Vigorous activation of monocytes in juvenile autoimmune liver disease escapes the control of regulatory T-cells†
Potential conflict of interest: Nothing to report.
fax: (44)-203-2993700
Abstract
Interface hepatitis, the histological lesion typical of autoimmune hepatitis (AIH), is composed of CD4 and CD8 T lymphocytes and of innate immunity cells, particularly monocytes. Studies in AIH have focused on autoreactive CD4 and CD8 T cells and impairment of CD4+CD25+ regulatory T cells (T-regs), whereas little is known about the role of monocytes and their relationship with T-regs. We have investigated 51 patients with autoimmune liver disease (AILD) and 27 healthy subjects, finding that monocytes were higher in number (P = 0.044), had a more vigorous spontaneous migration (P < 0.0005 in patients with inactive disease [ID], and P < 0.001 in those with active disease [AD]), displayed a higher tumor necrosis factor alpha (TNF-α) over interleukin (IL)-10 production (P = 0.07 in ID and P = 0.0005 in AD), and expressed higher levels of Toll-like receptor (TLR) 4 (P = 0.048 in ID and P = 0.03 in AD). Addition of conventional T-regs (cT-regs) in AILD enhanced monocyte migration (P = 0.05 in ID and P = 0.08 in AD), magnified TNF-α over IL-10 production (P = 0.0005 in ID and P = 0.006 in AD), and markedly increased TLR4 expression levels (P = 0.01 in ID and P = 0.004 in AD), whereas in normal subjects it either restrained or left unchanged monocyte function. Because a CD127-negative subpopulation within CD4+CD25+ T cells exerts the strongest regulatory activity, we performed additional experiments using purified CD4+CD25+CD127− T cells (true T-regs [tT-regs]). Addition of tT-regs to monocytes decreased monocyte migration (P = 0.03) and promoted IL-10 production (P = 0.009), leaving unchanged TLR4 expression in healthy subjects, whereas in patients with AILD it induced only a marginal increase in IL-10 production (P = 0.045 in ID and P = 0.13 in AD). Conclusion: Monocyte overactivation and inability of cT-regs and tT-regs to restrain it may contribute to the loss of immune tolerance and perpetuation of the autoimmune attack in AILD. (HEPATOLOGY 2009.)
Cells of the adaptive immune system have been implicated in the pathogenesis of autoimmune hepatitis (AIH), a liver disorder characterized by elevated transaminase levels, circulating autoantibodies, hypergammaglobulinemia, and a histological lesion known as interface hepatitis consisting of portal/periportal mononuclear cell infiltration.1-4 In AIH, CD4+CD25+ regulatory T cells (T-regs), a lymphocyte subpopulation central to the maintenance of immune homeostasis, are defective in number and unable to restrain CD4 and CD8 T cell proliferation and interferon gamma secretion,5-7 in vitro readouts of in vivo T cell effector function. In addition to CD4 and CD8 T lymphocytes, monocytes/macrophages, defined as CD11b+ cells,8 represent a major component of the portal/periportal cellular infiltrate in AIH; however, no studies have attempted to determine their contribution to the pathogenesis of the disease.
In other autoimmune conditions, monocytes have been shown to play a key role in disease initiation and progression. Block of monocyte migration through chemoattractant inhibition was found to lead to a reduction of disease severity in a murine model of autoimmune myocarditis.9 Moreover, monocyte release of proinflammatory cytokines (for example, interleukin [IL]-1, IL-6 tumor necrosis factor alpha [TNF-α]) was able to sustain the chronic inflammation in patients with rheumatoid arthritis.10, 11 Monocyte release of proinflammatory cytokines was also documented after in vitro stimulation with lipopolysaccharide (LPS), the natural ligand of Toll-like-receptor-4 (TLR4), in patients with primary biliary cirrhosis, where it was found to enhance T-cell–mediated immune responses.12
Akin to CD4 and CD8 T lymphocytes, monocytes isolated from healthy subjects are susceptible to T-reg control, as demonstrated by a decrease in the production of proinflammatory cytokines and in the ability to act as antigen-presenting-cells after coculture with CD4+CD25+ T cells.13 Monocytes also can influence T-reg function, as demonstrated in patients with rheumatoid arthritis, in whom activated monocytes abrogate T-reg suppressor function through production of TNF-α.14
The aim of the current study is to characterize phenotypically and functionally circulating monocytes in juvenile autoimmune liver disease (AILD) and to investigate the effect T-regs have on these cells.
Abbreviations
AD, active disease; AIH, autoimmune hepatitis; AILD, autoimmune liver disease; AST, aspartate aminotransferase; cT-regs, conventional regulatory T cells; FOXP3, forkhead winged/helix transcription factor box P3; HLA, human leukocyte antigen; ID, inactive disease; IgG, immunoglobulin G; IL, interleukin; LPS, lipopolysaccharide; %MM, monocyte migration; MMP, matrix metalloproteinase; mRNA, messenger RNA; PBMC, peripheral blood mononuclear cells; PE, phycoerythrin; SDF-1α, stromal cell–derived factor 1α TLR, Toll-like receptor; TNF-α, tumor necrosis factor alpha; tT-regs, true CD4+CD25+ regulatory T cells; T-regs, CD4+CD25+ regulatory T cells.
Patients and Methods
Patients and Controls.
Fifty-one patients with antinuclear antibody or smooth muscle antibody–positive AILD (AIH type 1 [AIH-1] or autoimmune sclerosing cholangitis) were studied; median age at diagnosis was 12.6 years (range, 3.6-16.6); aspartate aminotransferase (AST), 511 IU/L (range, 21-2,642); bilirubin, 54 μmol/L (range, 5-423); immunoglobulin G (IgG), 25.9 g/L (range, 9.55-68.9); antinuclear antibody titer, 1/320 (range, 1/20-1/10,240); and smooth muscle antibody titer 1/160 (range: 1/20-1/2,560). Clinical presentation was acute in 23 and chronic in 28. A liver biopsy performed at the time of or close to diagnosis showed histological features of interface hepatitis in all patients, with a median histological inflammatory activity index of 6 (range, 1-13), calculated as previously described.1, 15 Sixteen children with autoimmune sclerosing cholangitis had cholangiographic evidence of bile duct disease.15 All patients were studied while on immunosuppressive treatment (age at the time of study, 6.3-28 years; median, 14.5), having previously demonstrated that T-regs collected at disease presentation, before immunosuppressive treatment is started, are inefficient at suppressing the proliferation of target cells.5, 6 Thirty-two patients were studied while their disease was inactive (ID = normal levels of AST), and 22 while their disease was active (AD = elevated AST); three patients were studied on both occasions. Patients were receiving prednisolone (2.5-5 mg daily) with or without azathioprine (1-2 mg/kg/day). Twenty-seven healthy subjects served as normal controls. The study was approved by the Ethics Committee of King's College Hospital, London, United Kingdom. Demographic and laboratory data at the time of study are shown in Table 1.
| Study Group | Number of Subjects | Sex (F/M) | Age (years) | AST* | Bilirubin† | IgG‡ | Autoantibody Titer (Reciprocal) | ||
|---|---|---|---|---|---|---|---|---|---|
| ANA | SMA | LKM-1 | |||||||
| AILD | 51 | 31/20 | 6.3–28 (14.5) | ||||||
| Inactive disease | 32 | 18–50 (27) | 4–36 (10) | 5.29–37.3 (13) | 20–320 (160) | 20–80 (40) | Negative | ||
| Active disease | 22§ | 54–597 (109) | 8–145 (21) | 6.37–34.1 (16.8) | 40–2560 (2560) | 40–2560 (160) | Negative | ||
| Healthy subjects | 27 | 22/5 | 25–32 (28) | — | — | — | — | — | — |
- Data are presented as the range (median) unless noted otherwise.
