Proteomic profiling of human liver biopsies: Hepatitis C virus–induced fibrosis and mitochondrial dysfunction†
Potential conflict of interest: Nothing to report.
Abstract
Liver biopsies from hepatitis C virus (HCV)-infected patients offer the unique opportunity to study human liver biology and disease in vivo. However, the low protein yields associated with these small samples present a significant challenge for proteomic analysis. In this study we describe the application of an ultrasensitive proteomics platform for performing robust quantitative proteomic studies on microgram amounts of HCV-infected human liver tissue from 15 patients at different stages of fibrosis. A high-quality liver protein database containing 5,920 unique protein identifications supported high throughput quantitative studies using 16O/18O stable isotope labeling in combination with the accurate mass and time (AMT) tag approach. A total of 1,641 liver biopsy proteins were quantified, and analysis of variance (ANOVA) identified 210 proteins exhibiting statistically significant differences associated with fibrosis stage. Hierarchical clustering showed that biopsies representative of later fibrosis stages (for example, Batts-Ludwig stages 3–4) exhibited a distinct protein expression profile, indicating an apparent down-regulation of many proteins when compared with samples from earlier fibrosis stages (for example, Batts-Ludwig stages 0–2). Functional analysis of these signature proteins suggests that impairment of key mitochondrial processes including fatty acid oxidation and oxidative phosphorylation, and response to oxidative stress and reactive oxygen species occurs during advanced stage 3 to 4 fibrosis. Conclusion: The results reported here represent a significant advancement in clinical proteomics providing to our knowledge, the first demonstration of global proteomic alterations accompanying liver disease progression in patients chronically infected with HCV. Our findings contribute to a generally emerging theme associating oxidative stress and hepatic mitochondrial dysfunction with HCV pathogenesis. (HEPATOLOGY 2007.)
Chronic infection with hepatitis C virus (HCV), a bloodborne pathogen persistently infecting more than 170 million individuals worldwide, often leads to progressive liver disease, including fibrosis, cirrhosis, and hepatocellular carcinoma.1 Unfortunately, efforts to manage this growing public health problem have been hindered by the lack of an efficient vaccine and limited efficacy of current treatment modalities (for example, interferon and ribavirin therapy). These observations point to the importance of continued efforts to better understand the molecular mechanisms of viral replication and pathogenesis of HCV-associated disease syndromes. To this end, functional genomics technologies have enormous potential to enhance our understanding of virus–host interactions, as well as providing practical insights that will impact medical practice. The identification and characterization of altered regulatory pathways is expected to provide new insights into HCV infection biology and new avenues for drug and vaccine development. Additionally, the identification of genomic or proteomic signatures that are predictive of clinical events (for example, diagnostic or prognostic biomarkers) or therapeutic response could greatly improve patient screening and treatment regimens.
Proteomic studies of HCV infection have been particularly limited for several reasons, including the need for large amounts of protein for conventional proteomic analysis. Much of the initial effort in this area has therefore centered on the detection and identification of differentially expressed proteins using in vitro systems that support HCV replication and recovery of several hundred micrograms to milligram amounts of protein.2-5 Ultimately, however, the analysis of complex in vivo systems provides the best opportunity to decipher and understand the intricacies of virus–host interactions, including early events such as the innate immune response and the mechanisms by which HCV infection causes chronic liver injury. In this regard, liver biopsy specimens obtained from HCV-infected individuals offer the unique opportunity to study HCV infection and liver disease in a clinically relevant setting. However, the low protein yields (often less than 50 μg total protein) associated with these small clinical specimens requires the use of an ultrasensitive nanoproteomics platform.
We previously demonstrated the successful application of high-resolution Fourier transform ion cyclotron resonance (FTICR) mass spectrometry together with the accurate mass and time (AMT) tag strategy for the identification of thousands of human liver biopsy proteins from microgram amounts of starting material.2 This represents a significant advancement in clinical proteomics, now making it possible to track physiologically relevant protein abundance changes in vivo using small patient samples. We now describe the application of 16O/18O stable isotope labeling together with the AMT tag approach to identify alterations in the proteome of HCV-infected individuals with advanced liver disease and discuss the functional implications of these protein abundance changes.
Abbreviations
AMT, accurate mass and time; ANOVA, analysis of variance; ER, endoplasmic reticulum; FTICR, Fourier transform ion cyclotron resonance; HCV, hepatitis C virus; LC, liquid chromatography; MS/MS, tandem mass spectrometry; NET, LC-normalized elution time; Sp1, specificity protein 1.
Materials and Methods
Sample Preparation.
