Regulation of human liver δ-aminolevulinic acid synthase by bile acids†
Potential conflict of interest: Nothing to report.
Abstract
Aminolevulinic acid synthase 1 (ALAS1) is the rate-limiting enzyme of heme synthesis in the liver and is highly regulated to adapt to the metabolic demand of the hepatocyte. In the present study, we describe human hepatic ALAS1 as a new direct target of the bile acid–activated nuclear receptor farnesoid X receptor (FXR). Experiments in primary human hepatocytes and in human liver slices showed that ALAS1 messenger RNA (mRNA) and activity is increased upon exposure to chenodeoxycholic acid (CDCA), the most potent natural FXR ligand, or the synthetic FXR-specific agonist GW4064. Moreover, overexpression of a constitutively active form of FXR further increased ALAS1 mRNA expression. In agreement with these observations, an FXR response element was identified in the 5′ flanking region of human ALAS1 and characterized in reporter gene assays. A highly conserved FXR binding site (IR1) within a 175-bp fragment at −13 kilobases upstream of the transcriptional start site was able to trigger an FXR-specific increase in luciferase activity upon CDCA treatment. Site-directed mutagenesis of IR1 abolished this effect. Binding of FXR/retinoid acid X receptor heterodimers was demonstrated by mobility gel shift experiments. Conclusion: These data strongly support a role of bile acid–activated FXR in the regulation of human ALAS1 and, consequently, hepatic porphyrin and heme synthesis. These data also suggest that elevated endogenous bile acids may precipitate neuropsychiatric attacks in patients with acute hepatic porphyrias. (HEPATOLOGY 2007.)
Synthesis of heme is indispensable for life and takes place in every cell of the body except mature erythrocytes. The bulk of heme is synthesized in bone marrow for the production of hemoglobin and in liver, where it is incorporated into various heme proteins, such as cytochromes P450, catalases, peroxidases and respiratory cytochromes.1 Because either excess or deficiency of heme is detrimental to the cell, heme synthesis needs to be tightly controlled. In nonerythroid cells, the rate of synthesis is controlled at its first enzymatic step. Accordingly, the first enzyme of the synthesis pathway, δ-aminolevulinic acid synthase 1 (ALAS1), is highly regulated in different cellular contexts to ensure adequate levels of intracellular heme.1-3 Hereditary partial defects of enzymes of heme synthesis lead to rare metabolic diseases known as porphyrias.4, 5 Inducible, acute hepatic porphyrias are characterized by intermittent attacks of neuropsychiatric dysfunction precipitated by stimuli such as drugs, alcohol, sex steroids, or fasting. These attacks are invariably associated with increased hepatic ALAS1 activity. Acute attacks are treated by withdrawal of the causative agent, high carbohydrate load, and most efficiently by intravenous administration of hemin.
In recent years, progress has been made in understanding the molecular mechanisms of ALAS1 regulation in the liver. Members of the nuclear hormone receptor family of transcription factors, the xenosensors constitutive androstane receptor (CAR) and pregnane X receptor (PXR), were identified to mediate induction of ALAS1 by inducer drugs. We here characterized drug response elements in the far upstream 5′ flanking region of chicken, mouse, and human ALAS1, demonstrating direct transcriptional upregulation of ALAS1 by activators of CAR and PXR.6-8 Furthermore, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a transcriptional coactivator involved in mitochondrial biogenesis and energy homeostasis, was shown to be the master regulator of the fasting response of ALAS1 by acting via an insulin-sensitive Forkhead box protein O1A site in the ALAS1 promoter.9
For many years bile acids were mostly known as important products of cholesterol metabolism, acting as detergents for the intestinal absorption of nutrients. More recently, new biological functions of bile acids as signaling molecules in regulating their own synthesis and controlling lipid and glucose homeostasis have been discovered (for recent reviews see Kalaany and Mangelsdorf10 and Lee et al.11). Bile acid signaling in the liver is mostly mediated via the farnesoid X receptor (FXR), a nuclear receptor of the same subclass as CAR and PXR. The most potent natural ligand of FXR is chenodeoxycholic acid (CDCA), a primary bile acid. FXR heterodimerizes with the retinoid X receptor (RXR) and binds to consensus sequences, most commonly an IR1 (inverted hexameric nucleotide repeat separated by one nucleotide) in the flanking region of its target genes. FXR plays a critical role in bile acid homeostasis by repressing de novo synthesis12 and enhancing the metabolism and excretion of bile acids via transcriptional upregulation of enzymes involved in detoxification such as cytochrome P450 CYP3A4.13 One enzyme that is crucial for detoxification and is therefore coregulated with cytochrome P450 via CAR and PXR is ALAS1, ensuring sufficient heme for newly synthesized apocytochromes. Because bile acids induce heme proteins such as cytochrome P450, we hypothesized that a similar mechanism may affect heme synthesis.
Several lines of evidence indeed suggest such a connection between bile acid homeostasis and the regulation of hepatic heme synthesis. The bile acid precursors 5β-cholestan-3α,7α-diol and 5β-cholestan-3α,7α 12α-triol were shown to induce ALAS1 activity in chicken embryo hepatocytes.14 Moreover, in hepatobiliary diseases accompanied by cholestasis, increased urinary excretion of porphyrins is a common feature.15, 16
In this study, we investigated the effect of bile acids on ALAS1 in 2 human liver culture systems, primary culture of human hepatocytes, and human liver slices. We identified the bile acid–activated nuclear receptor FXR as a regulator of ALAS1, thereby connecting bile acid signaling and hepatic heme synthesis.