- * Normal: < 50 UI/L
- † Normal: < 20 μmol/L
- ‡ Normal: 6.5–17 g/L
- § Three patients were studied both in remission and during an episode of relapse.
Cell Separation.
Peripheral blood mononuclear cells (PBMCs) were obtained as previously described.3 Lymphocyte viability, determined by Trypan blue exclusion, exceeded 98%.
Flow Cytometry.
The frequency and phenotype of monocytes was determined by double-color flow cytometry, performed on fresh PBMCs. Unfractionated cells were stained with fluorescein isothiocyanate–conjugated anti-CD14 and phycoerythrin (PE)-conjugated anti–human leukocyte DR antigen (HLA)-DR, anti-CD86, anti-CD54 (all from BD Biosciences Discovery Labware, Oxford, UK), and anti-CD40 monoclonal antibodies (eBioscience, San Diego, CA). The expression of TLR4, whose engagement by its natural ligand LPS is key to the initiation of adaptive immune responses,16 was determined on purified monocytes (see Cell Purification) using PE-conjugated anti-TLR4 monoclonal antibodies (eBioscience). Monocyte TLR4 expression was assessed before and after LPS stimulation, in the absence or presence of T-regs (or CD4+CD25− T cells as control) (see Cell Purification). T-reg phenotype was assessed by triple-color flow cytometry using fluorescein isothiocyanate–conjugated anti-CD4, PE-conjugated anti-CD45RO, CD62L, and antigen-presenting cell–conjugated CD25 monoclonal antibodies (all from BD Biosciences) on purified CD4+CD25+ T cells. Because of recent evidence showing that absence or low expression levels of the activation markers CD127 (α chain of the IL-7 receptor) and CD154 (CD40L) define those CD4+CD25+ T cells with the strongest suppressor function (true T-regs),17-19 purified T-regs were further characterized using PE-conjugated anti-CD127 (BD Bioscience) and anti-CD154 monoclonal antibodies (eBioscience).
Cells were incubated at 4°C for 35 minutes in the dark, washed once with phosphate-buffered saline/1% fetal bovine serum, then resuspended and stored at 4°C in the dark until analysis. Expression of the forkhead winged/helix transcription factor box P3 (FOXP3), another T-reg marker,20, 21 was assessed by intracellular staining using PE-conjugated anti-human FOXP3 monoclonal antibodies (eBioscience), as previously described.22 Flow cytometry was performed on a Becton Dickinson FACScalibur (Becton Dickinson, Immunocytochemistry Systems, San José, CA), and CellQuest software (Becton Dickinson) was used for analysis. A minimum of 1 × 104 gated events was acquired for each sample.
Immunohistochemistry.
Paraffin-embedded liver sections, available from five biopsy specimens, were stained with anti-CD68 monoclonal antibody, which stains both resident and infiltrating macrophages.23, 24 Sections were also stained using anti-CD3 and anti-CD79, markers for T and B lymphocytes, respectively. To unmask the antigen, samples were deparaffinized by placing them in xylene, then in ethanol. After rehydration with water, sections were placed in a jar containing Tris-ethylenediaminetetra-acetic acid buffer (pH: 9) and microwaved for 25 minutes. After cooling for 15 to 30 minutes, sections were placed in 1× phosphate-buffered saline for 5 minutes. Staining was carried out using a Novolink Polymer Detection System (Novocastra, Newcastle upon Tyne, UK). After endogenous peroxidase blocking and treatment with protein block solution, sections were washed with 1× phosphate-buffered saline for 5 minutes and stained with monoclonal antibodies to CD68, CD3, and CD79 (DakoCytomation Ltd., Ely, UK). Anti-CD68 monoclonal antibodies were prediluted by the manufacturer, whereas anti-CD3 and anti-CD79 monoclonal antibodies were used at the dilution of 1/10 and 1/50, respectively. After 1 hour incubation at room temperature, sections were washed, treated with a post-primary block solution, and incubated for 30 minutes with anti-mouse IgG polymer–horseradish peroxidase–labeled antibody. After washing, 3,3′-diaminobenzidene chromogen solution was applied at 100 μL per milliliter substrate.
Cell Purification.
Monocytes were isolated from PBMCs by positive selection using microbeads conjugated with anti-human CD14 antibodies (Miltenyi Biotec, Bergisch-Gladbach, Germany). T-regs were obtained from PBMCs by CD4-negative selection followed by CD25-positive selection (Dynal Invitrogen, Oslo, Norway),7 and purified CD4+CD25+ T cells localizing in the CD4+CD25high cell gated area by cell sorting (model BD Vantage SE/DiVa, Immunocytochemistry Systems, San José, CA). CD4+CD25− T cells used in control experiments were obtained by negative selection (Dynal Invitrogen) as previously described.7 The purity of the three cell populations, assessed by flow cytometry, exceeded 95% for monocyte and T-reg subsets and 90% (range, 92%-97%) for CD4+CD25− T cells.
True T-regs (tT-regs), i.e., CD4+CD25+CD127− T cells, were further purified from total T-regs. Because of the large number of cells required, this additional purification step was limited to those subjects (11 AILD patients, six ID and five AD, and six healthy subjects) from whom sufficient numbers of CD4+CD25+ T cells were obtained. In brief, purified CD4+CD25+ cells were incubated with PE-conjugated anti-CD127 monoclonal antibodies for 30 minutes, then with microbeads conjugated to monoclonal anti-PE antibodies (Miltenyi Biotec) for a further 15 minutes at 4°C. CD4+CD25+CD127− and CD4+CD25+CD127+ T cell populations were purified by negative and positive selection, respectively, using MS columns (Miltenyi Biotec) according to the manufacturer's instructions. Their purity, assessed by flow cytometry, exceeded 90%. Unfractionated CD4+CD25+ T cells are henceforth referred to as conventional CD4+CD25+ T-regs (cT-regs) and CD4+CD25+CD127− T cells as true T-regs (tT-regs).
