Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver†
Potential conflict of interest: Nothing to report.
Abstract
Ethanol-induced liver injury is characterized by increased formation of reactive oxygen species (ROS) and inflammatory cytokines, resulting in the development of hepatic steatosis, injury, and cell death by necrosis and apoptosis. Thioredoxin (Trx), a potent antioxidant and antiinflammatory molecule with antiapoptotic properties, protects animals from a number of inflammatory diseases. However, the effects of ethanol on Trx or its role in ethanol-induced liver injury are not known. Female C57BL/6 mice were allowed ad libitum access to a Lieber-deCarli ethanol diet with 5.4% of calories as ethanol for 2 days to acclimate them to the diet, followed by 2 days with 32.4% of calories as ethanol or pair-fed control diet. Hepatic Trx-1 was decreased by ethanol feeding; daily supplementation with recombinant human Trx (rhTrx) prevented this ethanol-induced decrease. Therefore, we tested the hypothesis that administration of rhTrx during ethanol exposure would attenuate ethanol-induced oxidative stress, inflammatory cytokine production, and apoptosis. Mice were treated with a daily intraperitoneal injection of either 5 g/kg of rhTrx or phosphate-buffered saline (PBS). Conclusion: Ethanol feeding increased accumulation of hepatic 4-hydroxynonenal protein adducts, expression of hepatic tumor necrosis factor α, and resulted in hepatic steatosis and increased plasma aspartate aminotransferase and alanine aminotransferase. In ethanol-fed mice, treatment with rhTrx reduced 4-hydroxynonenal adduct accumulation, inflammatory cytokine expression, decreased hepatic triglyceride, and improved liver enzyme profiles. Ethanol feeding also increased transferase-mediated dUTP-biotin nick-end labeling-positive cells, caspase-3 activity, and cytokeratin-18 staining in the liver. rhTrx treatment prevented these increases. In summary, rhTrx attenuated ethanol-induced increases in markers of oxidative stress, inflammatory cytokine expression, and apoptosis. (HEPATOLOGY 2009.)
Thioredoxin-1 (Trx-1) is a 12-kDa endogenous protein found in micromolar concentrations in the cytosol of cells.1 Trx-1 interacts with a variety of disulfide/thiols in both prokaryotes and eukaryotes through its highly conserved active site (Cys-Gly-Pro-Cys).2 Once oxidized, Trx-1 can be reduced by thioredoxin reductase.2 Trx-1 has multiple functions in the cell, including antioxidant, antiinflammatory, and antiapoptotic activity. Although the molecular mechanisms for the cellular functions of Trx are complex and not fully understood, the cellular activity of Trx-1 likely involves its interaction with specific signaling cascades. For example, Trx-1 regulates apoptosis by preventing apoptosis signal-regulating kinase 1 (ASK-1), a mitogen-activated kinase kinase kinase (MKKK) in the p38 MAPK signaling cascade, from activating p38 MAPK.3 Under conditions of oxidative stress, Trx-1 translocates into the nucleus and acts as a transcriptional cofactor by modulating the binding activity of redox-sensitive transcription factors, such as p53, nuclear factor-κB, and activating protein-1.1, 4 In the nucleus, Trx-1 also serves as a proton donor for ribonucleotide reductase,4 making Trx-1 critical for DNA synthesis and embryonic development.5
Inflammatory diseases are characterized by increased oxidative stress and inflammatory stress, as well as apoptosis. Overexpression of Trx-1 or administration of recombinant human Trx (rhTrx) improves outcomes in several murine models of inflammatory diseases, such as pancreatitis6 and colitis.7 Transgenic overexpressing Trx mice have lower tissue concentrations of tumor necrosis factor α (TNFα), interleukin-6 messenger (m)RNA, and lower protein concentrations of TNFα, interferon-γ, and macrophage migration inhibitory factor at baseline compared to wild type controls.5, 7 Additionally, mice receiving exogenous Trx-1 and transgenic mice that overexpress Trx-1 are resistant to oxidative damage in response to viral infection.5