Abbreviations
ALAS1, δ-aminolevulinic acid synthase 1; CAR, constitutive androstane receptor; CDCA, chenodeoxycholic acid; FXR, farnesoid X receptor; IR1, inverted hexameric repeat spaced by 1 nucleotide; mRNA, messenger RNA; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; PXR, pregnane X receptor; RXR, retinoid X receptor; SHP, short heterodimer partner.
Materials and Methods
Chemicals.
All chemicals were purchased from Sigma (Buchs, Switzerland) unless stated otherwise. GW4064 was kindly provided by Dr. T. M. Willson (GlaxoSmithKline, Research Triangle Park, NC). Mammalian expression plasmids for human FXR and VP16FXR were as described previously.13 pcDNA3 human PGC-1α was a kind gift from Dr. A. Kralli (The Scripps Research Institute, La Jolla, CA).
Isolation and Culture of Primary Human Hepatocytes.
Cultures of primary human hepatocytes were obtained from patients undergoing liver surgery. All patients gave written consent. Cultures from 7 donors (donors D1–D7 as indicated in the figure legends) were prepared and cultured as described previously.17
Preparation and Culture of Human Liver Slices.
Human liver slices were prepared as described.18 Slices were incubated in the presence or absence of 10 or 100 μM CDCA for 8, 16, and 24 hours; they were then frozen in liquid nitrogen and stored at −80°C until RNA was prepared.
RNA Isolation, Reverse Transcription and Real-Time PCR.
Total RNA was isolated with the Trizol TM reagent (Invitrogen, Basel, Switzerland) and reverse-transcribed, and real-time PCR was performed as described.13 Primers and probes were optimized as indicated in Table 1. The levels of either 18S, cyclophilin, or glyceraldehyde 3-phosphate dehydrogenase were used for normalization.
| Gene | Primers | Purpose |
|---|---|---|
| h18S | 5′-AGTCCCTGCCCTTTGTACACA-3′ F | RT-PCR, Taqman |
| 5′-CGATCCGAGGGCCTCACTA-3′ R | ||
| 5′-CGCCCGTCGCTACTACCGATTGG-3′ Probe | ||
| hCyclophilin | 5′-GGAGATGGCACAGGAGGAA-3′ F | RT-PCR, SYBR |
| 5′-GCCCGTAGTGCTTCAGCTT-3′ R | ||
| hALAS1 | 5′-ATGATGCCAGGCTGTGAGATTT-3′ F | RT-PCR, Taqman |
| 5′-GCTGTTTCGAATCCCTTGGA-3′ R | ||
| 5′-TCTGATTCTGGGAACCATGCCTCCA-3′ Probe | ||
| hSHP | 5′-CTTCAATGCTGTCTGGAGTCCTT -3′ | RT-PCR, SYBR |
| 5′-GCACCAGGGTTCCAGGACTT-3′ | ||
| mGAPDH | 5′-CCAGAACATCATCCCTGCATC-3′ F | RT-PCR, Taqman |
| 5′-GGTCCTCAGTGTAGCCCAAGAT-3′ R | ||
| 5′-CCGCCTGGAGAAACCTGCCAAGTATG-3′ Probe | ||
| mALAS1 | 5′-GGCCTCCCGGTCATCC-3′ F | RT-PCR, Taqman |
| 5′-TGTTCTTAGCAGCATCGGCA-3′ R | ||
| 5′-CTGTCCGAGTCACATCATCCCTGTGC-3′ Probe | ||
| mSHP | 5′-TGAGCTGGGTCCCAAGGA-3′ F | RT-PCR, SYBR |
| 5′-AGGGCTCCAAGACTTCACACA-3′ R | ||
| 1.2 kb prom ALAS1 | 5′-cgGGTACCGAGCTCTCAGACCAAAGCCC-3′ F | Cloning |
| 5′-ccgCTCGAGCAAGTCGAGAAGTCCAAACG-3′ R | ||
| −13 kb ALAS1 | 5′-cgGGTACCGGTGGAGAATCTGAGGTCCA-3′ F | Cloning |
| 5′-ccgCTCGAGCTCTTCCTTGACCACCACCT-3′ R | ||
| Intron 10 ALAS1 | 5′-cgGGTACCAATGGCCTGTCTCTGATTGG-3′ F | Cloning |
| 5′-ccgCTCGAGGCCTCTGAAGGGCTTCAATTA-3′ F | ||
| Mut IR1 | 5′CATGTGTGCCTCTGTGACATCTAGACAGCCCCCGAGGCACGC-3′ | Mutagenesis |
| hA IR1 | 5′-TGTGACAAGGTCACAGCCCCCGAGGCA-3′ | EMSA |
| hA mt IR1 | 5′-TGTGACATCTAGACAGCCCCCGAGGCA-3′ | EMSA |
| Perfect IR1 | 5′-GCTTTTAGGTCAATGACCTAGCCCTC-3′ | EMSA |
- For oligonucleotides used for cloning, restriction enzyme cleavage sites and overhang nucleotides are shown in bold and lower case letters, respectively. Only the sense strands are shown for oligonucleotides used in electromobility shift assays. Abbreviations: EMSA, electromobility shift assay; RT-PCR, real-time polymerase chain reaction.
