Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes†
Potential conflict of interest: Nothing to report.
Abstract
Obesity and the metabolic syndrome are closely correlated with hepatic steatosis. Simple hepatic steatosis in nonalcoholic fatty liver disease can progress to nonalcoholic steatohepatitis (NASH), which can be a precursor to more serious liver diseases, such as cirrhosis and hepatocellular carcinoma. The pathogenic mechanisms underlying progression of steatosis to NASH remain unclear; however, inflammation, proinflammatory cytokines, and oxidative stress have been postulated to play key roles. We previously reported that patients with NASH have elevated serum levels of proinflammatory cytokines, such as interleukin-8 (IL-8), which are likely to contribute to hepatic injury. This study specifically examines the effect of hepatic steatosis on IL-8 production. We induced lipid accumulation in hepatocytes (HepG2, rat primary hepatocytes, and human primary hepatocytes) by exposing them to pathophysiologically relevant concentrations of palmitic acid to simulate the excessive influx of fatty acids into hepatocytes. Significant fat accumulation was documented morphologically by Oil Red O staining in cells exposed to palmitic acid, and it was accompanied by an increase in intracellular triglyceride levels. Importantly, palmitic acid was found to induce significantly elevated levels of biologically active neutrophil chemoattractant, IL-8, from steatotic hepatocytes. Incubation of the cells with palmitate led to increased IL-8 gene expression and secretion (both mRNA and protein) through mechanisms involving activation of nuclear factor kappaB (NF-κB) and c-Jun N-terminal kinase/activator protein-1. Conclusion: These data demonstrate for the first time that lipid accumulation in hepatocytes can stimulate IL-8 production, thereby potentially contributing to hepatic inflammation and consequent liver injury. (HEPATOLOGY 2007.)
Several studies suggest that elevated levels of circulating free fatty acids (FFAs) contribute to the complications of obesity and the metabolic syndrome by promoting excess fat deposition in nonadipose tissues not suited for fat storage, such as the liver.1 A strong correlation exists between obesity and fatty liver (hepatic steatosis); indeed, nonalcoholic fatty liver disease is considered to be the hepatic manifestation of the metabolic syndrome.2 Liver steatosis is often considered a benign condition, but it can progress to nonalcoholic steatohepatitis (NASH), which may be a precursor to more severe liver diseases such as cirrhosis and hepatocellular carcinoma.3
NASH is a common liver disease in the United States, and its prevalence is on the rise worldwide. NASH is characterized by microvesicular and macrovesicular steatosis, inflammation with mixed cellularity including neutrophils, hepatocyte degeneration and injury, and sometimes fibrosis.3 The pathogenesis of NASH and the mechanisms of progression of hepatic steatosis to NASH remain unclear.4 Current understanding supports the “multiple-hit model” for the development of NASH, wherein hepatic steatosis represents the “first hit,” whereas the “second” or “subsequent” hits must induce liver damage and promote inflammation with neutrophil infiltration. The nature of the subsequent hits is hypothesized to include direct lipid toxicity, mitochondrial dysfunction, oxidative stress, and inflammation due to proinflammatory cytokine production.3
One of the key proinflammatory cytokines involved in modulating the inflammatory response is the CXC chemokine interleukin-8 (IL-8), which functions as a critical chemoattractant and activator for neutrophils, basophils, and T cells.5 Elevated levels of IL-8 are associated with several diseases, including obesity, diabetes, atherosclerosis, and various forms of liver injury, suggesting that IL-8 may play a key role in the development and/or progression of these diseases by causing inflammation and tissue injury.6-8 Our group and others have demonstrated that hepatocytes are capable of producing significant amounts of biologically active IL-8, thereby initiating and/or enhancing hepatic inflammation and injury.9, 10 Our group and others have demonstrated that patients with NASH have significantly elevated serum levels of IL-8 compared with healthy individuals, suggesting a role for IL-8 in the pathogenesis of NASH.11, 12
In this study, we used an in vitro model of hepatic steatosis to investigate whether steatosis could play a role in the development of inflammation in the liver through regulation of IL-8 production by hepatic cells (HepG2, primary human, and rat hepatocytes). In rat hepatocytes, we have examined the production of the CXC chemokine CINC-3/Groβ/MIP-2, because it is considered the murine homolog of human IL-8.13 Our data demonstrate that exposure of hepatocytes to pathophysiologically relevant concentrations of palmitic acid results in the production of neutrophil chemoattractant, proinflammatory CXC cytokines, such as IL-8 by human cells and MIP-2 by rat cells. This increase in CXC cytokines induced by free fatty acids may play an important role in inflammatory liver injury and the development of steatohepatitis.