One hallmark of alcoholic liver disease (ALD) is increased inflammation. Early stages of ethanol-induced hepatocyte injury are characterized by steatosis, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST), increased formation of reactive oxygen species (ROS)8, 9 and increased expression of inflammatory cytokines.10 Nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase,11, 12 cytochrome P450 2E1 (CYP2E1),13 and xanthine oxidase11 contribute to ROS production after ethanol exposure and contribute, in part, to increased TNFα production after ethanol feeding.10 Mice deficient in the p47phox subunit of NADPH oxidase11 or CYP2E114 have decreases in inflammatory cytokine expression and fatty liver after ethanol exposure. Additionally, TNFα receptor I knockout mice are protected from ethanol-induced liver injury.15 Ethanol exposure also increases hepatocyte apoptotic cell death,16-18 which is likely due, at least in part to, ethanol-induced increases in ROS and TNFα.9, 17
Despite the clear role of ROS in the pathogenesis of ALD, treatment with antioxidants, such as N-acetylcysteine (NAC)19 or vitamin E20 during ethanol exposure, only partially protects against liver damage. Although antioxidants prevent oxidative damage, they do not directly protect against inflammation or apoptosis. In contrast to NAC and vitamin E, Trx has antioxidant properties, as well as specific functions in decreasing inflammatory cytokine production and apoptosis. Therefore, we tested the hypothesis that administration of rhTrx would reduce ethanol-induced liver injury in mice. Importantly, we found that rhTrx prevented ethanol-induced increases in hepatic oxidative stress, inflammatory cytokine production, and apoptosis.
Abbreviations
4-HNE, 4-hydroxynonenal; ALD, alcoholic liver disease; ALT; alanine aminotransferase; ASK-1, apoptosis signal regulating kinase-1; AST, aspartate aminotransferase; Ct, comparative threshold; CYP2E1, cytochrome P450 2E1; ERK, extracellular signal-regulated kinase; GSH, glutathione; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MKKK, MAP kinase kinase kinase; NAC, N-acetylcysteine; NADPH, nicotinamide adenine dinucleotide phosphate, reduced form; PCR, polymerase chain reaction; ROS, reactive oxygen species; rhTrx, recombinant human thioredoxin; TNFα, tumor necrosis factor α; TUNEL, transferase-mediated dUTP-biotin nick-end labeling; Trx, thioredoxin.
Materials and Methods
Materials.
Female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Lieber deCarli high-fat ethanol liquid diet was purchased from Dyets (Bethlehem, PA). rhTrx was purchased from Prospec-Tany Technogene (Rehovot, Israel). Additional information on materials can be found in the Supporting Information.
Ethanol Feeding and Treatment with rhTrx.
All procedures using animals were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Eight to 10-week-old female C57BL/6 mice were housed two per cage in shoebox cages with microisolator lids. Two ethanol-feeding models were used: a chronic and a short-term model. In the chronic model, mice were randomized into either ethanol-fed or pair-fed groups, gradually adapted to increasing ethanol concentrations, and then provided a diet containing 27% calories from ethanol for 4 weeks (see Supporting Information for more details). In order to investigate the early phases of ethanol-induced liver injury, we also used a short-term ethanol feeding model in which mice were exposed to ethanol over 4 days. In the short-term feeding model, the mice were first adapted to the control liquid diet for 2 days and then allowed free access to 5.4% of calories from an ethanol-containing diet for 2 days to acclimate them to the diet, followed by 2 days of 32.4% of calories from ethanol (defined as short-term). In the control diet, ethanol was isocalorically substituted with maltose dextrins. In some experiments, mice were treated with rhTrx during short-term ethanol exposure. In these experiments the mice were handled daily for 1 week prior to the start of the experiment to acclimatize them to the injection procedure. Mice were then randomized into pair-fed vehicle, pair-fed rhTrx, ethanol-fed vehicle, or ethanol-fed rhTrx groups. The half-life of rhTrx is 18 hours (unpubl. obs. by Prospec-Tany). rhTrx was tested for endotoxin (lipopolysaccharide, LPS) using a limulus amebocyte lysate kinetic-QCL kit (Lonza, Basel, Switzerland), and found to have negligible amounts of LPS. Therefore, mice were given 100 μL injections once a day of either sterile phosphate-buffered saline (PBS) or 5 g/kg rhTrx at the time food was supplied. Further details on the ethanol-feeding protocol for the chronic study (Fig. 1) can be found in the Supporting Information. Initial and final body weights did not differ between groups (Table 1).