Transfection of Primary Human Hepatocytes.
After overnight plating, cells were transfected with plasmid DNA using Effectene transfection reagent (Qiagen, Hombrechtikon, Switzerland) following the manufacturer's recommendations. Transfection mixes contained 1 μg of plasmid DNA (prepared using Endo Free Plasmid Maxi Kit, Qiagen), 8 μL enhancer buffer, and 10 μL Effectene per well to be transfected. Thirty-six hours later, cells were exposed to reagents for 10 hours followed by lysis and RNA preparation.
ALAS Activity Assay.
ALAS enzymatic activity was assayed as described19 with the following modifications. Primary human hepatocytes were plated at a density of 600,000 cells per well in 6-well plates. After culture under serum-free conditions for 12 hours, cells were induced overnight in the presence of 250 μM 4,6-dioxoheptanoic acid with the chemicals as indicated. Induction medium was replaced by 700 μL prewarmed glycine buffer19 and plates were incubated for additional 2 hours at 37°C. The assay was stopped by TCA (5% final) and supernatant was taken for subsequent analysis of aminolevulinic acid production according to Sinclair et al.19 Cells were lysed in standard lysis buffer for protein determination using the Bradford method. Activity was calculated as nanomoles aminolevulinic acid produced per milligram protein per hour and data are given as fold induction relative to vehicle.
In Silico Analysis of Human ALAS1 Flanking Region.
For cross-species sequence comparison, a total of 66.7 kilobase (kb) genomic sequence ranging from the 5′ to the 3′ neighboring annotated gene of human ALAS1 including 43.7 kb 5′-flanking, 16.3 kb genic, and 6.9 kb 3′-flanking sequence was used. Multispecies (human, chimpanzee, mouse, rat, and dog) sequence alignments produced by MULTIZ20 were retrieved from the browser of the Genome Informatics group of the University of California, Santa Cruz (genome.cse.ucsc.edu). A total of 16 blocks of conserved noncoding sequences located from −27 kb to +19kb from the transcriptional start site as identified by MULTIZ were then individually scanned for putative IR1 elements using a NUBIScan algorithm specifically designed for detection of nuclear receptor binding sites21 with a score threshold of >0.62 (www.nubiscan.unibas.ch).
Plasmid Construction.
Fragments of human ALAS1 flanking region were amplified via PCR as described previously.8 Primer sequences are shown in Table 1. The fragments, subfragments, and mutated constructs were cloned into pGL3 tk luciferase vector using standard cloning procedures. The 1.2-kb promoter was cloned into pGL3 basic. All constructs were verified by sequencing.
Transactivation Assay.
Transactivation assays were performed in CV-1 (monkey kidney) cells as described previously.13 All transfections were repeated 3 to 4 times in triplicate or quadruplicate.
Electromobility Shift Assay.
Electromobility shift assays were performed as previously described.22 For supershift experiments, 1 μL of antibody against FXR (Santa Cruz Biotechnology; sc-13063) or 0.5 μL of monoclonal anti-mouse–RXR rabbit antibody (kindly provided by P. Chambon, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Université Louis Pasteur, Illkirch, France) was used.
Animal Experiments.
C57/Bl6 wild-type animals were from a colony maintained at the Biozentrum and treated with GW4064 40 mg/kg as described previously.13 For the cholic acid feeding experiment, animals (n = 5) were either on standard laboratory chow or fed a 1% cholic acid diet for 1 week when animals were killed. Liver tissues were frozen in liquid nitrogen and stored at −80°C until further analysis. All experiments were approved by the Institutional Animal Care.
Isolation and Culture of Primary Mouse Hepatocytes.
The preparation and culture of mouse hepatocytes has been described previously.17
Statistical Analysis.
Statistical significance was defined in 2-tailed Student t test (P < 0.05 or P < 0.01).
Results
Bile Acids Induce ALAS1 Messenger RNA in Cultured Primary Human Hepatocytes.
To study whether hepatic heme synthesis is regulated by bile acids, cultures of human hepatocytes were exposed for 8 hours to the primary bile acids CDCA, cholic acid, and ursodeoxycholic acid (all at 50 μM) and the secondary bile acid lithocholic acid (25 μM) and analyzed for ALAS1 messenger RNA (mRNA) expression. As illustrated in Fig. 1, CDCA, ursodeoxycholic acid, and lithocholic acid—as well as the classical inducer phenobarbital—all increased ALAS1 mRNA expression, whereas cholic acid had no effect. All bile acids but ursodeoxycholic acid induced the expression of short heterodimer partner (SHP), a known bile acid–responsive gene.