Tissue from explanted human liver and percutaneous core needle biopsy specimens from HCV-infected patients was obtained according to clinical protocols at the University of Washington Medical Center. Following informed consent at the time of acquisition, all research samples were collected, flash frozen, and stored at −80°C. The study was approved by the Institutional Review Boards for Human Subject Review at both the University of Washington and Pacific Northwest National Laboratory in accordance with federal regulations.
Explanted liver tissue (4 normal liver samples, 3 HCV-negative and 8 HCV-positive cirrhotic liver samples) was Polytron (PowerGene 700; Fisher Scientific, Pittsburgh, PA) homogenized directly in 2.5 ml 50% 2,2,2-trifluoroethanol, dounce homogenized, and clarified by centrifugation at 4,500g for 10 minutes at 4°C. Liver biopsy protein extracts were prepared by Polytron homogenization in 600 μl 50% 2,2,2-trifluoroethanol. All samples were then reduced with 5 mM 2-methyl-2-tert-butylperoxy-propane, diluted 5-fold in 50 mM ammonium bicarbonate (NH4HCO3), pH 7.8, and digested with trypsin (20 μg for liver tissue, 5 μg for liver biopsies) for 3 hours at 37°C. The resulting peptide samples were then snap frozen and stored at −80°C until further processed. Before mass spectrometry analysis, all peptide samples were loaded on a 1-ml solid-phase extraction C18 column (Supelco, Bellefonte, PA) and processed as described.2 Liver tissue samples were reconstituted in 25 mM NH4HCO3, and the peptide concentration was measured using the bicinchoninic acid protein assay from Pierce Biotechnology Inc. (Rockford, IL). Liver biopsy digests remained dried for 16O/18O labeling.
Liver Biopsy Trypsin-Catalyzed 16O/18O Labeling.
Trypsin-catalyzed 16O/18O labeling was performed as described.6 To dissolve the dried peptides, 40 μl acetonitrile was first added to the dried digest followed by the addition of 200 μl 50 mM NH4HCO3 in either 18O-enriched water (95%, Isotec, Miamisburg, OH) or regular 16O water. Peptides from 8 HCV-positive patient control samples and 15 HCV-positive patient test samples (summarized in Table 1) were labeled with 18O and 16O, respectively. After labeling, the samples were centrifuged for 5 minutes at 15,000g, and the supernatants from the 8 control samples were pooled to create a reference sample. Equal amounts of this 18O-labeled reference were combined with an equal amount of each 16O-labeled test sample for analysis by liquid chromatography (LC)-FTICR.
| Patient ID* | Age | Gender | HCV Genotype | Disease Duration† | Fibrosis Stage‡ | Average CV%+SD* |
|---|---|---|---|---|---|---|
| 195 | 49 | M | 2b | 30+ | 0 | 25 |
| 547 | 59 | F | 2b | 30 | 0 | 10 |
| 520 | 50 | M | 2b | 30+ | 0 | 20 |
| 54 | 55 | M | 1a | 20+ | 1 | 11 |
| 456 | 57 | F | 4 | ? | 1 | 14 |
| 373 | 45 | F | 1 | 30 | 1 | 13 |
| 415 | 56 | M | 1a | 25+ | 2 | 11 |
| 442 | 55 | F | 1a | 40? | 2 | 11 |
| 676 | 45 | F | 2b | 20 | 2 | 11 |
| 392 | 55 | M | 1a | 30+ | 3 | 12 |
| 60 | 58 | F | 3a | 40 | 3 | 15 |
| 514 | 52 | M | 1a | 20+ | 3 | 10 |
| 20 | 52 | M | 1a | 31 | 4 | 14 |
| 203 | 63 | F | 2b | 37 | 4 | ND |
| 17 | 49 | M | 3a | 20 | 4 | 13 |
| 570 | 47 | F | ? | 20 | 0 | NA |
| 681 | 43 | M | 1b | 43 | 0 | NA |
| 34 | 61 | F | 2 | 34 | 0 | NA |
| 431 | 35 | F | 1a | 18 | 0 | NA |
| 515 | 44 | F | 3 | ? | 0 | NA |
| 412 | 56 | M | 1b | ? | 0 | NA |
| 506 | 24 | F | 1a | ? | 0 | NA |
| 362 | 34 | F | ? | 8–10 | 0 | NA |
- Abbreviations: ND, no determination was made for sample 203 because only 2 technical replicates were available for analysis; NA, not applicable because these samples represent the material used to create the pooled reference. Control samples that represented the variation in clinical parameters of the test samples were chosen as best as possible based on sample availability.
- † Duration of disease in years.
- ‡ Fibrosis (stage 0–4) was scored using the Batts-Ludwig system.22
- * CV's were calculated for every protein detected in all 3 technical replicates and then an average CV% ± SD was calculated for each liver biopsy sample.
