for PSC at this time. Several potential treatments have been evaluated in both controlled and uncontrolled trials, including methotrexate, corticosteroids, cyclosporin, tacrolimus, and colchicine but none have been found to be effective.3-8 Ursodeoxycholic acid (UDCA) has been tested in a randomized controlled trial of 105 patients at a dose of 13 to 15 mg/kg/day.9 There was biochemical improvement and a trend toward an improvement to time of treatment failure was noted, although the difference was not significant. A study testing a higher dose of UDCA in 23 patients receiving 20 mg/kg/day showed the drug to be well tolerated.10 There was improvement in liver biochemistries and stability of histologic staging on biopsy. A randomized trial with an even higher dose of UDCA (17 to 23 mg/kg/day) in 219 patients was recently published.11 A smaller number of patients than anticipated entered the study. There were favorable trends toward reduction, but no significant differences in death, transplantation, or development of cholangiocarcinoma. A pilot study using the Mayo risk score as a surrogate endpoint suggested that a dose of 25 to 28 mg/kg/day might improve survival.12 These results prompted us to initiate a multicenter placebo-controlled trial using a dose of 28 to 30 mg/kg/day in 2002. The results of this study are reported herein.
Abbreviations
PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid.
Materials and Methods
Patients were entered based on inclusion criteria with exceptions approved by our Institutional Review Boards.
Inclusion Criteria.
Primary sclerosing cholangitis was defined as present when all the following criteria were met: (1) chronic cholestatic disease of at least 6 months' duration; (2) serum alkaline phosphatase at least 1½ times the upper limits of normal; (3) retrograde, operative, percutaneous, or magnetic resonance cholangiography demonstrating intrahepatic and/or extrahepatic biliary duct obstruction, beading or narrowing consistent with PSC within 1 year of the study entry; (4) liver biopsy in the previous 1 year that was available for review and compatible with the diagnosis of PSC (7 patients did not have entry liver biopsy due to low platelet count and/or presence of cirrhosis). Compatible biopsy features included fibrous cholangitis, ductopenia with periportal inflammation, and biliary fibrosis.
Exclusion Criteria.
Patients were excluded if they had any of the following: (1) coexistent conditions such as preexisting advanced malignancies or severe cardiopulmonary disease that would limit their life expectancy to less than 2 years; (2) inability to provide consent; (3) treatment with UDCA, pentoxifylline, corticosteroids, cyclosporin, colchicine, azathioprine, methotrexate, D-penicillamine, budesonide, nicotine, pirfenidone, or tacrolimus in the 3 months prior to study entry; (4) inflammatory bowel disease patients requiring specific treatment in the preceding 3 months except for maintenance therapy with a 5-ASA compound; (5) anticipated need for liver transplantation within 2 years (expected survival of <80% at 2 years based on Mayo risk score)13; (6) recurrent variceal bleeds, spontaneous uncontrolled encephalopathy, international normalized ratio >1.5 uncorrected by vitamin K or resistant ascites that suggested an anticipated survival of less than 1 year; (7) pregnancy or lactation (patients who became pregnant during the study were discontinued and referred to their physicians); (8) age less than 18 years or greater than 75 years; (9) findings highly suggestive of liver disease of other etiology such as chronic alcoholic liver disease, chronic hepatitis B or C infection, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, Wilson's disease, congenital biliary disease, or cholangiocarcinoma; (10) previous intraductal stones or operations on the biliary tree, other than cholecystectomy, such as biliary drainage procedures preceding the diagnosis of PSC; or (11) recurrent ascending cholangitis requiring hospitalization occurring more than two times per year.
Screening Log.
A screening log was maintained for all patients screened and included patients', age, sex, race, date of screening, eligibility status, and randomization number if enrolled or reason for not enrolling.
Randomization and Stratification.
All patients found to be eligible and who provided written informed consent to participate in the study were randomized to one of two groups: 1) UDCA at a dose of 28 to 30 mg/kg/day or (2) an identical-appearing placebo.
The randomization was stratified by histologic stage I or II versus III or IV; presence or absence of varices; and Mayo risk score (<1.5, >1.5). Computer-based dynamic allocation was used to assign patients to study groups via the coordinating center in Rochester, MN.
Drug Administration.