Abbreviations
AP-1, activator protein-1; BSA, bovine serum albumin; CXC, family of chemokines with 2 conserved cysteine residues separated by a single amino acid; DNA, deoxyribonucleic acid; ELISA, enzyme-linked immunosorbent assay; FFA, free fatty acid; GAPDH, glyceraldehyde-3-phosphate-dehydrogenase; HepG2, hepatocytes; IL-8, interleukin-8; JNK, c-Jun N-terminal kinase; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor kappaB; PA, palmitic acid; PCR, polymerase chain reaction; TG, triglyceride.
Materials and Methods
Reagents.
Palmitic acid and common chemicals were purchased from Sigma Aldrich (St. Louis, MO). Anti-human IL-8 antibody was purchased from R&D Systems (Minneapolis, MN). Inhibitors for caspase (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone [z-VAD-fmk]) and JNK (SP600125) were obtained from BIOMOL (Plymouth Meeting, PA). Antibodies for phospho-c-Jun N-terminal kinase (JNK), IκBα and β-actin were purchased from Cell Signaling (Beverly, MA). Cell culture supplies were obtained from Invitrogen (Carlsbad, CA).
Cell Culture.
HepG2, a human hepatoma cell line, was obtained from American Type Culture Collection (Manassas, VA). The cells were maintained and grown as described previously.9 Primary human hepatocytes were obtained from ZenBio (Research Triangle Park, NC) and used according to company instructions. Rat primary hepatocytes were isolated as described earlier.14
Fatty Acid Treatment.
Palmitic acid complexed with bovine serum albumin (BSA) was made as follows: palmitic acid powder was added to a 10% solution of fatty acid free BSA and dissolved by shaking gently overnight at 37°C to yield an 8-mM solution of palmitic acid complexed to BSA. The final molar ratio of free fatty acid to BSA was 5.2:1.
Oil-Red-O Staining.
Subconfluent monolayers of HepG2 cells were exposed to palmitic acid or BSA as a control for 12 hours. Cells were stained with Oil-Red-O to examine the amount of fat accumulation in the cells. Briefly, dishes were washed with cold phosphate-buffered saline and fixed in 10% neutral formalin. After 2 changes of propylene glycol, Oil-Red-O was added with agitation for 7 minutes, followed by washing in 85% propylene glycol. The dishes were then rinsed in distilled water and counterstained with hematoxylin. For each dish, 3 images were photographed, and a representative image is shown.
Human IL-8 Assay and Rat MIP-2 Assay.
IL-8 and MIP-2 were measured in cell-free culture supernatants by highly specific enzyme-linked immunosorbent assay (ELISA) kits as per the manufacturer's directions (Biosource).
Neutrophil Chemotaxis Assay.
This was performed as described previously.9
RNA Isolation, Reverse Transcription Polymerase Chain Reaction, and Real Time Polymerase Chain Reaction Analysis.
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA). Real time polymerase chain reaction (PCR) was performed with an ABI prism 7000 sequence detection system and SYBR green I dye reagent in accordance with manufacturer instructions. All specific primers [human IL-8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), rat CXC-2/MIP-2, and GAPDH] were purchased from SuperArray (Frederick, MD). The relative gene expression for all genes was analyzed using 2−ΔΔCt method by normalizing with GAPDH gene expression in all experiments.
Electrophoretic Mobility Shift Assay.
Electrophoretic mobility shift assay was performed as described earlier.9
Western Blot Analysis.
Total cellular proteins (80 μg) were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis using a BioRad (Hercules,CA) electrophoresis system, followed by immunoblotting according to the manufacturer's instructions. Immunoreactive bands were visualized using enhanced chemiluminescence light detection reagents (Amersham, Arlington Heights, IL).