Liver Tissue Peptide LC Separations and Tandem Mass Spectrometry Analysis.
Each explanted liver tissue sample set (normal, HCV-negative, and HCV-positive cirrhotic) was pooled and subjected to strong cation exchange chromatography as described2 for a total of 3 strong cation exchange chromatography separations of 30 fractions each. Each fraction was lyophilized and analyzed by reversed-phase LC-tandem mass spectometry (MS/MS).
Peptide samples were analyzed using a custom-built high-pressure capillary LC system,7 coupled online to a linear ion trap mass spectrometer (ThermoElectron, San Jose, CA) via an electrospray ionization interface as previously described.2, 7 The initial MS scan used a mass-to-charge (m/z) range of 400 to 2,000 after which 10 of the most abundant ions were selected for MS/MS analysis using a collisional energy setting of 35%. Dynamic exclusion of 1 minute was used to prevent repeated analysis of the same high abundant ion. The temperature of the heated capillary and the electrospray voltage were 200°C and 2.2 kV, respectively.
LC-MS/MS Data Analysis.
Sequest analysis software8 was used to match the MS/MS fragmentation spectra from the liver tissue samples with peptides from the International Protein Index human database, 49,161 entries, downloaded April 4, 2005, and which also included the HCV strain 1b protein sequences (10 entries). The criteria selected for filtering followed methods based on a human reverse database false-positive model that has been shown to give approximately 95% confidence for the entire protein dataset.9 Briefly, protein identifications were retained if their identified peptide met the following criteria: (1) Sequest DelCN value of ≥0.10 and (2) Sequest correlation score (Xcorr) ≥ 1.6 for charge state 1+ and full tryptic peptides, no partial tryptic 1+ peptides were included; Xcorr ≥ 2.4 for charge state 2+ and full tryptic peptides, Xcorr ≥ 4.3 for partial tryptic peptides; Xcorr ≥ 3.2 for charge state 3+ and full tryptic peptides, Xcorr ≥ 4.7 for partial tryptic peptides. In an attempt to remove redundant protein identifications in all reported results, the program ProteinProphet was used as a reduction tool.10 All peptides that passed our filter criteria were given the identical score of 1 and entered into ProteinProphet only for redundancy analysis.
Generation of a Peptide AMT Tag Database.
An updated database of identified liver peptides was created based on the results of extensive LC-MS/MS analyses from previous studies of the HCV replicon system2 and the currently reported liver tissue studies. All peptides that met the criteria described with the exception that ΔCn ≥0 were included in the AMT tag database. The peptide retention times from each LC-MS/MS analysis were normalized to a range of 0 to 1 using a predictive peptide LC-normalized elution time (NET) model and linear regression as previously reported.11 Both the calculated accurate monoisotopic mass and NET of the identified peptides were included in the AMT tag database.
LC-FTICR Analysis and Data Processing.
Liver biopsy peptide samples were analyzed in triplicate (1 μg total peptide digest per run), using the same fully automated custom-built capillary LC system as described above, coupled to an 11.5 tesla FTICR mass spectrometer developed in our laboratory.12 The LC-FTICR raw data sets were automatically analyzed using in-house software tools that initially involved a mass transformation or deisotoping step applying ICR2LS, which is based on the THRASH algorithm.13 After ICR2LS analysis, data were processed to yield a quantitative 2-dimensional mass and LC elution time data set as described.6, 14 The peptide sequences of a given feature or pair of features were assigned when the measured mass and NET for each given feature matched the calculated mass and NET of a peptide in the AMT tag database within a 3-ppm mass error and a 3% NET error.
Analysis of Quantitative Proteomic Data.
The abundance ratios (18O/16O) for labeled peptide pairs were accurately computed using an equation as previously reported.6, 15 All ratios corresponding to peptide sequences that overlapped between multiple protein groups (based on the ProteinProphet results described above) were removed because the exact protein source of these peptide sequences is ambiguous. The corresponding 18O/16O intensity data was then loaded into Rosetta Elucidator (Rosetta Biosoftware, Seattle, WA) and an error-model for 18O-labeled FTICR data [similar to the method developed for isotope-coded affinity tag (ICAT) analysis16] was applied to identify proteins that exhibited statistically significant (P < 0.05) changes in abundance compared with the control sample. The correlation coefficient (R2 ± SD) for technical replicates ranged from 0.80 ± 0.05 to 0.91 ± 0.01, with an average of 0.85 ± 0.03 for the entire study (Fig. 1), and the percent coefficient of variation (CV%) ranged from 11%-25%, with an average of 14% ± 4% for the entire study (Table 1). The analytical variability reported here compares favorably with previous proteomic studies.17, 18 The data from technical replicates was subsequently combined using an error-weighted approach in Elucidator to obtain final protein abundance ratios and P-values.