Chemotaxis Assay.
A series of transwell experiments was performed to assess the migration of monocytes in response to a chemoattractant as an in vitro readout of the in vivo ability of these cells to migrate and be recruited to the sites of inflammation. Purified monocytes (2 × 105) were resuspended in chemotaxis medium (Roswell Park Memorial Institute 1640 containing 1% bovine serum albumin) and placed in the upper chamber of a transwell system, separated from the lower chamber by an 8-μm pore membrane (BD Biosciences Discovery Labware). Monocyte migration was assessed in both the presence and the absence of the chemoattractant stromal cell–derived factor 1α (SDF-1α), added to the lower chamber at a concentration of 100 ng/mL.25 After incubation for 1 hour at 37°C and 5% CO2, the number of monocytes that had migrated through the membrane was determined by 1-minute flow cytometry acquisition,26 using anti-CD14 monoclonal antibody. To test whether the migration of monocytes through the pore membrane was affected by T-regs, cT-regs, tT-regs, or CD4+CD25+CD127+ T cells, these cells were added at a ratio of 1/8 to autologous monocytes either in direct contact in the upper chamber or in the lower chamber, separated from them by the membrane. The 1/8 ratio was selected because it is capable of exerting a detectable regulatory function in preliminary experiments in which ratios of 1/16, 1/8, and 1/4 were compared. Control experiments were performed using CD4+CD25− T cells.
Chemotaxis in the Presence of Matrigel Matrix.
The chemotaxis assay was repeated after matrigel matrix was layered onto the transwell membrane, because matrigel matrix is a basement membrane preparation whose structure mimics that of blood vessel walls. Cell migration across it, occurring on enzymatic (for example, metalloproteinases) digestion,27 reflects the in vivo capability of the cells to cross the blood vessel barrier. Matrigel (BD Biosciences Discovery Labware) was used at a final concentration of 100 μg/cm2 according to the manufacturer's instructions. After thawing, matrigel was diluted in chemotaxis medium, layered onto 8-μm polycarbonate tissue culture inserts (Nunc, Roskilde, Denmark), and maintained at 37°C and 5% CO2 for 1 hour to gel. Cells were then added and incubated at 37°C for 2 hours; the number of monocytes that had migrated through the matrigel and transwell membrane was tested by flow cytometry. Monocyte migration was assessed in the presence and absence of chemoattractant and after addition of cT-regs, tT-regs, CD4+CD25+CD127+, or CD4+CD25− T cells as described previously.
Results of chemotaxis assays are presented as a percentage of monocyte migration calculated as the ratio of the number of monocytes from the lower chamber over the total number of monocytes seeded initially in the upper chamber. Changes between the percentage of monocyte migration in the presence and absence of the chemoattractant SDF-1α and before and after T-reg addition were calculated for each individual as fold increase/decrease.
Quantification of Gene Expression by Real-Time Polymerase Chain Reaction.
To investigate whether the ability of monocytes to migrate through matrigel was related to the expression of metalloproteinase enzymes, a series of preliminary experiments was performed to identify which was the most expressed matrix metalloproteinase (MMP)-encoding genes among MMP1, MMP9, MMP19, and MMP26,28 MMPs characteristically expressed by monocytes. MMP19 was the most frequently expressed MMP gene in monocytes from six AILD patients and three healthy subjects and was therefore selected for further testing. Total RNA was extracted from purified monocytes (1 × 105 to 5 × 105) using TRizol reagent (Invitrogen Life Technologies, Paisley, UK), and messenger RNA (mRNA) was reverse transcribed as previously described.7 MMP19 gene transcripts were quantified by real-time polymerase chain reaction (PCR) using gene-specific probes and TaqMan Master Mix (Applied Biosystems, Warrington, UK). Polymerase chain reaction amplification conditions were as previously described.22 Samples were run in triplicate using a real-time polymerase chain reaction thermocycler (ABI-Prism 7000 Sequence Detection System; Applied Biosystems, Foster City, CA), and results were analyzed by matched software. Relative gene expression was determined by normalizing to glyceraldehyde-3-phosphate-dehydrogenase gene expression in each set of samples according to the manufacturer's instructions.
Intracellular TNF-α and IL-10 Staining.
The ability of monocytes to produce proinflammatory and anti-inflammatory cytokines, namely, TNF-α and IL-10, was assessed before and after LPS stimulation, in the presence or absence of cT-regs, tT-regs, or CD4+CD25+CD127+ T cells. T-regs were added to autologous monocytes (3 × 105/well) at a ratio of 1/8. The two cell populations were cultured in Roswell Park Memorial Institute-1640 medium supplemented with 2 mM L-glutamine, 25 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 100 U/mL benzyl penicillin, 0.1 mg/mL streptomycin, 2.5 μg/mL amphotericin B, and 5% heat-inactivated fetal bovine serum in the absence or presence of 100 ng/mL LPS (Sigma-Aldrich Ltd., Ayrshire, UK). After overnight incubation, cells were exposed to Brefeldin A to block secretion of cytokines and induce their intracellular accumulation. After 5 hours at 37°C and 5% CO2, cells were washed and stained with fluorescein isothiocyanate–conjugated anti-CD14 monoclonal antibodies, permeabilized, and fixed with Cytofix/Cytoperm, counterstained with PE-conjugated anti–TNF-α (BD Biosciences) or anti–IL-10 (BD Biosciences) monoclonal antibodies, washed, and analyzed by flow cytometry. Changes between the percentage of TNF-α–producing and IL-10–producing monocytes before and after T-reg addition were calculated in each individual as fold increase/decrease, expressed as the ratio between the levels of cytokine production after T-reg addition over baseline levels. Percentages of TNF-α–producing and IL-10–producing monocytes are determined after gating the monocyte subpopulation in the forward/side scatter plot.
Statistical Analysis.
The normality of variables distribution was assessed by the Kolmogorov-Smirnov goodness-of-fit test and, once the hypothesis of normality was accepted (P > 0.05), comparison was performed by paired and unpaired Student t tests as appropriate. If the values were not normally distributed, analysis was performed by Wilcoxon rank-sum test or Mann-Whitney test as appropriate. Correlation analysis was determined by Pearson's or Spearman's correlation coefficient. A value of P < 0.05 was considered significant. Results are expressed as mean ± standard error of the mean.
Results
Monocyte Frequency and Phenotype.
The frequency of monocytes (CD14+ cells) in ID (15.7 ± 2.1) and AD (19.6 ± 2.5) patients was higher than in healthy subjects (10.2 ± 1.6; P = 0.044 for both). The frequency of monocytes in ID patients was lower than in AD patients (P = 0.017).