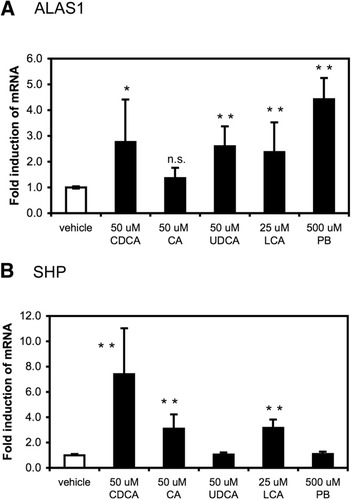
Bile acids induce ALAS1 mRNA expression in cultured primary human hepatocytes. Primary human hepatocytes were prepared and plated as described in Experimental Procedures. After 24-hour incubation in serum-free conditions, cells were challenged with vehicle (DMSO 0.1%); the primary bile acids CDCA, and cholic acid (CA), ursodeoxycholic acid (UDCA) (all at 50 μM); the secondary bile acid lithocholic acid (LCA) (25 μM); or phenobarbital (PB) (500 μM). Eight hours later, RNA was isolated and relative mRNA expression of (A) ALAS1 or (B) short heterodimer partner (SHP) was determined. Values are given as the mean ± standard deviation (2 donors, D1, D2, assays done in triplicate). *P < 0.05, **P < 0.01 (treated versus vehicle control).
Bile Acids Increase ALAS1 Expression in Human Liver Slices.
We next examined if ALAS1 also is induced in a system with intact tissue architecture. We tested the effect of bile acids on gene expression in precision-cut human liver slices, a well-established ex vivo model for liver physiology.23 Slices of 3 different donors were incubated for time intervals of 8, 16, and 24 hours with CDCA at low or near physiological concentration of 10 μM and at the toxic concentration of 100 μM. SHP expression was again measured as a positive control and was found to be increased in all 3 donors at both concentrations tested (data not shown). As depicted in Fig. 2, CDCA treatment increased ALAS1 mRNA expression in all 3 donors. Maximal induction was seen after 16 hours (donor A) or 24 hours (donors B and C). An increase in ALAS1 transcripts could be observed already at 10 μM CDCA (donors A and B at 16 and 24 hours, respectively; donor C at 24 hours) suggesting the physiological relevance of our findings.
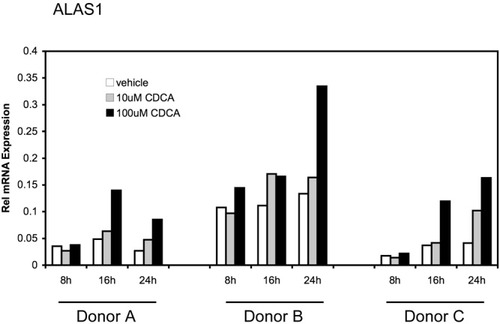
CDCA induces ALAS1 mRNA expression in human liver slices. Human liver slices were prepared as described in Experimental Procedures. Slices of 3 different donors A, B, and C were incubated with vehicle (0.1% DMSO), 10 μM, or 100 μM CDCA. After 8-hour, 16-hour, and 24-hour treatment, RNA was extracted and relative expression levels for ALAS1 were determined. Values are given as means of polymerase chain reaction duplicates for each donor separately.
The FXR Agonist GW4064 and Overexpression of Constitutively Active FXR Increase ALAS1 mRNA.
To prove the involvement of FXR, we used its synthetic selective agonist GW4064. As shown in Fig. 3A, 1 μm of GW4064 significantly increased SHP as well as ALAS1 mRNA expression in cultures of primary human hepatocytes. When primary human hepatocytes were cultured with increasing doses of GW4064, ALAS1 mRNA levels were dose-dependently induced (Fig. 3B). To further confirm the role of FXR in this pathway, the cultures were transiently transfected with a constitutively active form of FXR (VP16 huFXR), and subsequently challenged with GW4064 to achieve maximal induction. As illustrated in Fig. 3C, VP16 huFXR alone or in combination with GW4064 increased ALAS1 mRNA expression as well as the expression of the known FXR targets SHP and bile salt export pump (data not shown). These data confirm the role of FXR in inducing ALAS1.
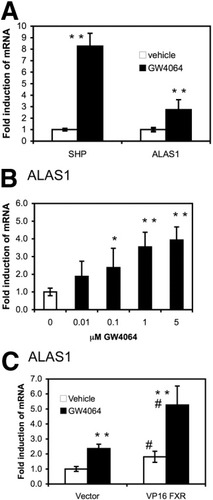
Activation of FXR induces ALAS1 mRNA expression in cultures of primary human hepatocytes. (A, B) GW4064 induces ALAS1 mRNA. Primary human hepatocytes were treated and analyzed as in Fig. 1, except that the specific FXR agonist GW4064 was used at (A) 1 μM or (B) increasing doses from 10 nM to 5 μM. (A) We found that 1 μM GW4064 significantly increased SHP and ALAS1 mRNA expression. Values are given as the mean ± standard deviation (6 donors, D1–D6, assays done in triplicate). (B) GW4064 dose dependently increased ALAS1 mRNA expression. Values are given as the mean ± standard deviation (2 donors, D3, D4). *P < 0.05, **P < 0.01 (GW4064 versus vehicle control). (C) Constitutive active FXR increases ALAS1. After overnight plating, primary human hepatoytes were transfected with VP16 human FXR or the vector control. Thirty-six hours later cells were induced with either vehicle or 1 μM GW4064 for 8 hours when RNA was isolated and relative ALAS1 mRNA expression was determined. Values are given as the mean ± standard deviation (2 donors, D2, D5). *P < 0.05, **P < 0.01 (GW4064 versus vehicle control). #P < 0.01 (VP16 FXR versus vector control).
FXR Activation Increases ALAS1 Enzymatic Activity.