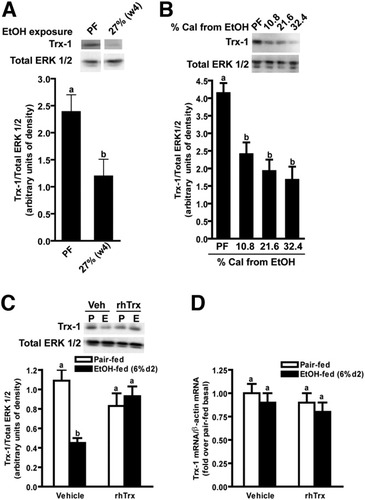
Hepatic Trx-1 protein concentration and mRNA in mice after ethanol feeding. Immunoreactive Trx-1 protein was measured by Western blot in livers from pair-fed control mice and (A) mice allowed free access to an ethanol containing diet (27% of calories from ethanol for 4 weeks) or (B) 5.4% for 2 days followed by 2 days of 10.8%, 21.6%, or 32.4% of calories from ethanol. (C,D) Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle (PBS). Trx-1 protein was measured by Western blot in liver lysates. (D) Trx-1 mRNA was measured by real-time PCR and normalized to β-actin. Values are expressed as fold over pair-fed and were normalized to the ΔCt of pair-fed vehicle. The ΔCt of pair-fed vehicle Trx-1 = 3.4 ± 0.1. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
| Characteristic | Vehicle | rhTrx | ||
|---|---|---|---|---|
| Pair-Fed | EtOH-Fed | Pair-Fed | EtOH-Fed | |
| Body weight Initial (g) | 18.0 ± 0.5 | 18.2 ± 0.4 | 18.7 ± 0.7 | 18.1 ± 0.5 |
| Final (g) | 19.4 ± 0.7 | 18.8 ± 0.0 | 20.0 ± 0.8 | 18.0 ± 0.3 |
| Plasma EtOH (mM) | nd | 49.1 ± 10.6 | nd | 55.2 ± 7.2 |
| Plasma adiponectin (μg/mL) | 47.1 ± 2.6 | 46.8 ± 3.3 | 43.6 ± 6.1 | 47.1 ± 3.6 |
| Plasma GSH (μM) | 29.2 ± 2.3 | 28.7 ± 1.0 | 31.8 ± 1.4 | 26.1 ± 1.1 |
| Plasma homocysteine (μM) | 6.4 ± 0.3a | 14.5 ± 1.1b | 6.7 ± 0.3a | 12.9 ± 1.4b |
- Pair-fed n = 4, ethanol-fed n = 6, ethanol-fed rhTrx n = 5. Values represent means ± SEM. Nd, not determined. Values with different superscripts are significantly different from each other, P < 0.05.
Plasma ALT and AST Activity.
Plasma samples were assayed for ALT and AST using commercially available enzymatic assay kits (Diagnostic Chemicals, Oxford, CT).
Hepatic Triglycerides.
Total liver triglycerides were measured biochemically using the Triglyceride Reagent Kit from Pointe Scientific (Lincoln Park, MI).
Immunohistochemistry.
Immunohistochemical analysis of 4-hydroxynonenal (4-HNE), transferase-mediated dUTP-biotin nick-end labeling (TUNEL), and cytokeratin-18 were performed in liver sections as previously published.21, 22 Additional information can be found in the Supporting Information.
Liver Lysate Preparation.
Frozen liver was homogenized as described.23 Protein concentrations were measured using a BCA kit (Pierce, Rockford, IL). Liver lysates were used to measure hepatic cytokine concentrations, or normalized to protein concentration and prepared in Laemmli buffer and boiled for 5 minutes prior to Western blot analysis.
Western Blotting.
Laemmli buffer samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes for Western blotting. Proteins of interest were detected by Western blotting as described23 using total extracellular signal-regulated kinase (ERK)1/2 as a loading control. Bound antibody was detected by chemiluminescence. Immunoreactive protein quantity was assessed by scanning densitometry. Antibody sources can be found in the Supporting Information.