The frequency of HLA-DR–positive monocytes in ID (66.9 ± 3.8) and AD (64.1 ± 4.4) patients was lower than in healthy subjects (94.1 ± 4.5; P < 0.001 for both), whereas the frequency of CD86-positive, CD54-positive, and CD40-positive monocytes did not differ amongst the three groups, exceeding 90% for CD86 and CD54 and 70% for CD40 in all subjects.
In AILD, there was a positive correlation between the frequency of monocytes and AST (R = 0.35; P = 0.03) and IgG (R = 0.75; P < 0.001) levels and a negative correlation between the frequency of HLA-DR–positive monocytes and AST (R = −0.51; P = 0.001) and IgG (R = −0.53; P = 0.002) levels.
Immunohistochemistry.
CD68+, CD3+, and CD79+ cells were detected in the portal tracts of the liver biopsy specimens from all five AIH patients (two ID and three AD). Figure 1 shows CD68+ monocyte staining in one ID (Fig. 1A) and one AD (Fig. 1B) patient. Portal infiltration by T and B cells (Fig. 1C, D) is also shown for the AD patient.
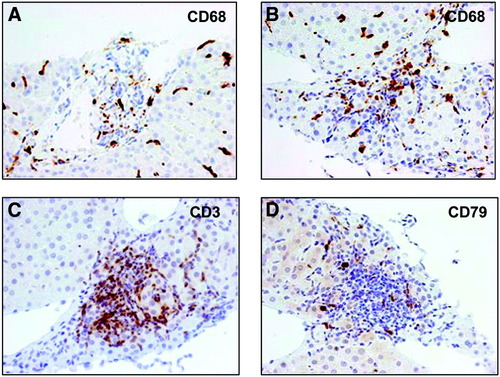
Immunohistochemical staining of liver sections. CD68+ monocytes infiltrating the portal tract in the liver sections from two AIH patients with inactive (A) or active (B) disease. (C, D) infiltrating T (CD3+) and B (CD79+) lymphocytes in the biopsy of the patient with active disease; original magnification ×40.
T-reg Phenotype.
In both AILD and healthy subjects, cells with high expression of CD25 (CD25high), CD45RO (CD45ROhigh), and CD62L (CD62Lhigh) and low expression of CD154 (CD154low) were also positive for FOXP3, whose expression was lower in patients than in healthy subjects, in accordance with our previous findings.7 The frequency of CD127+ cells within T-regs and the density of CD127 molecules on individual cells (expressed as mean fluorescence intensity) were higher in AILD (38.4% ± 6.9 and 28.1 ± 7.7) than in healthy subjects (19.3% ± 5% and 16.2 ± 3.2; P = 0.03 for both). Figure 2 shows the expression of conventional T-reg markers in one representative ID AIH-1 patient and in one healthy subject.
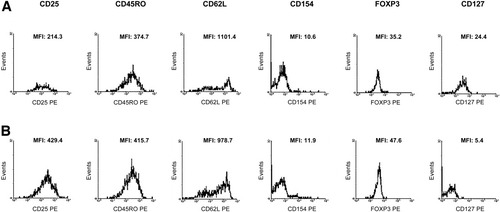
Phenotype of purified CD4+CD25+ T cells. (A) Expression of cT-reg markers in one representative AIH patient with inactive disease and (B) in one representative healthy subject. Histograms represent the mean fluorescence intensity of CD25, CD45RO, CD62L, CD154, FOXP3, and CD127 within CD4+CD25+ T cells. In both patient and healthy subjects, purified CD4+CD25+ T cells were CD25high, CD45ROhigh, CD62Lhigh, and CD154low. FOXP3 mean fluorescence intensity was lower in AIH than in health, whereas CD127 mean fluorescence intensity was higher in AIH than in health.
Chemotaxis Assay.
Monocyte migration (%MM) through the transwell membrane was assessed in 22 AILD patients (13 ID, 9 AD) and in 12 healthy subjects (Table 2). In the absence of chemoattractant, the %MM in patients was higher than in healthy subjects, in both ID (P < 0.0005) and AD (P < 0.001). In the presence of chemoattractant, the %MM remained unchanged in ID patients, it decreased in AD patients (1.2-fold, P = 0.07), and it increased in healthy subjects (three-fold, P < 0.0005). In the presence of chemoattractant and after addition of cT-regs, the %MM increased in both ID (1.4-fold, P = 0.05) and AD (1.5-fold, P = 0.08) patients, whereas it decreased in healthy subjects (1.4-fold, P = 0.017). Addition of cT-regs to the lower chamber, separated from the monocytes by the membrane, had no effect on the %MM in either the presence or absence of chemoattractant. Addition of CD4+CD25− T cells instead of cT-regs either directly to the monocytes or into the lower chamber had no effect on the %MM in the presence or absence of chemoattractant. When tT-regs were added to monocytes, %MM in the presence of chemoattractant decreased in both AILD patients (1.2-fold in both ID and AD) and healthy subjects (1.8-fold; P = 0.03), whereas it increased after addition of CD4+CD25+CD127+ T cells in patients (1.3-fold in ID [P = 0.05] and 1.2-fold in AD) and decreased in healthy subjects (1.4-fold [P = 0.06]), though less markedly than in the presence of tT-regs (Table 2).
| % MM (mean ± SEM) | |||||
|---|---|---|---|---|---|
| Absence of Chemoattractant | Presence of Chemoattractant | ||||
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | ||
| Patients with AILD (n = 22) | |||||
| Inactive disease (ID) (n = 13) | 23.9 ± 1.6* | 22.1 ± 2.5 | 31.5 ± 4.9†‡ | 18.3 ± 2.3§,∥ | 28.6 ± 1.8¶,#,**,†† |
| Active disease (AD) (n = 9) | 19.9 ± 3.5‡‡ | 16.4 ± 2.9§§ | 25.2 ± 3.4∥∥,¶¶ | 13.9 ± 2.4## | 20.4 ± 3.3*** |
| Healthy subjects (HS) (n = 12) | 5.9 ± 0.9 | 17.9 ± 2.9††† | 12.5 ± 2.6‡‡‡ | 9.9 ± 2.2§§§ | 12.9 ± 2.8∥∥∥ |
| IDversusHS:*P < 0.0005 | IDversus HS: †P = 0.003 | IDversus HS: §P = 0.026 | IDversus AD: ¶P = 0.05 | ||
| ADversusHS: ‡‡P < 0.001 | ADversus HS: ∥∥P = 0.01 | IDversus AD: #P < 0.001 | |||
- Absence versus presence of chemoattractant before T-reg addition in AD: §§P = 0.07 and in HS: †††P < 0.0005.
- Presence of chemoattractant: Monocytes only versus monocytes plus cT-regs in ID: ‡P = 0.05, AD: ¶¶P = 0.08, and in HS: ‡‡‡P = 0.017.
- Monocytes only versus monocytes plus tT-regs in HS: §§§P = 0.03.