Having shown that activation of FXR increases ALAS1 gene transcription, we investigated whether the activity of the enzyme also was increased. ALAS1 enzymatic activity was assayed after exposure of cultures of primary human hepatocytes to the drugs indicated in Fig. 4, in the presence of the aminolevulinic acid dehydratase inhibitor 4,6-dioxoheptanoic acid, which prevents further usage of aminolevulinic acid. Phenobarbital, as well as natural and synthetic FXR agonists (50 μM CDCA and 1 μM GW4046), increased the enzymatic activity of ALAS1. These data clearly demonstrate that not only ALAS1 mRNA levels but also the corresponding protein and its activity increase upon FXR activation.
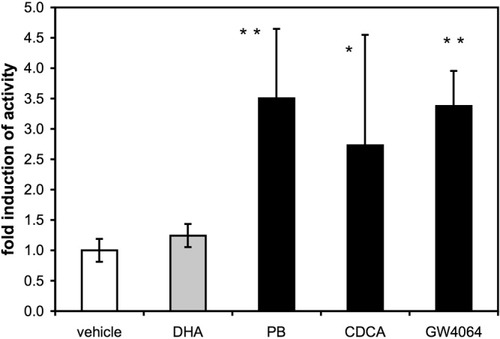
FXR activation increases ALAS activity in cultures of primary human hepatocytes. Cultures of primary human hepatocytes were prepared and plated as described in Experimental Procedures. After 12-hour incubation in serum-free medium, cells were exposed overnight to vehicle (0.15% DMSO), 250 μM 4,6-dioxoheptanoic acid alone or together with 50 μM CDCA, 1 μM GW4064, or 500 μM phenobarbital (PB). Cells were then cultured in reaction buffer for 2 hours when aminolevulinic acid production in the supernatant was measured. Activity was calculated as nanomoles aminolevulinic acid produced per milligram protein per hour. Values are expressed as fold induction and are given as the mean ± standard deviation (2 donors, D6, D7). *P < 0.05, **P < 0.01 (drug treatment versus 4,6-dioxoheptanoic acid control).
Identification of an FXR-Responsive Sequence in the 5′ Flanking Region of ALAS1.
Having established that the human ALAS1 mRNA as well as its enzymatic activity is induced upon FXR activation, we searched for putative FXR response elements in the ALAS1 gene. A combined in silico approach was chosen; a total of 67 kb of sequence containing the human ALAS1 locus was filtered via cross-species sequence comparison to retain only fragments that are conserved between different species. These sequences were then individually scanned for putative FXR binding sites using the NUBIScan algorithm, which is specifically designed to detect nuclear receptor binding sites.21 Two conserved nonprotein-coding regions contained high-scoring FXR response elements (score >0.62). These regions were located at minus 13 kb and within intron 10, respectively (−13 kb ALAS1 and ALAS1 intron).
To see if these regions were able to confer a response to FXR, they were cloned in front of a thymidine kinase–driven luciferase reporter construct for subsequent testing in CV-1 transactivation assays. Because FXR response elements have been identified in proximal promoter regions in other studies, a construct encompassing 1.2 kb of human ALAS1 promoter was included in the analysis, even though no putative FXRE was identified by NUBIScan with a threshold of 0.62.
As illustrated in Fig. 5B, CDCA increased the reporter activity of the 795-base pair (bp), −13-kb ALAS1 construct in the presence of exogenous FXR. In contrast, the promoter construct as well as the construct spanning intron 10 were unaffected by CDCA treatment. To verify if the response of the −13 kb ALAS1 fragment to CDCA was FXR-specific, the fragment was cotransfected with expression constructs of either FXR or PXR in the absence or presence of PGC-1α, a coactivator also shown to coactivate various nuclear receptors, including FXR.24 Again, CDCA augmented reporter gene activity of the −13 kb ALAS1 fragment only when FXR was present. Cotransfection of PXR had no effect on the CDCA response of the fragment. As expected, the CDCA induction of reporter activity via FXR was further increased by PGC-1α up to 5-fold (Fig. 5C).
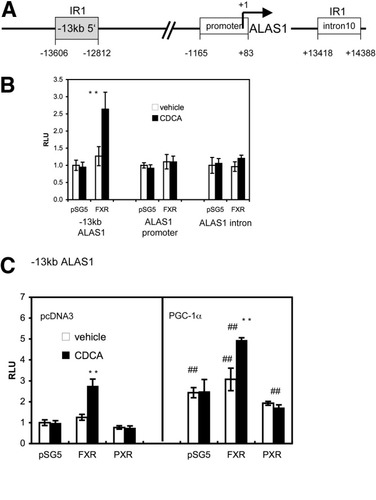
Identification of FXR response element in the 5′ flanking region of human ALAS1. (A) Schematic representation of human ALAS1 gene. The NUBIScan algorithm identified 2 high-scoring IR1 elements within cross-species conserved nonprotein-coding sequences located at −13 kb and within intron 10, respectively. The 1.2-kb promoter construct used in (B) is shown as a white box. (B) The 795-bp, −13-kb ALAS1 construct confers an FXR response. CV1 cells were transfected with the indicated constructs, together with expression plasmids of huFXR or vector control. Twenty-four hours later, cells were treated with vehicle (0.1%DMSO) or 50 μM CDCA for 24 hours. CDCA in the presence of FXR increased the activity of the −13-kb ALAS1 construct but not of the promoter or of the intron construct. (C) FXR but not PXR activates the 795-bp, −13-kb ALAS1 construct. CV1 cells were cotransfected with the −13-kb ALAS1 fragment together with expression plasmids of huFXR or huPXR with or without huPGC-1α. Twenty-four hours later, cells were treated with vehicle (0.1% DMSO) or 50 μM CDCA for 24 hours. CDCA increased the activity of the −13-kb construct only in the presence of FXR and not PXR. This increase was further augmented by the coactivator PGC-1α. Luciferase activity of each construct is expressed in relation to values obtained in DMSO-treated controls and is shown as the ratio of luciferase to β-galactosidase activities. Values are given as the mean ± standard deviation. All transfections were performed in at least 3 different experiments in triplicate or quadruplicate. **P < 0.01 (CDCA versus vehicle control). #P < 0.05, ##P < 0.01 (PGC-1α versus vector control).