Patients received UDCA at a dose of 28 to 30 mg/kg/day (combination of 500- and 250-mg tablets of URSO, Axcan Pharma, Mont-St. Hiliare, Canada) in divided doses given with meals and a bedtime snack or an identical placebo. The dose was gradually tapered upward, beginning with one tablet a day and increasing by one tablet every 3 days until the full dose was reached. If a patient required cholestyramine for pruritus, they were instructed to take the study drug at least 2 hours before or 2 hours after the dose of cholestyramine. The study drug and the placebo were provided at no charge to the patient. The physician, study coordinator, and patient were blinded as to whether active drug or placebo was being administered. Drug or placebo was continued even after reaching a primary endpoint, except for liver transplantation or death.
Monitoring.
Liver enzymes were assessed by mailed containers or patient visit every 3 months to monitor for possible toxicity and to assess biochemical response. Patients were examined annually. Upper endoscopy to assess for varices was done at 2 years and endoscopy, cholangiography, and liver biopsy were scheduled to be repeated 5 years after entry. Treatment was stopped if liver transplantation was required.
Patient Compliance and Termination.
Patient compliance was determined by the nurse coordinator at each site with regular telephone calls and bottle and pill counts done at various times by the study monitor.
Noncompliant patients were censored from the study but still followed (intent to treat). Three patients were determined to be noncompliant, being off study medication for time periods ranging from 6 to 16 months.
Data Collection.
Data were collected prospectively at each clinical center and forwarded to Mayo Clinic Rochester, which served as the coordinating center. The data were entered into computers at the coordinating center once the initial quality assurance audits were completed at the originating study site.
Laboratory values, such as serum liver biochemistries, were normalized by dividing the actual value by the upper limits of normal for the clinical laboratory in which the test was performed.
Statistical Analyses.
Baseline characteristics were calculated as the median (range) for continuous variables, and the number and percent in each group were tabulated for categorical variables. The primary endpoint of the trial was a comparison between the two arms based on the initial assignment of drug (intention-to-treat). Only patients who withdrew from the study before receiving any study medication (and without knowledge of the treatment arm) were excluded. The primary endpoint was time to first failure (death, transplantation, meeting minimal listing criteria, development of varices, cholangiocarcinoma, or progression to cirrhosis) and was assessed using a Cox model. Patients were censored at the day they went off the protocol. A second set of models also assessed time to death, transplantation, or meeting minimal listing criteria alone. Both these models were adjusted for the stratification variables (Mayo risk score, baseline presence of varices and histologic stage). We further assessed both endpoint sets to compare the effect of study medication while adjusting for a variety of baseline characteristics: Mayo risk score, varices, histologic stage, age, sex, inflammatory bowel disease, alkaline phosphatase, aspartate aminotransferase, and bilirubin. Finally, within the full primary endpoint model, we tested every two-way interaction between study medication and the baseline characteristics. All analyses used a 5% two-sided type I error rate. Analyses were performed with the SAS statistical software package (Version 9.1; SAS Institute Inc., Cary, NC).
Sample Size.
Sample size calculations were made assuming that UDCA would halve the risk of a primary endpoint that was based on projections from our pilot study.12 Based on our previous study, we expected 35% of patients to reach a primary endpoint in 5 years.9 With α = 0.05 and power = 80%, we estimated a need to recruit at least 149 patients.
Results
One hundred fifty patients were entered over a 3-year period from seven sites as shown in Table 1. The process for arriving at these 150 patients from the 455 assessed for eligibility is shown in Fig. 1. Of the 305 patients who were screened but not enrolled, 11 were eligible but declined, 141 were not eligible, and 153 had unknown eligibility. The majority of the 141 patients were not eligible due to inadequate alkaline phosphatase elevation and exclusionary medication use; other reasons included advanced liver disease, age, and complicating medical conditions. Eligibility was not known in 153 patients, because these patients declined further testing for reasons including cost, the randomized nature of the trial, concern about side effects, and unknown reasons.