- Monocytes plus cT-regs versus monocytes plus tT-regs in ID: ∥P = 0.032 and in AD: ##P = 0.026.
- Monocytes only versus monocytes plus CD4+CD25+CD127+ T-cells in ID: **P = 0.05 and in HS: ∥∥∥P = 0.06.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells inID: ††P = 0.006 and in AD: ***P = 0.03.
Chemotaxis Assay in the Presence of Matrigel Matrix.
Monocyte migration through the matrigel-coated transwell membrane was tested in 21 AILD patients (11 ID and 10 AD) and in 11 healthy subjects (Table 3). In the absence of chemoattractant, the %MM did not differ significantly between the three groups. In its presence, the %MM increased in ID patients (1.4-fold, P < 0.001) and in healthy subjects (2.7-fold, P = 0.002), whereas it decreased in AD patients (1.3-fold, P = 0.09). In the presence of chemoattractant and after cT-reg addition, the %MM increased marginally in ID (1.1-fold) and in AD (1.3-fold) patients, whereas it decreased in healthy subjects (1.7-fold, P = 0.006). Addition of cT-regs or CD4+CD25− T cells to the lower chamber, separated from the monocytes by the membrane, and of CD4+CD25− directly to monocytes had no effect on the %MM in the presence or absence of chemoattractant. Addition of tT-regs decreased the %MM in both patients (1.2-fold in ID and 1.3-fold in AD) and healthy subjects (1.6-fold, P = 0.09), whereas addition of CD4+CD25+CD127+ T cells increased the %MM in patients (2.3-fold in ID [P < 0.001]; 1.7-fold in AD), leaving it unchanged in healthy subjects (Table 3).
| % MM (mean± SEM) | |||||
|---|---|---|---|---|---|
| Absence of Chemoattractant | Presence of Chemoattractant | ||||
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | ||
| Patients with AILD (n = 21) | |||||
| Inactive disease (ID) (n = 11) | 9.8 ± 0.8 | 13.9 ± 1*,†,‡ | 16.2 ± 1.6§,∥ | 11.3 ± 1.6¶,# | 31.7 ± 0.8**,††,‡‡,§§,∥∥ |
| Active disease (AD) (n = 10) | 8 ± 1.7 | 5.9 ± 1.9¶¶,## | 8 ± 2 | 4.6 ± 2.1*** | 10.1 ± 3.8†††,‡‡‡ |
| Healthy subjects (HS) (n = 11) | 7.4 ± 0.7 | 20.3 ± 3.5§§§ | 12.1 ± 2.8∥∥∥ | 12.7 ± 3¶¶¶ | 21.3 ± 3.1###,**** |
| IDversus AD: *P = 0.001 | IDversus AD: §P = 0.008 | IDversus AD: ¶P = 0.03 | IDversus AD: **P = 0.006 | ||
| IDversus HS: †P = 0.09 | IDversus HS: ∥P = 0.09 | ADversus HS: ***P = 0.05 | IDversus HS: ††P = 0.02 | ||
| ADversus HS: ¶¶P = 0.002 | ADversus HS: †††P = 0.048 | ||||
- Absence versus presence of chemoattractant before T-reg addition in ID: ‡P < 0.001, AD: ##P = 0.09, and in HS: §§§P = 0.002.
- Presence of chemoattractant: Monocytes only versus monocytes plus cT-regs in HS: ∥∥∥P = 0.006.
- Monocytes only versus monocytes plus tT-regs in HS: ¶¶¶P = 0.09.
- Monocytes plus cT-regs versus monocytes plus tT-regs in ID: #P = 0.05.
- Monocytes only versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ‡‡P < 0.001.
- Monocytes plus cT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: §§P < 0.001 and in HS: ###P = 0.049.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ∥∥P < 0.001, AD: ‡‡‡P = 0.04, and in HS: ****P = 0.001.
Quantification of MMP19 Gene Expression.
The MMP19 gene, a surrogate marker of monocyte ability to cross barriers, was quantified in monocytes purified from 19 patients with AILD (9 ID and 10 AD) and in 13 healthy subjects. MMP19 transcripts were detectable in both AILD and healthy subjects, the relative level of MMP19 mRNA in ID being higher than in AD patients (4.9 ± 1.2 versus 2.6 ± 1.1 [P = 0.054]) and similar to that observed in healthy subjects (5.2 ± 1.9). Whereas no correlation between the expression of MMP19 and the ability of monocytes to migrate was noted in AILD, a positive correlation was observed in healthy subjects (R = 0.8, P = 0.016).
TNF-α and IL-10 Intracellular Staining.
The ability of monocytes to produce proinflammatory and anti-inflammatory cytokines, namely, TNF-α and IL-10, and the effect of T-regs on their production were assessed in 23 AILD patients (12 ID and 11 AD) and in 12 healthy subjects. Before LPS stimulation, the percentage of TNF-α–producing and IL-10–producing monocytes was negligible and was not affected by cT-reg addition. After LPS stimulation (Table 4), the frequency of TNF-α–producing monocytes in AD was higher than in ID (P = 0.0001) patients and in healthy subjects (P = 0.017). After cT-reg addition, the frequency of TNF-α–producing monocytes increased in both AILD patients (3.4-fold [P < 0.001] in ID and 1.5-fold [P = 0.01] in AD) and healthy subjects (2.4-fold [P = 0.016]), remaining higher in AD compared with ID (P = 0.022) patients and healthy subjects (P = 0.055).
| % TNF-α−Producing Monocytes (Mean± SEM) | ||||
|---|---|---|---|---|
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | |
| Patients with AILD (n = 23) | ||||
| Inactive disease (ID) (n = 12) | 2.1 ± 0.4*,† | 7.1 ± 0.9‡,§ | 2.9 ± 0.6∥,¶,# | 6.4 ± 1.3**,††,‡‡,§§ |
| Active disease (AD) (n = 11) | 11.3 ± 2∥∥ | 17.3 ± 3.6¶¶,## | 12.6 ± 1.4*** | 27.5 ± 3.5†††,‡‡‡,§§§,∥∥∥ |
| Healthy subjects (HS) (n = 11) | 3.8 ± 0.5 | 9.1 ± 2.1¶¶¶ | 4.2 ± 0.7**** | 11.3 ± 2.3††††,‡‡‡‡ |
| IDversus AD: *P = 0.0001 | ID versus AD: ‡P = 0.022 | ID versus AD: ∥P < 0.001 | ID versus AD: **P = 0.002 | |
| IDversus HS: †P = 0.01 | ADversus HS: ¶¶P = 0.055 | ID versus HS: ¶P = 0.14 | ID versus HS: ††P = 0.03 | |
| ADversus HS: ∥∥P = 0.017 | AD versus HS: ***P < 0.001 | ADversus HS: †††P = 0.004 | ||
- Monocytes only versus monocytes plus cT-regs in ID: §P < 0.001, AD: ##P = 0.01 and in HS: ¶¶¶P = 0.016.