Intracellular Triglyceride Assay.
Intracellular triglyceride (TG) content was determined by an enzymatic kit (Sigma-Aldrich., St. Louis, MO).
Apoptosis/Deoxyribonucleic Acid Fragmentation Assay.
To measure cell apoptosis, the treated cells were lysed after 24 hours. Deoxyribonucleic acid (DNA) fragmentation was quantified using the cell death ELISA kit (Roche, Indianapolis, IN).
Results
To investigate the relation between hepatic steatosis and IL-8 expression, we used a palmitic acid-induced in vitro fatty liver model. We induced lipid accumulation in hepatic cells by exposing them to pathophysiologically relevant concentrations of the common plasma free fatty acid, palmitic acid (PA), to simulate excessive influx of free fatty acids into hepatocytes. The experiments were performed using palmitic acid complexed with BSA at a free fatty acid to BSA (FFA:BSA) ratio of 5.2:1. The normal physiologic ratio of FFA to albumin is approximately 2:1; however, in disease states, serum FFA levels are elevated, yielding ratios as high as 7.5:1.15 Thus, the experimental conditions used in this study mimic pathophysiological states in which unbound FFA concentrations are high. The concentrations of palmitic acid used here are within the reported pathophysiologic range.16, 17
Exposure to Palmitic Acid Results in Intracellular Lipid Accumulation in Hepatocytes.
Human hepatoma cells (HepG2) were cultured with different concentrations of palmitic acid for varying intervals. As a control, some cells were treated with equimolar BSA carrier protein. Intracellular lipid vacuoles were seen by phase-contrast microscopy and confirmed by Oil Red O staining. Lipid accumulation was observed on addition of PA in a dose-dependent and time-dependent manner. Fig. 1 shows a representative Oil-Red-O staining in which HepG2 cells treated for 24 hours with 0.3 mM palmitic acid exhibited significant lipid droplet accumulation (Fig.1C), while minimal staining for lipids was seen in untreated cells (Fig. 1A) and in cells treated with the BSA control (Fig.1B). Accumulation of lipid droplets was observed equally in almost all PA-treated cells.
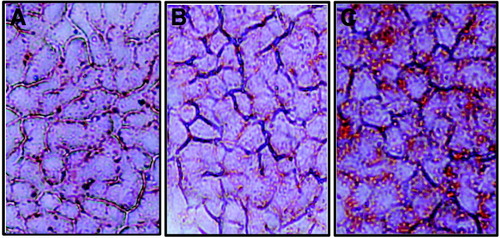
Exposure to palmitic acid results in intracellular lipid accumulation in hepatocytes. HepG2 (human hepatoma) cells were untreated (A), cultured with 0.3 mM BSA control (B) or 0.3 mM palmitic acid (C). After 24 hours, the cells were stained with Oil Red O to measure intracellular lipid accumulation. Cells were examined by light microscopy at a magnification of 50×.
Intracellular Triglyceride Levels Are Increased on Palmitic Acid Supplementation.
An increased availability of FFAs with increased uptake is expected to augment hepatic triglyceride synthesis. We measured intracellular triglyceride content in HepG2 cells that were exposed to 0.3 mM and 0.4 mM palmitic acid for 24 hours (Fig. 2). Compared with untreated cells, palmitate-treated cells accumulated greater than 2.5-fold more TGs. No significant difference was seen between the TG content of cells treated with 0.3 mM and 0.4 mM PA.
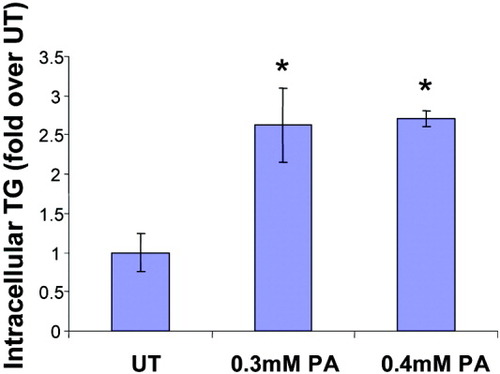
Intracellular triglyceride levels are increased on palmitic acid supplementation. HepG2 cells were untreated (UT) or exposed in triplicate to 0.3 mM and 0.4 mM palmitic acid for 24 hours, and intracellular triglyceride content was measured. Absorbance readings were normalized by protein concentrations and expressed as fold over UT (average untreated absorbance value of 0.140 was set to 1). Data represent mean ± standard deviation, n = 3. *P < 0.05 compared with UT.