Hepatic Cytokine Concentration.
Liver lysates were used to measure the concentrations of TNFα by enzyme-linked immunosorbent assay (ELISA); 20 to 30 μg of hepatic protein were used in ELISAs to measure TNFα.
Real-Time Polymerase Chain Reaction (PCR).
Total RNA was isolated and reverse-transcribed and real-time PCR amplification was performed as described.23 The relative amount of mRNA was determined using the comparative threshold (Ct) method by normalizing target cDNA levels to β-actin. Fold induction ratios were calculated relative to pair-fed vehicle conditions using the formula: 2−ΔΔCt. Primer sequences can be found in the Supporting Information.
Caspase-3 Activity.
Caspase-3 activity was measured as described,24 with some modifications. Frozen liver was homogenized, as described above, except that the lysis buffer contained 10 mM Tris-HCl pH 7.5, 10 mM NaH2PO4, 130 mM NaCl, 1% Triton X-100, and 10 mM sodium pyrophosphate. Lysates were incubated on ice for 15 minutes and then centrifuged at 16,000g at 4°C for 15 minutes. Samples were incubated in reaction buffer containing 20 μM Ac-DEVD-AMC for 1 hour at 37°C. Fluorescence was measured at excitation wavelength of 380 nm and an emission range of 430-460 nm.
Serum Glutathione and Homocysteine.
Glutathione and homocysteine were measured using high-performance liquid chromatography with fluorescence as described.25
Statistical Analysis.
Values reported are means ± standard error of the mean (SEM). Data were analyzed by general linear models procedure (SAS, Cary, NC). Data were log-transformed if needed to obtain a normal distribution. Follow-up comparisons were made by least square means testing.
Results
Chronic ethanol feeding (4 weeks at 27% of calories as ethanol) in mice decreased hepatic Trx-1 protein by 50% (Fig. 1A). In a 4- day ethanol-feeding model, Trx-1 protein was reduced by 50% after 2 days on a diet containing 10.8%, 21.6%, or 32.4% of calories as ethanol (Fig. 1B). Daily injections of rhTrx prevented the decrease in hepatic Trx-1 in mice receiving 32.4% ethanol for 2 days (Fig. 1C). Because the anti-Trx-1 antibody recognizes both endogenous and rhTrx forms of Trx-1, the source of hepatic Trx-1 could not be determined. Neither ethanol feeding nor rhTrx administration affected Trx-1 mRNA accumulation, suggesting that the decrease in Trx-1 protein involved posttranscriptional regulation (Fig. 1D).
If the depletion of Trx contributes to the ethanol-induced increase in oxidative stress, inflammation, and apoptosis, then treatment with rhTrx should prevent these effects. Ethanol feeding increased the accumulation of the oxidized lipid-protein adduct, 4-HNE, in the liver. Treatment with rhTrx prevented this increase (Fig. 2A). Induction of CYP2E1 during ethanol exposure is an important contributor to ethanol-induced oxidative stress.9 Short-term ethanol feeding increased immunoreactive CYP2E1 concentrations in the liver compared to pair-fed controls; rhTrx treatment did not prevent this increase (Fig. 2B). Plasma ethanol, measured 2 hours into the dark cycle on the first day of the 32.4% ethanol diet, was ≈50 mM; rhTrx had no effect on plasma ethanol concentration (Table 1). Taken together, these data suggest that rhTrx did not alter ethanol metabolism, but did reduce lipid peroxidation.