| Site | UDCA | Placebo | Total |
|---|---|---|---|
| Mayo Clinic, Rochester, MN | 33 | 33 | 66 |
| Mayo Clinic, Jacksonville, FL | 3 | 3 | 6 |
| Mayo Clinic, Scottsdale, AZ | 8 | 8 | 16 |
| University of Washington, Seattle, WA | 12 | 11 | 23 |
| University of Nebraska, Lincoln, NE | 7 | 6 | 13 |
| Saint Louis University, St. Louis, MO | 4 | 4 | 8 |
| Virginia Commonwealth University, Richmond, VA | 9 | 9 | 18 |
| Total | 76 | 74 | 150 |
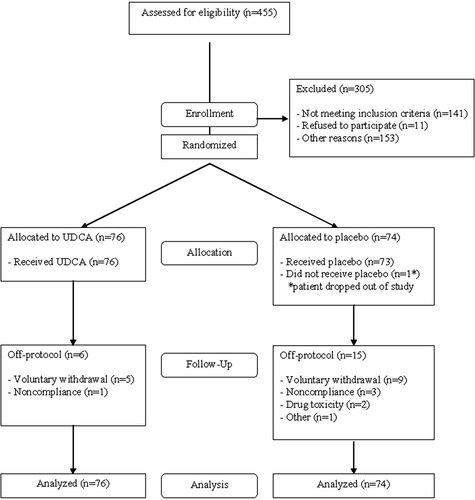
Diagram of flow of patients through the study beginning with those assessed for eligibility through enrollment, randomization, and follow-up.
The baseline characteristics of these patients in both groups are shown in Table 2. The two treatment groups were well balanced at enrollment. The average age in the treatment group was 47.9 (range, 20.5-75.6) years; among patients in the control group, the average age was 44.5 (range, 17.9-73.9) years. Thirty-eight (50%) of the UDCA patients were female, compared with 26 (35%) on placebo. Colitis was present in 116 (77%) of patients.
| Characteristic | UDCA (n = 76) | Placebo (n = 74) | P Value |
|---|---|---|---|
| Age, years | 47.9 (20.5–75.6) | 45.3 (17.9–73.6) | 0.219 |
| Duration of disease, years | 1.3 (0.1–13.4) | 1.0 (0.0–49.5) | 0.833 |
| Female sex, n (%) | 38 (50) | 26 (35) | 0.066† |
| Colitis, n (%) | 55 (72) | 61 (82) | 0.14 |
| Varices, n (%) | 13 (17) | 13 (18) | |
| Histologic stage, n (%) | |||
| I | 27 (36) | 23 (31) | 0.564† |
| II | 19 (25) | 21 (28) | 0.640† |
| III | 20 (26) | 17 (23) | 0.635† |
| IV | 10 (13) | 13 (18) | 0.454† |
| Alkaline phosphatase* | 3.3 (0.7–11.2) | 3.2 (0.5–16.9) | 0.814 |
| Aspartate aminotransferase* | 2.0 (0.5–6.9) | 2.3 (0.5–9.4) | 0.684 |
| Bilirubin, mg/dL* | 0.8 (0.2–3.2) | 1.0 (0.2–5.5) | 0.100 |
| Mayo risk score | 0.3 (−1.4–2.4) | 0.3 (−1.5–2.5) | 0.483 |
- Data are presented as the median (range) unless otherwise indicated.
- * Values represent multiples of the upper limits of normal.
- † The chi-square test of independence was used to determine statistical significance for categorical data. For the remaining (continuous) variables, the Wilcoxon rank sum test was used.
After a planned analysis once 75% of expected endpoints had been reached, the Data Safety and Monitoring Board reviewed the data and terminated the study due to futility.
The biochemical responses for alkaline phosphatase, aspartate aminotransferase, and bilirubin over a 3-year period are shown in Table 3. Table 4 shows the total number of primary endpoints per treatment group. Table 5 shows the hazard ratio for the effect of UDCA for both Cox models. In both models, the hazard ratio favors placebo over UDCA with statistically significant results. Analyzing the primary endpoints yielded a hazard ratio of 2.27 (95% confidence interval: 1.24-4.16) in favor of placebo. The Kaplan-Meier curve for time until reaching primary endpoints is shown in Fig, 2, and time to death or transplantation is shown in Fig. 3.