Palmitic Acid Exposure Leads to Induction of Biologically Active IL-8 Expression in HepG2 Cells.
We examined the effects of PA on the production of IL-8 by HepG2 cells. Starting at 0.2 mM PA, the cells produced increasing amounts of IL-8 with increasing concentrations of PA, whereas the control BSA supplemented cells did not make any more IL-8 than untreated cells (Fig. 3A). Importantly, the level of IL-8 (483 pg/ml) produced in response to 0.3 mM PA approximated the level induced by 500 U/ml tumor necrosis factor (508 pg/ml), a known inducer of IL-8 in hepatocytes. Additionally, production of IL-8 was detectable starting at 6 hours and reached a maximum value at 48 hours after PA addition (Fig. 3B). These results demonstrate that HepG2 cells express large amounts of IL-8 in response to palmitic acid, in a time-dependent and concentration-dependent manner. The biological activity of the IL-8 produced in response to PA exposure was determined by the neutrophil chemotaxis assay, using cell culture supernatants from untreated HepG2 cells and those treated with 0.3 mM PA. Substantially more (3.5-fold) neutrophil migration was observed in PA-treated cells as compared with untreated cells (Fig. 3C). A “no-HepG2 cell” control demonstrated that PA was not a direct chemoattractant for neutrophils. Neutrophil chemotaxis induced by the PA-treated supernatant was significantly inhibited (approximately 43%) on incubation with anti–IL-8 antibody and not a control IgG (not shown), showing the specific role of IL-8 in this process.
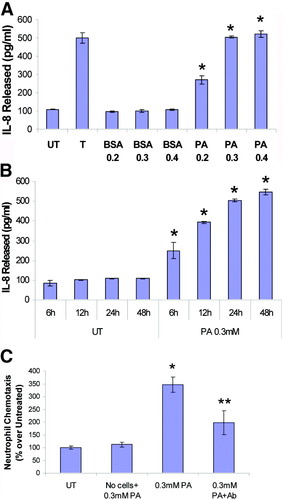
Palmitic acid exposure leads to dose-dependent and time-dependent production of biologically active IL-8. (A) HepG2 cells were untreated (UT), treated with 500 U/ml tumor necrosis factor (T), or exposed to increasing concentrations of palmitic acid (0.2 mM, 0.3 mM, and 0.4 mM PA) or BSA carrier control (0.2 mM, 0.3 mM, 0.4 mM BSA) for 24 hours. (B) HepG2 cells were untreated (UT) or exposed to 0.3 mM palmitic acid for 6 hours, 12 hours, 24 hours, or 48 hours. Cell-free culture supernatants were assayed for IL-8 by commercial ELISA kit. The data represent a mean of 3 experiments ± standard deviation. *P < 0.05 compared with UT. (C) The biological activity of the IL-8 produced in response to palmitic acid exposure was determined by the neutrophil chemotaxis assay using cell-free culture supernatants obtained from untreated HepG2 cells (UT) and those treated with 0.3 mM PA for 24 hours (0.3 mM PA), which contained 483 pg/ml IL-8 as determined by ELISA. The numbers of cells that crossed the membranes were normalized to the untreated values (19 cells, set to 100%) and are expressed as a percentage of UT (mean ± standard deviation, n = 3). PA incubated in culture media without HepG2 cells served as negative control (No cells + PA). Neutrophil chemotaxis induced by the PA-treated culture supernatant was significantly inhibited by IL-8 antibody (0.3 mM PA + Ab) showing specificity. *P < 0.05 compared with UT. **P < 0.05 compared with PA.
Palmitic Acid Supplementation Enhances IL-8 mRNA Levels.