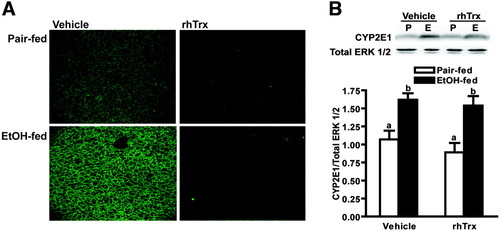
Hepatic 4-HNE and CYP2E1 expression in mice after short-term ethanol feeding. Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle. (A) Immunoreactive 4-HNE adducts were visualized by immunohistochemistry in formalin-fixed liver sections. Images are representative of at least four mice per treatment group and are shown at ×10 magnification. (B) Immunoreactive CYP2E1 protein in liver lysates was assessed by Western blot analysis. Images are representative of at least four mice per treatment group. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
Because increased expression of the proinflammatory cytokine TNFα is critical for hepatic injury in response to ethanol exposure,26 we next investigated the interactions between ethanol and rhTrx treatment on expression of TNFα. Short-term ethanol feeding increased hepatic TNFα mRNA accumulation and protein concentration in mice; rhTrx normalized the effect of ethanol exposure (Fig. 3A,B). Under basal conditions, Trx binds to ASK-1 preventing MAPK activation3; MAPK activation contributes to ethanol-induced increases in TNFα expression.26 Short-term ethanol feeding increased hepatic p38, ERK1/2, and c-Jun N-terminal kinase (JNK) phosphorylation; treatment with rhTrx attenuated this increase (Fig. 4A -C).
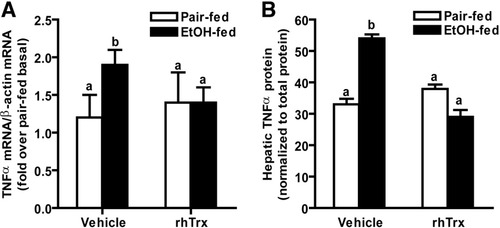
Hepatic TNFα expression after short-term ethanol feeding. Hepatic TNFα (A) mRNA accumulation and (B) protein in vehicle and rhTrx-treated mice after ethanol feeding (short-term). Livers were perfused with saline and frozen in liquid nitrogen or stored at −20°C in RNA later. (A) Total RNA was isolated and accumulation of TNFα mRNA was measured by real-time PCR. TNFα is expressed relative to β-actin mRNA. Values were normalized to ΔCt for pair-fed vehicle. TNFα ΔCt in pair-fed vehicle treated mice was 10.1 ± 0.4. (B) TNFα protein was measured by ELISA. Data expressed relative to pair-fed vehicle. TNFα concentration in pair-fed vehicle treated mice was 36.2 ± 2.7 pg/mg protein. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
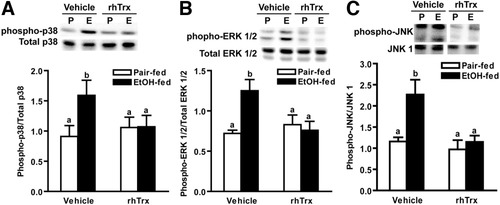
Hepatic MAPK phosphorylation in mice after short-term ethanol feeding. Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle. Immunoreactive phosphorylated (A) p38, (B) ERK1/2, and (C) JNK protein in liver lysates was assessed by Western blot analysis. Inset illustrates a representative blot. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
Kupffer cells are the initial source of cytokine production in response to ethanol exposure.26 Therefore, using a well-characterized model of primary rat Kupffer cells, we investigated the mechanism of action of rhTrx in Kupffer cells from pair- and ethanol-fed rats. Ethanol feeding increased LPS-stimulated TNFα expression by Kupffer cells, associated with increased activation of TLR4-mediated signals, including increased phosphorylation of ERK1/2 and p38, as well as increased Egr-1 expression (supporting data).29 Pretreatment with rhTrx decreased these LPS-stimulated responses and normalized TNFα expression in Kupffer cells from ethanol-fed rats (supporting data).
Oxidative stress and increased inflammatory cytokines are thought to contribute to hepatocyte injury and apoptosis in response to ethanol exposure. Because Trx has antiapoptotic functions,3 we examined whether rhTrx protected mice from ethanol-induced apoptosis. Caspase-3 activity was increased 4-fold by ethanol feeding (Fig. 5A); treatment with rhTrx normalized caspase-3 activity. TUNEL-positive cells were increased in livers of ethanol-fed mice and treatment of mice with rhTrx attenuated this increase (Fig. 5B).