| n | Alkaline Phosphatase | Aspartate Aminotransferase | Bilirubin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UDCA | Placebo | UDCA | Placebo | P Value | UDCA | Placebo | P Value | UDCA | Placebo | P Value | |
| Baseline | 76 | 73 | 3.3 (0.7–11.2) | 3.2 (0.5–16.9) | 0.814 | 2.0 (0.5–6.9) | 2.3 (0.5–9.4) | 0.684 | 0.8 (0.2–3.2) | 1.0 (0.2–5.5) | 0.100 |
| 12 Months | 70 | 63 | 1.9 (0.6–9.1) | 2.9 (0.6–13.6) | <0.001 | 1.0 (0.5–10.5) | 1.9 (0.6–8.7) | <0.001 | 0.8 (0.2–6.3) | 0.9 (0.3–8.2) | 0.074 |
| 24 Months | 65* | 59† | 1.8 (0.6–8.5) | 2.6 (0.5–11.9) | 0.001 | 1.1 (0.4–8.4) | 1.9 (0.5–13.3) | <0.001 | 0.8 (0.2–7.2) | 1.0 (0.2–8.6) | 0.393 |
| 36 Months | 56‡ | 53 | 1.7 (0.6–16.5) | 2.4 (0.4–12.1) | 0.012 | 1.1 (0.3–7.2) | 1.7 (0.5–14.8) | <0.001 | 0.8 (0.2–15.9) | 0.9 (0.3–9.6) | 0.037 |
- Data are presented as the median (range) unless otherwise indicated.
- * At 24 months, only 64 patients were tested for bilirubin in the UDCA group.
- † At 24 months, only 58 patients were tested for alkaline phosphatase in the placebo group.
- ‡ At 36 months, only 55 patients were tested for bilirubin in the UDCA group.
| Primary Endpoints | UDCA | Placebo |
|---|---|---|
| Death | 5 | 3 |
| Liver transplantation | 11 | 5 |
| Minimal listing criteria for liver transplantation | 13 | 10 |
| Development of cirrhosis | 6 | 4 |
| Esophageal and/or gastric varices | 15 | 5 |
| Cholangiocarcinoma | 2 | 2 |
| Total endpoints | 52 | 29 |
| Number of patients reaching a primary endpoint | 30 | 19 |
| Number of patients reaching death, orthotopic liver transplantation, minimal criteria listing | 22 | 15 |
| Hazard Ratio (UDCA versus Placebo) | (95% Confidence Interval) | P Value | |
|---|---|---|---|
| Adjusted for stratification variables: Mayo risk score, baseline presence of varices, and histologic stage | |||
|
Primary endpoints (Fig. 2) |
2.27 | (1.24–4.16) | 0.008 |
|
Death, liver transplantation or minimal criteria for listing (Fig. 3) |
2.11 | (1.04–4.28) | 0.038 |
| Adjusted for Mayo risk score, varices, histologic stage, age, sex, inflammatory bowel disease, alkaline phosphatase, aspartate aminotransferase, and bilirubin | |||
| Primary endpoints | 2.73 | (1.37–5.38) | 0.004 |
| Death, liver transplantation or minimal criteria for listing | 2.85 | (1.26–6.49) | 0.012 |
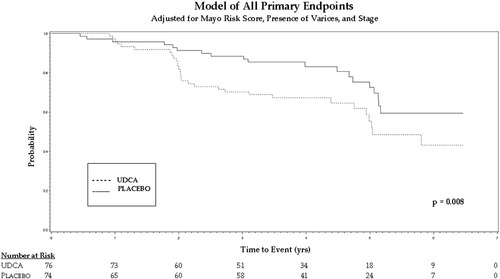
Kaplain-Meier curve for time until reaching primary endpoints.
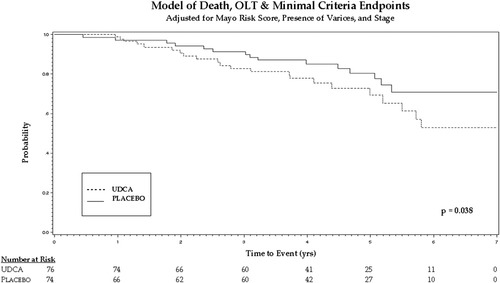
Kaplan-Meier curve showing time to death or transplantation.