- Monocytes plus cT-regs versus monocytes plus tT-regs in ID: #P = 0.005 and in HS: ****P = 0.044.
- Monocytes only versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ‡‡P = 0.0008, AD: ‡‡‡P = 0.001, and in HS: ††††P < 0.001.
- Monocytes plus cT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in AD: §§§P = 0.07.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: §§P = 0.004, AD: ∥∥∥P = 0.003, and in HS: ‡‡‡‡P = 0.01.
After LPS stimulation, the frequency of IL-10–producing monocytes (Table 5) in AD was higher than in ID (P = 0.034) patients and similar to healthy subjects (P = NS). After cT-reg addition, the frequency of IL-10–producing monocytes increased in both AILD patients (twofold [P = 0.014] in ID; 2.1-fold [P = 0.002] in AD) and healthy subjects (2.6-fold [P = 0.006]). No changes in TNF-α–producing and IL-10–producing monocytes were observed when CD4+CD25− T cells were added instead of cT-regs. Before cT-reg addition, the TNF-α–producing/IL-10–producing monocyte ratio was higher in AILD (1 ± 0.1 in ID and 1.8 ± 0.2 in AD, P = 0.007) than in healthy subjects (0.77 ± 0.1; P = 0.07 and P = 0.0005, respectively); after cT-reg addition, it remained higher in AILD (1.05 ± 0.04 in ID and 1.14 ± 0.1 in AD) than in healthy subjects (0.7 ± 0.1; P = 0.0005 and P = 0.006, respectively).
| % IL-10−Producing Monocytes (Mean± SEM) | ||||
|---|---|---|---|---|
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-cells | |
| Patients with AILD (n = 23) | ||||
| Inactive disease (ID) (n = 12) | 3.3 ± 0.9*,† | 6.7 ± 0.7‡,§,∥ | 5.9 ± 1.7#,**,†† | 2 ± 0.4‡‡,§§,∥∥,¶¶ |
| Active disease (AD) (n = 11) | 6.6 ± 1.1 | 4.2 ± 1.9*** | 9.3 ± 2.2†††,‡‡‡ | 6.4 ± 1.5§§§,∥∥∥ |
| Healthy subjects (HS) (n = 11) | 5.2 ± 0.6 | 13.8 ± 2.9¶¶¶ | 9.5 ± 1.6### | 6.5 ± 1.6****,†††† |
| IDversus AD: *P = 0.034 | IDversus AD: ‡P = 0.002 | ID versus AD: #P = 0.01 | IDversus AD: ‡‡P = 0.039 | |
| IDversus HS: †P = 0.018 | IDversus HS: §P = 0.035 | IDversus HS: **P = 0.008 | ID versus HS: §§P = 0.036 | |
- Monocytes only versus monocytes plus cT-regs in ID: ∥P = 0.014, AD: ***P = 0.002, and in HS: ¶¶¶P = 0.006.
- Monocytes only versus monocytes plus tT-regs in ID: ††P = 0.045, AD: †††P = 0.13, and in HS: ###P = 0.009.
- Monocytes plus cT-regs versus monocytes plus tT-regs in AD: ‡‡‡P = 0.13.
- Monocytes plus cT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ∥∥P = 0.0018, AD: §§§P = 0.019, and in HS: ††††P = 0.045.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ¶¶P = 0.059, AD: ∥∥∥P = 0.018, and in HS: ****P = 0.03.
Addition of tT-regs did not affect the frequency of TNF-α–producing monocytes (Table 4) before T-reg addition, whereas it increased that of IL-10–producing monocytes (1.8-fold in ID [P = 0.045]; 1.4 in AD [P = 0.13]; 1.8-fold in healthy subjects [P = 0.009]) (Table 5). In contrast, addition of CD4+CD25+CD127+ T cells increased the frequency of TNF-α–producing monocytes in both AILD (threefold in ID [P = 0.0008]; 2.4 in AD [P = 0.001]) and healthy subjects (2.9-fold [P < 0.001]) (Table 4) but had no effect on that of IL-10–producing monocytes (Table 5). After tT-reg addition, the TNF-α–producing/IL-10–producing monocyte ratio decreased from 1 ± 0.1 to 0.71 ± 0.2 in ID patients, from 1.8 ± 0.2 to 1.5 ± 0.3 in AD patients and from 0.77 ± 0.1 to 0.44 ± 0.03 (P = 0.02) in healthy subjects. In contrast, after CD4+CD25+CD127+ T cell addition, the TNF-α–producing/IL-10–producing monocyte ratio increased to 2.8 ± 0.1 (P < 0.001) in ID to 5.4 ± 1.2 (P < 0.001) in AD and to 1.8 ± 0.2 (P < 0.001) in healthy subjects.
Monocyte TLR4 Expression.
The expression of monocyte TLR4, whose engagement is key to initiation of adaptive immune responses, was assessed on purified monocytes from 27 patients with AILD (15 ID and 12 AD) and 13 healthy subjects. Irrespective of LPS stimulation, the percentage of TLR4-expressing monocytes was greater than 95% in both AILD patients and healthy subjects. Monocyte TLR4 molecule density before LPS stimulation and in the absence of cT-regs was higher in AILD than in healthy subjects (P = 0.048 in ID and P = 0.03 in AD) (Table 6). After cT-reg addition, the TLR4 density increased more in AILD patients (1.5-fold [P = 0.01] in ID; 2.1-fold [P = 0.01] in AD) than in healthy subjects (1.3-fold [P = 0.015]). No change in monocyte TLR4 expression was observed when CD4+CD25− T cells were added instead of cT-regs. Addition of tT-regs had a negligible effect on monocyte TLR4 expression in AILD and decreased it marginally in healthy subjects (1.2-fold [P = 0.15]), whereas addition of CD4+CD25+CD127+ T cells increased it in both AILD (1.5-fold in ID [P = 0.003]; 1.4-fold in AD [P = 0.001]) and in healthy subjects (1.3-fold [P = 0.04]) (Table 6).
| TLR4 MFI (Mean± SEM) | ||||
|---|---|---|---|---|
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | |
| Patients with AILD (n = 27) | ||||
| Inactive disease (ID) (n = 15) | 41.5 ± 3.1* | 60.7 ± 7.1†,‡,§ | 41.5 ± 2.3∥,¶ | 62.1 ± 5.2#,**,†† |
| Active disease (AD) (n = 12) | 42 ± 2.3‡‡ | 89.9 ± 21.3§§ | 41.7 ± 2.7∥∥,¶¶ | 60.2 ± 2.9##,***,†††,‡‡‡ |
| Healthy subjects (HS) (n = 13) | 33.5 ± 2.7 | 44.8 ± 3.4§§§ | 28.4 ± 2∥∥∥,¶¶¶ | 45.3 ± 4.8###,**** |
| IDversus HS: *P = 0.048 | IDversus AD: †P = 0.051 | IDversus HS: ∥P = 0.018 | IDversus HS: #P = 0.044 | |
| ADversus HS: ‡‡P = 0.03 | IDversus HS: ‡P = 0.056 | ADversus HS: ∥∥P = 0.0013 | ADversus HS: ##P = 0.037 | |
- Monocytes only versus monocytes plus cT-regs in ID: §P = 0.01, AD: §§P = 0.01, and in HS: §§§P = 0.015.