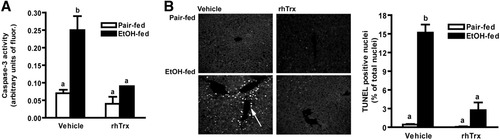
Caspase-3 activity and hepatic TUNEL staining in mouse livers after short-term ethanol feeding. Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle. (A) Caspase-3 activity was detected using the fluorogenic substrate Ac-DEVD-AMC in liver lysates. (B) TUNEL-positive nuclei were visualized in formalin-fixed liver sections. Images are representative of at least four mice per treatment group and are shown at ×40 magnification. Cells that had colocalization of DAPI and TUNEL were quantified. Images are representative of at least four mice per treatment group. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
Hepatocytes are sensitized to TNFα-mediated apoptosis after ethanol exposure27; therefore, using an antibody specific to cytokeratin-18, a caspase cleavage product found only in hepatocytes,22 we assessed the effects of ethanol and rhTrx on hepatocyte apoptosis. Ethanol feeding increased the number of cytokeratin-18-positive cells. In contrast, cytokeratin-18-positive hepatocytes were not detected in mice treated with rhTrx (Fig. 6).
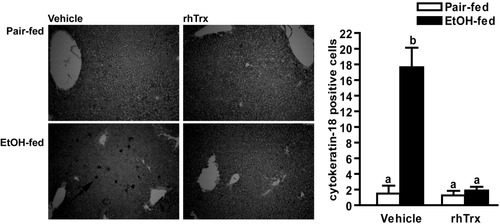
Cytokeratin-18 staining in mice after short-term ethanol feeding. Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle. Cytokeratin-18 positive cells were visualized in formalin-fixed liver sections. Images are representative of at least four mice per treatment group and are shown at ×10 magnification. Quantified cells that were cytokeratin-18-positive. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
Increases in circulating liver enzymes are an indicator of hepatocyte injury. Plasma AST and ALT activities were increased in ethanol-fed vehicle controls when compared to their pair-fed controls (Fig. 7A,B); these concentrations observed after short-term ethanol feeding were comparable to those observed after 6 weeks on the Lieber-deCarli high fat ethanol diet.21, 28 Treatment with rhTrx decreased AST activity by 35% and ALT activity by 25%; however, the difference in ALT was not statistically significant. Short-term ethanol feeding increased plasma homocysteine; rhTrx did not prevent this increase. Plasma GSH was not affected by ethanol or rhTrx (Table 1). Short-term ethanol feeding also resulted in hepatic steatosis. Treatment with rhTrx only modestly decreased total hepatic triglycerides in the ethanol-fed mice (Fig. 7C). Short-term ethanol feeding had no effect on plasma adiponectin (Table 1).
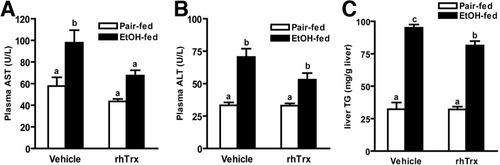
Plasma AST, ALT, and hepatic triglycerides in mice after short-term ethanol feeding. Pair-fed and ethanol-fed mice (short-term) were treated daily with an intraperitoneal injection of either 5 g/kg rhTrx or vehicle. (A) AST and (B) ALT activity was measured enzymatically. (C) Total hepatic triglyceride levels were measured using the Trinder reagent. Values represent means ± SEM. Pair-fed n = 4, ethanol-fed n = 6. Values with different superscripts are significantly different from each other, P < 0.05.
Discussion
Chronic alcohol consumption leads to ALD in ≈20% of individuals.29 Increased production of ROS and TNFα, as well as hepatocyte apoptosis, contribute to the progression of ethanol-induced liver injury.9, 10 Based on the pathophysiology of ethanol-induced liver injury, it is reasonable to hypothesize that effective treatment strategies for ALD need to combat oxidative stress, inflammation, and apoptosis. It is well established that Trx has antioxidant, antiinflammatory, and antiapoptotic properties. The purpose of this study was to test the hypothesis that rhTrx treatment reduces oxidative stress, inflammatory cytokine expression, and apoptosis in mice exposed to short-term ethanol feeding. We found that rhTrx effectively decreased ethanol-induced lipid peroxidation and hepatic inflammatory cytokine concentration, as well as prevented apoptosis.