To identify the exact sequences conferring the FXR response, the −13 kb ALAS1 construct was digested and the resulting fragments were cloned into the reporter vector as illustrated in Fig. 6A. The 175-bp middle fragment D retained the response to CDCA in the presence of FXR with or without PGC-1α, whereas there was no significant response in the flanking fragments A and C (Fig. 6A). This middle region contains the IR1 element initially identified with NUBIScan (Fig. 6B).
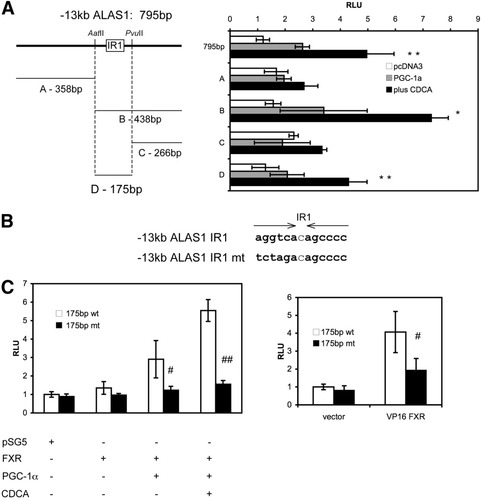
Characterization of a functional IR1 within the −13-kb ALAS1. (A) Subcloning of the 795-bp, −13-kb ALAS1 construct. CV1 cells were cotransfected with the indicated constructs together with expression plasmids of huFXR with or without huPGC-1α. Twenty-four hours later, cells were treated with vehicle (DMSO 0.1%) or 50 μM CDCA and analyzed for luciferase light units relative to β-galactosidase 24 hours later. The 438-bp fragment B and the 175-bp subfragment D retained the response to CDCA and PGC-1α in the presence of FXR, whereas there was no significant response observed in the flanking fragments A and C. (B) Sequence of wild-type and mutated IR1. (C) Site-directed mutagenesis of IR1. CV1 cells were transfected with wild-type or mutated 175-bp fragment together with expression constructs for FXR and PGC-1α and subsequently stimulated with CDCA as in (A) (left panel) or CV1 cells were cotransfected with the constitutively active VP16 FXR (right panel). Mutation resulted in a significant loss of the FXR response to CDCA and PGC-1α (left panel) as well as to VP16 huFXR (right panel). Values are given as the mean ± standard deviation. All transfections were performed in at least 3 different experiments in triplicate. *P < 0.05, **P < 0.01 (CDCA versus vehicle control). ##P < 0.01 (wild-type versus mutated fragment).
To demonstrate the importance of the identified IR1 in the FXR response, site-directed mutagenesis was performed. As compared with the wild-type construct, the response of the mutated 175-bp fragment was reduced when stimulated with CDCA in the presence of FXR (Fig. 6C, left panel). Moreover, the activation of the 175-bp fragment by the constitutively active FXR (VP16 huFXR) was significantly reduced by the mutation (Fig. 6C, right panel). These data clearly demonstrate that the identified region confers a response to FXR.
The FXR/RXR Heterodimer Binds to IR1 in the ALAS1 Enhancer.
To assess whether FXR is able to bind the newly identified functional IR1 element, electromobility shift assays were performed. Incubation of in vitro translated FXR and RXR proteins together with 32P-labeled double-stranded oligonucleotides derived from the ALAS1 responsive sequence (Table 1) or a perfect IR1 as a positive control resulted in a specific DNA protein complex (Fig. 7A, lanes 4 and 15). This binding was successfully competed by the addition of unlabeled oligonucleotide dimers in increasing concentrations (25–200-fold excess) as shown in lanes 5–8 and lanes 10–13. In contrast to this, no competition was observed when the mutated IR1 was used as a competitor (lane 9). The addition of an antibody against FXR abolished the formation of a DNA protein complex at the ALAS1 IR1 and the perfect IR1 (Fig. 7A, lanes 14 and 16; Fig. 7B, lane 3). An anti-RXR antibody successfully super-shifted the DNA protein complex (Fig. 7B, lane 2). Taken together, these data indicate that the FXR/RXR heterodimer binds to the identified ALAS1 IR1.