- Monocytes only versus monocytes plus tT-regs in HS: ∥∥∥P = 0.15.
- Monocytes plus cT-regs versus monocytes plus tT-regs in ID: ¶P = 0.019, AD: ¶¶P = 0.05, and in HS: ¶¶¶P = 0.005.
- Monocytes only versus monocytes plus CD4+CD25+CD127+ T-cells in ID: **P = 0.003, AD: ***P = 0.001, and in HS: ###P = 0.04.
- Monocytes plus cT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in AD: †††P = 0.04.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ††P = 0.004, AD: ‡‡‡P = 0.003, and in HS: ****P = 0.007.
After LPS stimulation and in the absence of cT-regs, monocyte TLR4 expression intensity increased in AILD, though significantly only in AD patients (1.2-fold [P = 0.02]), and also in healthy subjects (1.2-fold [P = 0.024]). After LPS stimulation and cT-reg addition, monocyte TLR4 expression increased in both AILD (P = 0.01 in ID and P = 0.004 in AD) and healthy subjects (P = 0.037), this increase being more evident in the former (1.35-fold in ID; 2.1-fold in AD) than in the latter (1.2-fold) (Table 7). Addition of tT-regs had negligible effect on monocyte TLR4 expression in both AILD and healthy subjects (1.2-fold [P = 0.14]). In contrast, addition of CD4+CD25+CD127+ T cells increased monocyte TLR4 expression in both AILD and controls, though more markedly in the former (1.6-fold in ID [P = 0.01]; twofold in AD [P = 0.0007]) than in the latter (1.2-fold [P = 0.04]).
| TLR4 MFI (Mean± SEM) | ||||
|---|---|---|---|---|
| Monocytes Only | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | |
| Patients with AILD (n=27) | ||||
| Inactive disease (ID) (n = 15) | 47.8 ± 3.5 | 64.7 ± 5.4*,† | 48.9 ± 2.9‡,§ | 76.1 ± 14.1∥,¶ |
| Active disease (AD) (n = 12) | 49.6 ± 1.8# | 106.2 ± 19.9**,†† | 47.4 ± 3.8‡‡,§§ | 98.6 ± 23.8∥∥,¶¶,## |
| Healthy subjects (HS) (n = 13) | 42 ± 2.3 | 52.9 ± 4.5*** | 35.4 ± 4†††,‡‡‡ | 50.8 ± 3.3§§§,∥∥∥ |
| ADversus HS: #P = 0.018 | IDversus AD: *P = 0.035 | IDversus HS: ‡P = 0.022 | ADversus HS: ∥∥P = 0.022 | |
| AD versus HS: **P = 0.008 | ADversus HS: ‡‡P = 0.059 | |||
- Monocytes only versus monocytes plus cT-regs in ID: †P = 0.01, AD: ††P = 0.004, and in HS: ***P = 0.037.
- Monocytes only versus monocytes plus tT-regs in HS: †††P = 0.14.
- Monocytes plus cT-regs versus monocytes plus tT-regs in ID: §P = 0.02, AD: §§P = 0.018, and in HS: ‡‡‡P = 0.01.
- Monocytes only versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ∥P = 0.01, AD: ¶¶P = 0.0007, and in HS: §§§P = 0.04.
- Monocytes plus tT-regs versus monocytes plus CD4+CD25+CD127+ T-cells in ID: ¶P = 0.08, AD: ##P = 0.03, and in HS: ∥∥∥P = 0.014.
Table 8 represents a summary of monocyte immune responses in AILD and in health. Results were similar for patients with AIH-1 or autoimmune sclerosing cholangitis.
| Monocytes Only (Absence of Chemoattractant or LPS) | Monocytes Only (Presence of Chemoattractant or LPS) | Monocytes Plus cT-regs | Monocytes Plus tT-regs | Monocytes Plus CD4+CD25+CD127+ T-Cells | |
|---|---|---|---|---|---|
| % MM | |||||
| AILD—Inactive disease | ++ | - | ↑ | ↓ | ↑ |
| AILD—Active disease | ++ | ↓ | ↑↑ | ↓ | ↑ |
| Healthy subjects | + | ↑↑↑↑ | ↓ | ↓↓ | ↓ |
| % MM through matrigel matrix | |||||
| AILD—Inactive disease | + | ↑ | - | ↓ | ↑↑↑ |
| AILD—Active disease | + | ↓ | ↑ | ↓ | ↑↑ |
| Healthy subjects | + | ↑↑↑↑ | ↓↓ | ↓↓ | - |
| % TNF-α−producing monocytes | |||||
| AILD—Inactive disease | ND | + | ↑↑↑↑ | - | ↑↑↑↑ |
| AILD—Active disease | ND | ++ | ↑↑ | - | ↑↑↑ |
| Healthy subjects | ND | + | ↑↑↑ | - | ↑↑↑↑ |
| % IL-10−producing monocytes | |||||
| AILD—Inactive disease | ND | + | ↑↑↑ | ↑↑ | - |
| AILD—Active disease | ND | ++ | ↑↑↑ | ↑ | - |
| Healthy subjects | ND | ++ | ↑↑↑↑ | ↑↑ | - |
| TLR4 MFI | |||||
| AILD—Inactive disease | ++ | NA | ↑↑ | - | ↑↑ |
| AILD—Active disease | ++ | NA | ↑↑↑ | - | ↑ |
| Healthy subjects | + | NA | ↑ | ↓ | ↑ |
| TLR4 MFI in the presence of LPS | |||||
| AILD—Inactive disease | NA | ++ | ↑* | - | ↑↑ |
| AILD—Active disease | NA | ++ | ↑↑↑ | - | ↑↑↑ |
| Healthy subjects | NA | + | ↑† | ↓ | ↑ |
- Values indicated as * refer to monocyte function in the absence of T-regs.
- -, unchanged.
- ↑ or ↓: 1.2 ≤ fold increase/decrease < 1.5.
- ↑↑ or ↓↓ : 1.5 ≤ fold increase/decrease < 2.
- ↑↑↑ or ↓↓↓ : 2 ≤ fold increase/decrease < 2.5.
- ↑↑↑↑ or ↓↓↓↓ : fold increase/decrease ≥ 2.5.
- MM, monocyte migration; ND, not detectable; NA, not applicable.
- * 1.35-fold increase.