Previous studies have focused on the potential prophylactic effects of exogenous antioxidants during ethanol exposure, as ethanol compromises endogenous antioxidant systems. For example, treatment with NAC, a precursor to glutathione (GSH), has been investigated in the prevention of ethanol-induced liver disease. Loss of GSH, the most abundant endogenous antioxidant, after chronic ethanol feeding contributes to an increased sensitivity of hepatocytes to TNFα-induced cell death.30 NAC prevents the decrease in GSH, decreases markers of oxidative damage and ALT, but is not able to normalize TNFα mRNA accumulation.19 Similarly, vitamin E supplementation decreases lipid peroxidation, but does not protect from fat accumulation, necrosis, or inflammation in the liver, as assessed by histology.20 Overall, dietary supplements with only antioxidant function offer minimal protection against ethanol-induced liver injury.
Here we report that ethanol exposure depleted hepatic Trx-1 protein; this depletion of hepatic Trx-1 is another indicator of redox imbalance resulting from ethanol exposure. Because Trx-1 has antioxidant, antiinflammatory, and antiapoptotic functions, ethanol-induced decreases in Trx-1 protein would be expected to increase the vulnerability of the liver to injury. Although ethanol decreased Trx-1 protein, there was no effect of ethanol on Trx-1 mRNA, suggesting that the decrease in Trx-1 was posttranscriptionally regulated. Although the mechanism for this decrease is not clear, it is possible that ethanol feeding increases the rate of Trx-1 protein degradation. Under conditions of increased oxidative stress, Trx-1 dimerizes; Trx-1 dimers are more sensitive than the monomer to degradation.31 Although the cytosolic environment is generally a reducing environment, as ethanol is metabolized and free radicals are produced, the resulting shift in redox balance in the hepatocyte to a more oxidized state8 would thus favor Trx-1 dimerization and degradation.
We tested the hypothesis that, like the antioxidants NAC and vitamin E, rhTrx would reduce markers of oxidative stress. Daily injections of rhTrx prevented the ethanol-induced decrease in hepatic Trx-1 protein. During ethanol exposure there is an accumulation of lipid peroxidation products, such as 4-HNE, which is considered a “dosimeter” of oxidative stress.32 4-HNE impairs cellular function through several mechanisms. For example, by covalently binding to proteins, 4-HNE serves as a signal for degradation of proteins by the 26S proteasome.33 4-HNE adducts also induce expression of proapoptotic genes in hepatic stellate cells.34 Oxidized lipid adducts can also trigger opening of the mitochondrial permeability transition pore, resulting in mitochondrial dysfunction and apoptosis in isolated cells.35 Here, treatment with rhTrx reduced 4-HNE adduct accumulation in the livers of ethanol-fed mice, indicating a potential protective effect of rhTrx during alcohol consumption. Reduced 4-HNE adducts in response to rhTrx may be due to decreased production of ROS and/or a reversal of protein oxidation. Unlike most traditional antioxidants that act solely to decrease ROS, Trx can also use its reducing power to reverse protein oxidation. rhTrx may prevent losses in protein function that occur with oxidation, and, therefore, potentially prevent the deleterious downstream consequences of protein oxidation.36
In addition to its antioxidant functions, Trx has antiinflammatory and antiapoptotic properties that could also contribute to the protective effect of rhTrx in the liver during ethanol exposure. Overexpression of Trx-1 and treatment of mice with rhTrx decreases inflammatory cytokine expression.5, 7 By preventing ASK-1 signaling and subsequent activation of p38 MAPK,37 which regulates expression of a number of inflammatory cytokines, Trx-1 acts as an antiinflammatory molecule.3, 37 TNFα is necessary for the progression of ethanol-induced liver injury.10, 26 However, because TNFα is also necessary for hepatocyte proliferation and wound healing after liver injury, therapeutic strategies blocking TNFα activity can have negative side effects.38, 39 rhTrx normalized ethanol-induced increases in mRNA accumulation and protein concentration both in vivo and in isolated Kupffer cells. rhTrx also prevented the phosphorylation of the MAPKs p38, ERK1/2, and JNK, all of which contribute to increased TNFα transcription. These data suggest that rhTrx might be a useful therapeutic approach for normalizing, rather than eliminating, appropriate activation of inflammatory cytokine production during ethanol exposure.