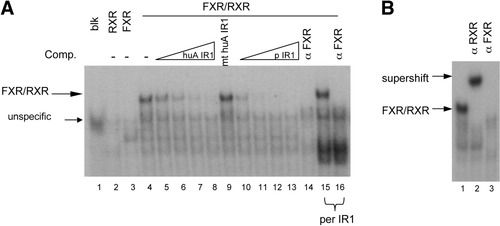
FXR/RXR heterodimer binds to the IR1 element of human ALAS1. Electromobility shift assays were performed using the radiolabeled probes of human ALAS1 (huA IR1) [lanes 1–14 in (A) and lanes 1–3 in (B)] or a perfect IR1 (per IR1) (lanes 15–16). The sequences of the wild-type and mutated probes are listed in Table 1. (A) FXR/RXR heterodimer binds to huA IR1 with high affinity (lane 4). The protein DNA complex disappears with increasing doses of competing wild-type unlabeled probe (lanes 5–8) or unlabeled perfect IR1 (lane 10–13), but not with mutated huA IR1 (lane 9). An antibody against human FXR blocks the formation of an FXR/RXR DNA complex at huA IR1 and at per IR1 [lanes 14 and 16 in (A) and lane 3 in (B)]. The FXR/RXR complex is successfully supershifted by an antibody against RXR [lane 2 in (B)].
FXR Activation of ALAS1 is “Human-Specific” and Does Not Occur in Mice.
To determine if FXR-dependent induction of ALAS1 also occurs in rodents, C57Bl/6 mice were treated with the FXR agonist GW4064 and analyzed for hepatic transcript levels. GW4064 treatment significantly repressed ALAS1 mRNA expression (Fig. 8A). This inhibitory effect on ALAS1 expression was also seen when mice were fed a 1% cholic acid diet for 1 week (Fig. 8B). To exclude that the dissimilar findings in human liver tissue in vitro and in mice in vivo were due to methodological differences, primary mouse hepatocytes were incubated with 50 μM CDCA and analyzed for ALAS1 expression. Again, the known FXR target gene SHP was markedly induced by CDCA treatment, while mouse ALAS1 transcript was significantly repressed (Fig. 8C). Taken together, these data show that both natural and synthetic FXR ligands affect hepatic ALAS1 levels in a species-specific manner. Interestingly, the identified human IR1, even though flanked by sequences showing high cross-species sequence identity, is not conserved in rodents (Fig. 8D).
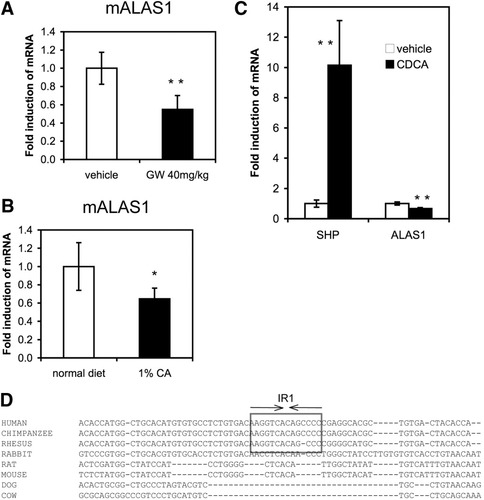
FXR activation of ALAS1 is “human-specific” and does not occur in mice. FXR activation by GW4064 or by cholic acid feeding represses hepatic ALAS1 in mice. (A) C57Bl/6 mice were injected with 40 mg/kg GW4064 intraperitoneally and sacrificed 16 hours later, at which point the livers were excised and analyzed for ALAS1 mRNA expression. GW4064 significantly repressed ALAS1 mRNA expression in mouse liver. (B) C57Bl/6 mice were fed normal chow or a 1% cholic acid diet for 1 week. Hepatic ALAS1 mRNA expression was repressed in mice fed cholic acid (n = 4–5 animals per group). *P < 0.05, **P < 0.01 (treated versus untreated control). (C) CDCA represses ALAS1 mRNA in primary mouse hepatocytes. After 24-hour culturing in serum-free conditions, primary mouse hepatocytes were incubated with either vehicle or 50 μM CDCA for 8 hours, when RNA was extracted and mRNA levels of SHP and ALAS1 were determined. CDCA exposure increased SHP mRNA expression and repressed ALAS1 mRNA expression in mouse hepatocyte culture. Values are given as the mean ± standard deviation in triplicate. **P < 0.01 (CDCA versus vehicle control). (D) The IR1 element is conserved in primate species and is not present in rodents. Multispecies (human, chimpanzee, rhesus monkey, rabbit, mouse, rat, dog, and cow) sequence alignment produced by MULTIZ covering the characterized human IR1 element was retrieved from gemone.cse.ucsc.edu. The functional IR1 element conserved in primates is highlighted by a box.
Discussion
The present study identifies human hepatic ALAS1 as a novel direct target of FXR. The evidence for this finding is as follows. (1) The natural FXR ligand CDCA, as well as the specific agonist GW4064, induce ALAS1 mRNA expression in primary human hepatocytes as well as in human liver slices. (2) ALAS1 enzyme activity is increased upon FXR activation. (3) The human ALAS1 gene contains a highly conserved FXR-binding element (IR1) that is able to confer an FXR response and bind FXR.