To determine whether the increase in IL-8 expression was a result of induction of the IL-8 gene, steady-state levels of IL-8 mRNA were quantified. Cells were treated for varying amounts of time with 0.3 mM PA, a dose that caused significant IL-8 production, and total RNA was isolated and analyzed by real time-PCR. A time-dependent increase in the steady-state level of IL-8 mRNA was observed starting as early as 2 hours, and reached a maximum (16-fold over UT) at 3 hours after PA addition (Fig. 4). The mRNA level remained almost 3-fold elevated after 24 hours.
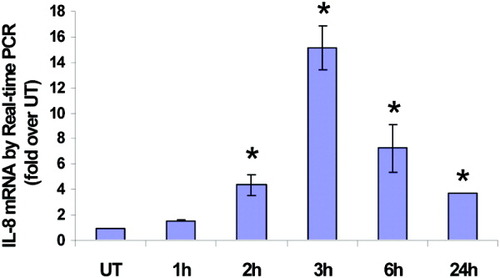
Palmitic acid supplementation increases IL-8 mRNA levels. IL-8 specific mRNA was measured by real-time PCR in untreated cells (UT) and cells treated for varying amounts of time (1 hour, 2 hours, 3 hours, 6 hours, and 24 hours) with 0.3 mM PA. The data are normalized using GAPDH as control and expressed as fold over untreated (mean ± standard deviation, n = 3). P < 0.05 compared with UT.
Exposure to Palmitic Acid Activates Nuclear Factor KappaB and Activator Protein 1.
Because the promoter region of IL-8 contains DNA binding sites for inducible transcription factors, nuclear factor kappaB (NF-κB) and activator protein 1 (AP-1),5 we examined the effect of PA on AP-1 and NF-κB activation. Nuclear extracts from cells treated with 0.3 mM PA for various intervals were analyzed by electrophoretic mobility shift assay (Fig. 5A). Exposure to PA resulted in a dramatic increase in AP-1 activity at 15 minutes of PA exposure and was sustained beyond 90 minutes. The upstream activator of AP-1, namely JNK, was also phosphorylated and activated in a similar manner (Fig. 5B). Nuclear factor kappaB was elevated at 15 and 30 minutes after PA, and returned to baseline at 60 minutes (Fig.5A). Correspondingly, degradation of IκBα was observed at 15, 30 (maximum), and 60 minutes in cytoplasmic extracts by Western blotting (Fig. 5B). Thus, PA induced a substantial increase in NFκB and AP-1 activity in HepG2 cells.
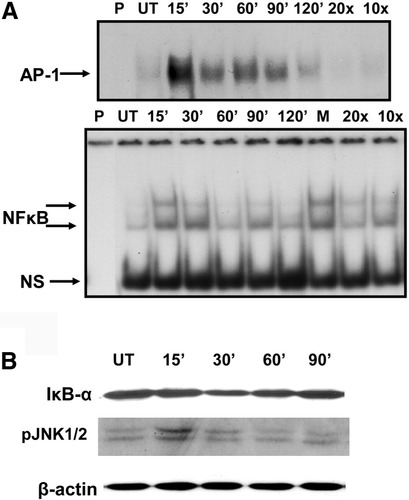
Exposure to palmitic acid upregulates DNA binding activity of AP-1 (by activation of JNK), and NF-κB (by degradation of IκBα). Cells were untreated (UT) and/or treated with 0.3 mM PA for increasing time intervals (15 minutes up to 120 minutes). (A) electrophoretic mobility shift assay to measure DNA binding activity was performed in nuclear extracts. Competition assay was performed to evaluate specificity, using the 15-minute sample, with 10-fold or 20-fold molar excess of unlabeled probe (10× and 20×) or 10-fold excess of mutant probe (M). NS denotes nonspecific binding, and P represents a control with no protein. (B) Western blot analysis of cytoplasmic extracts was used to determine kinetics of degradation of IκBα and activation of JNK 1/2 (as phospho-JNK 1/2). Beta-actin serves as loading control.
Palmitic Acid–Induced IL-8 Production Is Attenuated by Inhibitors of JNK and NF-κB.