Because of the early stopping of the study, only 16 UDCA-treated patients and 15 placebo-treated patients had biopsy of the liver at 5 years. The difference between the two groups in change from baseline histologic stage was −0.25 ± 1.0 versus −0.07 ± 0.70 (P = 0.76). Follow-up cholangiograms were available in only 23 UDCA-treated patients and 17 patients on placebo. Colonic dysplasia developed in three UDCA-treated patients and 5 placebo-treated patients (P value not significant).
Serious adverse events were more common in the drug treated group. Many of these related to the development of primary endpoints, and no unusual or new side effects were identified. When analyses for treatment response in various subgroups were performed, the presence or absence of inflammatory bowel disease, age, and sex were not associated with different treatment responses. Patients with a greater Mayo risk score and advanced histologic stage at entry had, as expected, poorer clinical outcomes, but this was irrespective of the treatment group to which they were assigned (data not shown).
Discussion
UDCA, when used for treatment of PSC, was associated with poorer clinical outcomes when compared with placebo. More patients developed varices, died, or became eligible for liver transplantation in the group receiving UDCA compared with the placebo group, despite improvement in liver tests. The likelihood of developing these adverse events was not predicted by a biochemical response, and as expected was predicted irrespective of treatment by higher Mayo risk score or presence of cirrhosis on entry biopsy (data not shown).
A statistically significant biochemical response was observed for alkaline phosphatase and aspartate aminotransferase (Table 3). However, response at 6 months was not associated with development of primary endpoints.
Previous studies led us to believe that UDCA would be safe and beneficial.9-12 Patients with primary biliary cirrhosis, in whom a dose of 13 to 15 mg/kg/day has been approved by the Food and Drug Administration, have not had more adverse events when treated with higher doses up to 25 or 30 mg/kg/day.14 Pilot studies in patients with PSC using doses ranging from 17 to 25 mg/kg/day did not have an increased risk of adverse events. Reassessment of the initial UDCA study, which used a dose 13 to 15 mg/kg/day in 105 patients, showed a trend toward improved survival free of transplantation in the treated group. Hence, the findings in this study were quite unanticipated.
The surprising results in this study led us to examine the data in several ways. The primary analysis is represented in the first row of Table 5 (assessing all primary endpoints and adjusting for baseline stratification variables). However, we were concerned that the study groups differed in follow-up visits, and that some endpoints could only be observed through particular tests. Therefore, in addition to assessing primary endpoints, we also assessed transplantation, meeting minimal listing criteria, and death alone. Both of these models, as well as the adjusted models, provided hazard ratios that were not only in the same, unexpected direction, but represented a clinically relevant increase in worse outcomes for patients on UDCA compared with placebo.
It is unclear how a drug that has a reputation for such safety would have these paradoxical effects in this condition. Analysis of the actual drug supply for contaminants failed to disclose any unusual compounds within the study supply of the drug. It is unclear whether higher doses of UDCA allowed unabsorbed drug to enter the colon and be modified into hepatotoxic bile acids. The possibility of hepatotoxic bile acids being produced from unabsorbed UDCA remains a potential explanation and deserves further evaluation.15 In an animal model, UDCA aggravated bile infarcts and hepatocyte necroses in the setting of biliary obstruction; this may also explain why the results in PSC, where biliary obstruction occurs, were different than in PBC.16
UDCA may also modulate apoptosis.17 It is possible that the high dose of UDCA used in this study prevented apoptosis of activated stellate cells, which continued to be active in fibrogenesis, leading to the advanced liver disease found in this study.
At this time, UDCA at a dose of 25 to 30 mg/kg/day for patients with PSC should not be used because of the increased risk of clinically important adverse endpoints. There is no treatment that can be recommended at this time, and only therapy in the context of prospective trials should be considered. Continued testing of drugs in pilot studies is reasonable, but the findings of this study mandate that any positive biochemical response be confirmed within a randomized controlled trial. In the absence of such randomized controlled data, it is likely that the adverse events detected in this study would have been attributed to the progressive nature of the liver disease and not recognized as related to the drug. It is hoped that in the near future, a safe and effective therapy for patients with PSC will be developed, but high-dose UDCA cannot be recommended despite findings in a recent pilot study.18 The results of this study caution against empiric therapy for patients with PSC and highlight the need for confirmation of promising pilot studies by adequate controlled trials.