- † 1.2-fold increase.
Discussion
This study investigates monocyte immune responses in juvenile AILD and documents important links between these innate immunity cells and T-reg populations. We found that monocytes in AILD are overactivated, this over-activation being enhanced by conventional T-regs and, in contrast to health, not restrained even by true T-regs.
When compared with normal, monocytes in AILD are increased in number and display a more vigorous spontaneous migration that cannot be further augmented by exposure to migration-inducing stimuli such as the chemokine SDF-1α. This chemokine, in fact, induces a reduction in migration ability when added to monocytes obtained in patients with active disease. This reduced migration may be attributable to the fact that monocytes have already undergone maximal activation in vivo, because, when exposed to the high concentration of SDF-1α reported in the plasma of AIH patients,29 they were unresponsive and even inhibited when further stimulated in vitro. A reduction in monocyte migration after SDF-1α addition was again observed during active disease when we evaluated the ability of these cells to pass through barriers, a metalloproteinase-dependent function. The low level of MMP19 during active disease, the principal metalloproteinase favoring monocyte migration through barriers, may account for this.
In addition to their vigorous spontaneous migration, monocytes from AILD patients are skewed toward a proinflammatory phenotype, as indicated by the higher TNF-α over IL-10 production and by the elevated level of expression of TLR4. Monocyte overactivation was more evident in patients with active disease when a higher TNF-α over IL-10 production was noted. The finding of a marked monocyte activation during the active phase of the disease, a time when CD4 and CD8 T cell autoimmune responses are at their highest in terms of proliferation and interferon gamma production,3, 4 suggests a monocyte participation in the pathogenesis of liver damage in AILD, possibly promoted by autoreactive cells belonging to the adaptive arm of the immune system. The correlation between monocyte percentage and biochemical and serological indices of disease activity gives strength to this suggestion, further corroborated by the finding of a sizeable monocyte infiltration within the portal tracts alongside cells of the adaptive immune system (Fig. 1).
In previous studies we showed that cT-regs regulate adaptive immune responses by controlling CD4 and CD8 T cell proliferation and effector function in health, this ability also being detectable but highly reduced in patients with AIH.7 In the current study, we find that cT-regs behave differently when in contact with cells of the innate immune system, because they inefficiently control monocyte function in normal but, unexpectedly, they enhance monocyte activation in AILD. This pattern is clearly and consistently documented by the increase in monocyte migration, TNF-α over IL-10 production, and TLR4 expression after coculture with cT-regs. Two possible non–mutually exclusive explanations can be invoked to explain this apparently paradoxical phenomenon: T-regs may be hampered in their inhibitory activity by the monocytes themselves, or the cT-reg population may contain a subset with nonregulatory but activating functions.
An inhibitory effect of monocytes on T-regs could be attributable to the observed low expression of HLA-DR on monocytes from AILD patients, which may prevent a full engagement of suppressor T cell function. The need for HLA class II products on monocytes for the induction of suppressor T cell function has been demonstrated in patients with multiple sclerosis, in whom monocytes expressing low levels of HLA-DR were able to activate autologous T cell proliferation, but unable to induce suppressive function.30 In keeping with a pathogenic role for reduced HLA-DR monocyte expression is our finding that monocyte HLA-DR expression correlates negatively with biochemical indices of liver damage in AILD, HLA-DR expression being the lowest when the AST and IgG levels are the highest. In addition to low HLA-DR expression, the high levels of TNF-α produced by monocytes during active disease may affect the regulatory function of T-regs. As noted in patients with rheumatoid arthritis, this cytokine, present at high levels in the synovial fluid, can significantly reduce CD4+CD25+ T-reg-mediated suppression.14 That TNF-α may compromise T-reg function was indirectly demonstrated by another study in rheumatoid arthritis, in which treatment with anti–TNF-α led to a significant increase in the number of T-regs in the circulation.31
An enhancement of monocyte proinflammatory profile after cT-reg addition may be explained by the presence of an elevated number of CD127+ cells within cT-regs. After further purification of CD4+CD25high according to CD127 expression, we have defined two functionally distinct CD4+CD25+ T cell populations: a CD4+CD25+CD127− fraction that dampens and a CD4+CD25+CD127+ fraction that stimulates monocyte activation. We show that in health the CD127− fraction exerts a regulatory function on monocytes, being capable of controlling their migration and of increasing their IL-10 production, while leaving unchanged their TLR4 expression. CD25+CD127low/(−) cells were previously shown to express high levels of FOXP3, a finding confirmed by our data (Fig. 2), and, more importantly, to inhibit vigorously proliferation and interferon gamma production by effector CD4 and CD8 T cells.17, 18, 32 In those studies, however, no direct functional comparison between conventional T-regs and their CD25+CD127low(−) subfraction was performed nor was the effect of the CD25+CD127low(−) cells on cells of the innate immune system tested. In our study, in contrast to healthy subjects, the CD127− fraction obtained from AILD patients induced only a marginal increase in monocyte IL-10 production and failed to control monocyte migration.
At variance with the CD127− fraction, the CD127+ population, while retaining the conventional T-reg phenotype, that is, highly expressing CD25, CD45RO, and CD62L markers, was not only ineffective at containing monocyte functions but promoted a more inflammatory profile. In previous investigations conducted on healthy subjects,17, 18 the activating capabilities of the CD25+CD127high T cell subpopulation were documented by showing that the addition of CD25+CD127high T cells markedly increased proliferation of effector CD4 T cells.32 In our study, enhancement of monocyte function after CD4+CD25+CD127+ T cell addition was especially evident in AILD, where these cells promoted monocyte migration, markedly increased TNF-α over IL-10 production, and enhanced TLR4 expression. In this context, it should be noted that CD4+CD25+CD127+ T cells are more frequent in AILD than in health.
The findings of this study are of relevance to the design of immunotherapies aimed at restoring tolerance in autoimmune hepatitis—and possibly other autoimmune disorders—through T-reg infusion. Reduction of inflammation, including the overactivation of monocytes, is a likely prerequisite to therapeutic T-reg infusion. Thus, in a murine model of autoimmune encephalomyelitis,33 the proinflammatory cytokine milieu had to be contained to enable T-regs to dampen efficiently autoimmune reactions in the target organ. Moreover, our data suggest that a further purification step to exclude CD127+ cells within the T-reg population will ameliorate the suppressor function of T-regs.
In conclusion, we provide evidence that in juvenile AILD monocytes are overactivated and that this overactivation is enhanced by cT-regs and inefficiently controlled by true T-regs. It is likely that T-reg impairment leads to loss of immune tolerance and perpetuation of the autoimmune attack by favoring monocyte involvement in the liver damage.
Acknowledgements
The authors thank Ann Rayner, Section of Histopathology, Institute of Liver Studies, for technical assistance with paraffin-embedded liver sections.