We examined the effect of inhibition of AP-1 and NFκB on IL-8 expression in response to PA (Fig. 6). Pharmacological inhibition of JNK and AP-1 activation in HepG2 cells was accomplished by using SP600125, a relatively selective inhibitor of the JNK proteins (JNK1, JNK2, and JNK3). NF-κB activation was blocked using (1) Bay-7085, a specific inhibitor of IκB phosphorylation and degradation, or (2) helenalin, which inhibits NFκB nuclear translocation by alkylating p65. The solvent dimethylsulfoxide (0.1%) was used as a vehicle, and control. PA-stimulated IL-8 production was significantly attenuated by Bay, Helenalin as well as the JNK inhibitor, showing that fatty acid induced IL-8 expression involves activation of NF-κB and AP-1. Dimethylsulfoxide had no effect on PA-induced IL-8 production.
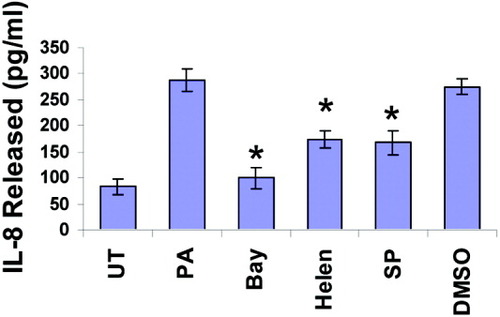
Effect of NF-κB and AP-1 inhibitors on palmitic acid–induced IL-8 production. HepG2 cells were untreated (UT) or exposed to 0.3 mM palmitic acid (PA) for 24 hours, either without or with pretreatment for 2 hours with 10 μM Bay-7085 (Bay), 10 μM Helenalin (Helen), 50 μM SP600125 (SP), or 0.1% dimethylsulfoxide (DMSO, as vehicle control). IL-8 production was measured by ELISA. Each experiment was done in triplicate. Data are mean ± standard deviation. *P < 0.05 compared with PA.
Induction of IL-8 by Palmitic Acid Is Independent of Cell Death.
Apoptotic hepatocytes might function as a trigger for neutrophil transmigration with subsequent hepatotoxicity. Hence, the interdependence, if any, between apoptotic cell death and IL-8 expression was investigated. As reported by Malhi et al,18 palmitic acid was clearly lipotoxic and induced a dramatic, dose-dependent increase in apoptotic cell death in HepG2 cells (Fig. 7A). Interestingly, although PA-induced hepatocyte lipotoxicity was significantly attenuated by the pancaspase inhibitor z-VAD-fmk (Fig. 7B), caspase inhibition had little to no effect on IL-8 expression (Fig. 7C). These data demonstrate that more distal apoptotic signaling events are not causally linked to IL-8 production in this in vitro model of hepatic steatosis and suggest that the inflammatory response to PA is not necessarily dependent on PA-induced lipotoxicity in hepatocytes.
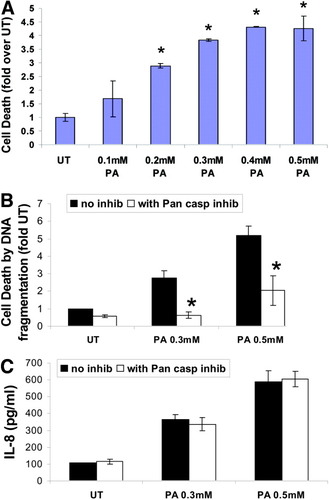
Induction of IL-8 by palmitic acid is independent of cell death. (A) Cells were untreated (UT) or treated with 0.1 mM-0.5 mM PA for 24 hours. Apoptotic cell death was measured by DNA ELISA in 3 separate experiments. Data are expressed as mean ± standard deviation (n = 3). *P < 0.05 compared with UT. (B) Cells were untreated (UT) or treated with 0.3 mM PA for 24 hours, with or without pretreatment for 2 hours with z-VAD-fink, a pancaspase inhibitor (50 mM). Apoptotic cell death was measured in 3 separate experiments. Data are expressed as mean ± standard deviation (n = 3). *P < 0.05 compared with treatments without inhibitor. (C) Cells were treated as in (B) and IL-8 released into the cell free culture supernatant was assayed by ELISA. The data are expressed as pg/ml and represented as a mean ± standard deviation from at least 3 experiments.
Primary Hepatocytes Produce Proinflammatory, CXC Cytokines on PA Exposure.