ALAS1 is the rate-limiting enzyme of heme synthesis and is subject to various stimuli to adapt to ever-changing demands of the cell for heme. More than 80% of hepatic heme synthesis is required for incorporation of heme into cytochrome P450, the enzyme essential for detoxifying drugs and xenobiotics but also responsible for the synthesis and metabolism of endogenous substances such as steroid hormones, cholesterol, and bile acids.25 Cytochrome P450 CYP3A4 accounts for the metabolism of more than 50% of prescribed drugs and is a prerequisite for the hydroxylation and therefore detoxification of endogenous bile acids. The corresponding gene has also been identified as a direct target of FXR only recently.13 This finding and the identification of other FXR target genes involved in the elimination and metabolism of bile acids, such as UDP-glucuronosyltransferases,26 sulfotransferases,27 or transport proteins, such as multidrug-resistant protein 2,28 illustrate the central role of FXR in coordinating detoxification processes of potentially toxic bile acids. Because functional CYP3A4 contains heme as a prosthetic group, a sufficient supply of the hepatocytes with heme is required whenever bile acids accumulate. In this context, this study proposes a new model of coordinated induction of apocytochrome CYP3A4 and heme synthesis by bile acid–activated FXR.
A similar coordinated response has been suggested for the xenosensors CAR and PXR.8 Activated by drugs, CAR and PXR bind to response elements in the flanking region of genes of the CYP3As and 2B families as well as to ALAS1 drug response elements. Therefore, ALAS1 is not only essential for enzymes involved in the detoxification of exogenous drugs but also for elimination of potentially toxic endogenous bile acids.
Substantial crosstalk between CAR and PXR and bile acid receptor FXR has been established recently.29-32 First, PXR itself is activated by bile acid precursors33 as well as by the secondary toxic bile acid lithocholic acid and its metabolites.34, 35 Second, although different mechanisms are used, all 3 nuclear receptors repress bile acid synthesis12, 36, 37 and share a common set of target genes involved in detoxification and elimination of potentially toxic compounds from the organism. Third, PXR gene transcription has been found to be a direct target of FXR, further supporting the connection between these factors.22 Together, these findings lead to the concept of a multifactorial and elaborate detoxification system the liver has developed to adapt to the accumulation of exogenous and endogenous toxic compounds. FXR activated by low or near physiological concentrations of primary bile acids represents the first line of defense, whereas toxic concentrations of bile acids—in particular the secondary bile acid lithocholic acid—push the response to a maximal level via CAR and PXR. We now add ALAS1 to the target genes, which are concomitantly regulated by CAR, PXR, and FXR.
Although overlapping substrate specificity for FXR and PXR have been reported, a pharmacological discrimination between the 2 nuclear receptors is possible. The induction of ALAS1 by an FXR-specific pathway is underlined by the data of the present study. Relatively low concentrations of CDCA induced ALAS1 transcripts in human liver slices and cultures of primary hepatocytes (10 μM and 50 μM, respectively). Apparently, these concentrations are unable to activate PXR.35 The specific FXR agonist GW4064 induced ALAS1, and transfected exogenous FXR was able to trigger the induction of ALAS1. Taken together, these results support an independent role of FXR in the regulation of ALAS1.
Interestingly, ALAS1 mRNA induction by FXR was not observed in mice. By contrast, FXR activation resulted in a significant repression of ALAS1 mRNA expression in vivo in mouse liver and in primary mouse hepatocytes. The identified human IR1, even though flanked by nucleotides showing high cross-species sequence identity, is indeed not present in rodents (Fig. 8D). This finding reflects the experimentally observed species difference at the genomic nucleotide level. The reason for this species-specific regulation remains speculative; however, the existence of species differences in genes regulated by nuclear receptors including FXR is well documented.10
The activation of human ALAS1 by bile acids is also in line with a well-known but incompletely understood clinical observation: increased urinary porphyrin excretion in humans is commonly observed in hepatobiliary diseases accompanied by cholestasis.15, 16, 38 Impaired biliary excretion of porphyrins in these conditions is one obvious explanation. In humans, the main bile acid retained in cholestasis is CDCA. We identified CDCA as a potent inducer of human ALAS1, which implies that increased porphyrin synthesis in cholestatic conditions may contribute to the phenomenon of elevated urinary porphyrin excretion as has been suggested previously.16, 38 Definite proof of this concept will require measurement of ALAS activity in liver biopsies of those patients.
Clinical implications of the data reported here most likely manifest in situations where ALAS1 is more sensitive to induction, as in acute hepatic porphyrias. Known triggering factors include drugs that induce P450 hemeproteins as well as alcohol, fasting, sex steroids, and all forms of stress.39 Unfortunately, the triggering events of most acute attacks remain obscure. It has long been assumed that ALAS1 is only indirectly upregulated via a diminishing regulatory heme pool upon heme consuming stimuli.1, 2 However, our previous work has established that most precipitating factors directly upregulate ALAS1 at the transcriptional level: phenobarbital type drugs stimulate ALAS1 via PXR and CAR acting through 2 distal enhancer elements.8 Additionally, the coactivator PGC-1α drives fasting response via a Forkhead box protein O1A site,9 probably in concert with a cyclic adenosine monophosphate response,40 within the proximal promoter of ALAS1. With the identification of an enhancer element conferring an FXR-specific response of ALAS1, we propose an additional mechanism having the potential to trigger acute porphyric attacks in susceptible patients.
Acknowledgements
The authors thank the group of G. M. Groothuis, in particular M. G. Elferink for providing samples of human liver slices, Nadia Gorman for information on the ALAS1 activity assay, F. Delobel for the preparation of mouse hepatocytes, A. Kralli and P. Chambon for providing expression plasmids, and M. Podvinec for providing the 1.2-kb human ALAS1 promoter construct.