The antiapoptotic properties of Trx are well documented. In the cytosol Trx-1 binds to and inhibits ASK-1 activation.3 Another downstream target of ASK-1 is caspase-3.40 HepG2E47 cells, a hepatocyte line that overexpresses CYP2E1, cultured with ethanol, as well as primary hepatocytes from ethanol-fed rats, are more susceptible to TNFα-induced activation of caspase-3 and apoptosis.27 Ethanol exposure also sensitizes hepatocytes to apoptosis mediated by Fas, an upstream regulator of caspase-3.41 Here we report that short-term ethanol feeding increased caspase-3 activity, as well as TUNEL staining; these increases were normalized by rhTrx treatment. Increased cytokeratin-18 suggests that hepatocytes were a target of ethanol-induced apoptosis.
There are several potential mechanisms by which rhTrx protects hepatocytes from apoptosis. First, rhTrx decreases oxidative damage and TNFα expression, two major contributors to apoptosis.9, 17 Second, rhTrx may also act to protect the mitochondria from ethanol and prevent apoptosis. Ethanol increases mitochondrial depolarization in hepatocytes by releasing cytochrome c, an upstream regulator of caspase-3, into the cytosol.27 Other agents that prevent ethanol-induced apoptosis in liver include betaine and zinc. Supplementation with betaine, a methyl donor for methionine metabolism, prevents apoptosis.42 However, betaine does not prevent ethanol-induced increases in TNFα expression.42 Zinc supplementation prevents both apoptosis and increased TNFα expression,43 at least in part by maintaining the integrity of the intestine during ethanol exposure.44
The earliest hallmark of ethanol-induced liver injury is an accumulation of hepatic triglyceride. Interestingly, whereas rhTrx treatment reduced the impact of ethanol on oxidative stress, inflammation, and cell death in the liver, it had only a modest impact on the ethanol-induced steatosis. Initially, accumulation of triglyceride in the liver results from an inhibition of β-oxidation of fatty acids in the mitochondria due to an increase in the ratio of NADH/NAD during ethanol metabolism.8 After longer periods of ethanol exposure, changes in the expression of genes regulating fatty acid synthesis and oxidation further contribute to hepatic steatosis.45 However, during the short duration of ethanol feeding used in the present study, it is likely that decreases in β-oxidation, rather than changes in gene expression, were responsible for increased hepatic triglycerides, as neither the expression of fatty acid synthase or acetyl co-A carboxylase were affected by short-term ethanol feeding (data not shown).
rhTrx ameliorated ethanol-induced oxidative stress, increased inflammatory cytokine expression, and apoptosis; however, there was only minimal protection from necrosis, as evident from the modest reductions in plasma AST and ALT, suggesting that ethanol feeding caused additional injury not targeted by rhTrx. Ethanol-induced changes in methionine metabolism are one possible mechanism of additional injury, as rhTrx did not affect ethanol-induced increases in circulating homocysteine. Ethanol exposure disrupts normal methionine metabolism, decreasing hepatic S-adenosylmethionine and GSH concentration, and increasing S-adenosylhomocysteine and homocysteine.42 In other disease states, supplementation with NAC and betaine restore balance to this pathway and decreases circulating homocysteine.42, 46, 47 NAC treatment during ethanol exposure restores GSH content in cultured hepatocytes30 and normalizes ALT in vivo,19 suggesting that the imbalance in methionine metabolism contributes to hepatocyte necrosis. S-adenosylmethionine has been successfully used to prevent ethanol-induced liver injury in both mouse48 and rat models49; however, clinical trials in humans are not conclusive and treatment with S-adenosylmethionine is not approved for general use in the treatment of ethanol-induced liver injury.50 Collectively, these data suggest that treatment with rhTrx, which acts to decrease ROS, TNFα expression, and apoptosis, in combination with a treatment that can normalize methionine metabolism and prevent necrosis, might be a useful combination therapy to prevent ethanol-induced liver injury.