To confirm these findings in noncancerous cell lines, we conducted similar experiments in primary rat and human hepatocytes. Expression of the CXC chemokine CINC-2/MIP-2 (murine homolog of IL-8) was measured at both mRNA and protein levels in freshly isolated rat primary hepatocytes. We observed increased MIP-2 expression on exposure to palmitic acid both at the protein level by ELISA (Fig. 8A) and at the message level by real time PCR (Fig. 8B). Likewise, we treated cryopreserved primary human hepatocytes with palmitic acid (0.3 mM) and examined its effect on IL-8 production by ELISA. Again, we observed that palmitic acid enhanced IL-8 production (Fig. 9), confirming that our findings were common to hepatoma cells as well as primary hepatocytes.
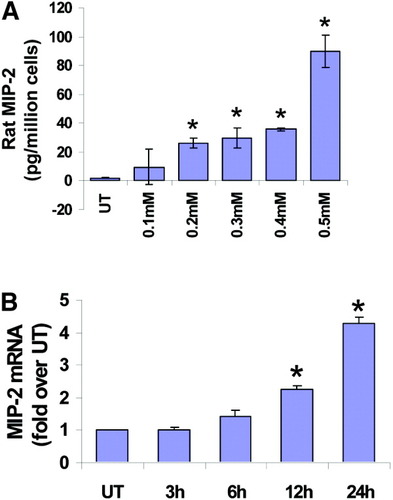
Primary rat hepatocytes express CINC-2/MIP-2 in response to palmitic acid. Freshly isolated rat primary hepatocytes were plated on collagen-coated plates and treated with increasing concentrations of palmitic acid for varying times. (A) MIP-2 levels were measured by ELISA in untreated cells (UT) and cells treated for 24 hours with different doses of PA. The data are expressed as picograms per milliliter cell-free culture supernatant, and represent a mean of 2 experiments ± standard deviation. *P < 0.05 compared with UT. (B) MIP-2 mRNA was examined by real-time PCR in untreated cells (UT) and cells treated for varying amounts of time (1 hour, 2 hours, 3 hours, 6 hours, and 24 hours) with 0.3 mM PA. The data are normalized using GAPDH as control and expressed as fold over untreated (mean ± standard deviation, n = 2). P < 0.05 compared with UT.
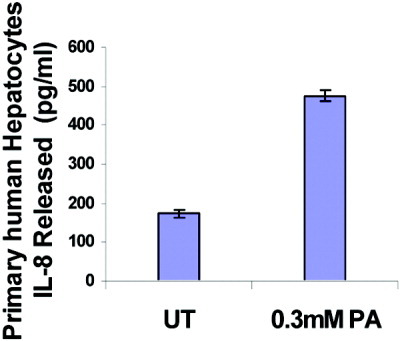
Primary human hepatocytes express IL-8 in response to palmitic acid. Cryopreserved human hepatocytes were treated with 0.3 mM PA for 18 hours, and IL-8 was assayed in culture supernatants by ELISA. The data are expressed as picogram per milliliter cell-free culture supernatant and represent a mean ± standard deviation. *P < 0.05 compared with UT.
Discussion
A strong correlation exists between obesity, increased circulating FFAs, and liver steatosis. Nonalcoholic fatty liver disease patients have increased lipolysis and increased delivery of FFAs to the liver. Higher concentrations of FFAs are associated with more severe liver disease.19, 20 Recently, Nehra et al.21 reported increased serum concentrations of FFAs in patients with nonalcoholic fatty liver disease that correlated with the development of more severe liver disease. Thus, high levels of FFAs may be a contributing factor for the development/progression of NASH.
Fatty acids in the liver are derived through de novo lipogenesis, or circulating FFAs obtained from lipolysis of stored triglycerides (TGs) in adipocytes and dietary fat. Mounting evidence suggests a “2-hit” hypothesis for the development of NASH in which the steatotic liver caused by the accumulation of fat in hepatocytes (first hit) is susceptible to secondary insults, including inflammatory cytokines, that promote hepatocellular damage, inflammation, and progressive liver disease.22, 23 Increased supply of FFAs to the liver may play a major role in the development of hepatic inflammation. Exposure to free fatty acids is known to result in secretion of cytokines from several different cell types.24, 25 Recent work demonstrates that FFAs can induce the activation of the key regulator of inflammation, NF-κB, as well as the inflammatory cytokine tumor necrosis factor alpha, in hepatocytes, while simultaneously causing JNK-dependent lipotoxicity.26
In this study, we supplemented hepatic cells with palmitic acid to mimic the influx of excess FFAs into hepatocytes, giving rise to hepatic steatosis. For most experiments, we used the human hepatoma cell line HepG2, which retains biochemical and morphological properties characteristic of hepatocytes and has proved useful for studying hepatocyte/liver injury and metabolism. Additionally, we have confirmed our major findings in primary rat and human hepatocytes.
Our data demonstrate that exposure of hepatic cells (HepG2) to pathophysiologically relevant concentrations of FFA (palmitic acid) results in increased IL-8 expression and secretion. This is in agreement with several studies that suggest a link between IL-8 and obesity-related complications.6, 27, 28 Importantly, recent evidence from our group11 and others12 shows that IL-8 is elevated in patients with NASH, suggesting that this inflammatory cytokine also may contribute to the development of NASH. Production of IL-8 by hepatocytes is likely to result in recruitment of T cells, and neutrophil activation, transmigration, and binding to adhesion molecules, such as intercellular adhesion molecule 1, on hepatocytes, with subsequent release of toxic factors such as reactive oxygen species and proteases, which can lead to enhanced inflammation and steatohepatitis and potentiate liver injury. Infiltrating neutrophils are known to play a mechanistic and causative role in many forms of liver injury, including those induced by endotoxins/sepsis, ischemia–reperfusion, certain drugs, shock, and alcohol,29 and are likely to be critically important in the development of NASH.
Both NF-κB and AP-1 are known to be important transcription factors for IL-8 expression and appear to regulate IL-8 gene expression in a cell type–specific and stimulus-specific manner. Previous studies have demonstrated both NF-κB-dependent30 and NF-κB-independent31 IL-8 expression. We recently demonstrated that inhibition of proteasome function leads to JNK and AP-1 activation–dependent IL-8 expression in hepatocytes, in the absence of NF-κB.9 In the current study, we found that palmitic acid induces IL-8 expression and activation of both NF-κB and AP-1 in human hepatocytes. The PA-induced IL-8 expression was attenuated by NF-κB and AP-1 inhibitors to a lesser or greater degree, strongly implicating both transcription factors in the regulation of PA-induced IL-8 expression. Further, U0126, a MEK inhibitor, also down regulated PA-induced IL-8 expression (data not shown), suggesting additional complex regulatory mechanisms, which are being studied. Previous work has shown that JNK activation plays a significant role in the development of obesity-related steatosis32 as well as FFA-induced hepatocyte lipotoxicity.18, 33 Our results further show the involvement of FFA-induced JNK in IL-8 production, which may play a contributory role in the evolution of NASH.
Our study clearly demonstrates that exposure to excess FFA (palmitic acid) induces apoptosis and IL-8 production in hepatocytes. The exact relation between IL-8 production, neutrophil infiltration, and hepatocyte apoptosis is not yet clear. Certainly, apoptosis is the mechanism contributing to the progression of many forms of liver disease, including NASH,34, 35 and recent studies demonstrate that several paradigms of hepatic apoptosis are associated with neutrophil infiltration, suggesting that apoptosis may be the trigger for neutrophil transmigration and subsequent parenchymal cell attack.29, 36 Hence, the interrelation between apoptotic cell death and IL-8 production may be an important factor in many forms of hepatotoxicity. Our data suggest that IL-8 expression and apoptotic death caused by excess FFAs are independent, distinct events that work simultaneously to cause liver injury. Thus, exposure to palmitic acid is sufficient, and hepatocyte apoptosis is not essential for IL-8 expression in our in vitro model of hepatic steatosis.
In conclusion, we show for the first time that the common plasma FFA, palmitic acid, stimulates human hepatocytes to produce IL-8 in a dose-dependent and time-dependent manner, via activation of JNK/AP-1, and NF-κB. Our findings suggest that steatotic hepatocytes play an important role in the genesis and progression of NASH during hyperlipidemic conditions via the production of IL-8, leading to neutrophil infiltration and inflammatory liver injury.