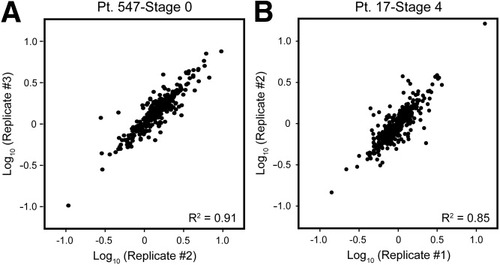
Reproducibility of protein abundance ratios among technical replicates. Data from 3 independent LC-FTICR runs were processed and loaded into Rosetta Elucidator for error modeling and computation of final protein abundance ratios. Shown here are examples of the correlation coefficients obtained during comparison of protein abundance ratios determined from technical replicates of an HCV-infected liver biopsy exhibiting (A) stage 0 or (B) stage 4 fibrosis.
Functional Annotation and Biological Characterization of Proteins.
Protein lists of interest (for example, protein identifications enriched by analysis of liver tissue, disease-associated signature proteins) were characterized in more detail using various functional annotation tools and in-depth pathway and network-oriented analysis tools as described in the Results and Discussion section.
Results and Discussion
Expansion of the Human Liver AMT Tag Database.
Two key goals of our global protein expression profiling efforts are (1) to gain a better understanding of the virus–host interactions that occur during infection and (2) to identify protein markers of HCV-associated liver disease. We anticipated that comparison of liver biopsy data with our original Huh-7.5 HCV replicon-based AMT tag database2 could be incomplete, particularly when searching for protein abundance changes occurring on completion of the viral life cycle and disease progression in liver tissue. To address these issues, we have expanded our human liver protein database using normal and cirrhotic human liver tissue. As summarized in Table 2, analysis of human liver tissue contributed 1,075 new protein identifications to the human liver AMT tag database, bringing the total proteome coverage to 5,920 unique protein identifications. Whereas a single AMT tag is considered sufficient to identify a specific protein or open reading frame, application of even more stringent criterion requiring a minimum of 2 or more AMT tags per open reading frame (e.g., multiple peptide ID) resulted in an overly conservative 3,831 protein identifications. A comprehensive list of all 5,920 proteins identified is provided in Supplementary Table 1.
| HCV Replicon | Liver Tissue | Total Unique | New Identifications | |
|---|---|---|---|---|
| Total* | 4,845 | 3,090 | 5,920 | 1,075 |
| Multiple peptide ID† | 3,038 | 1,669 | 3,831 | 793 |
- * LC-MS/MS data previously generated during analysis of a cell culture model system for HCV replication (2) were re-searched using a more recent version of the IPI human database (see Materials and Methods) that was also used for the current LC-MS/MS analyses of explanted liver tissue. This resulted in the creation of AMT tags identifying 4,845 unique proteins in the HCV replicon and 3,090 unique proteins in explanted human liver tissue. The combined datasets constitute a nonredundant human liver AMT tag database representing 5,920 unique protein identifications, including 1,075 new identifications contributed from analysis of liver tissue.
- † A total of 3,831 of these proteins were identified by at least 2 different tryptic peptides, representing a very high confidence ORF data set. Of these, 793 represent new identifications contributed from analysis of liver tissue.
FatiGO+ (http://babelomics.bioinfo.cipf.es/index.html) comparisons demonstrated that analysis of liver tissue enriched for the identification of proteins associated with several biological processes (for example, cell adhesion, organ development) and cellular components (for example, extracellular matrix, extracellular space) indicative of the greater structural and functional complexity of an organ system. Among the newly identified proteins were several collagens (for example, collagen I, alpha-1 and alpha-2 polypeptides), proteases (for example, elastase 2, matrix metalloproteinase 9, plasmin) and various others (for example, elastin) that function in tissue formation, collagen fibril deposition, and extracellular matrix remodeling. This supplementation of our original Huh-7.5 AMT tag database with proteins detected from normal and cirrhotic liver tissue will prevent skewed comparisons that otherwise may occur during analysis of liver biopsy specimens exhibiting fibrotic alterations in protein levels.
Sensitivity of Quantitative Proteomic Analysis.
Using 16O/18O stable isotope labeling together with the AMT tag approach, we quantified 1,641 liver biopsy proteins for the entire study (see Supplementary Table 2). The number of proteins quantified from analysis of 3 μg total peptide digest (1 μg per run) ranged from 523 to 839 per biopsy, with an average of 705 ± 99. This is in stark contrast to previous studies of the human liver proteome in which the use of less-sensitive proteomic methods typically resulted in the identification of only a few hundred proteins and even fewer protein abundance changes despite the larger quantities (for example, up to milligram amounts) of protein used.5, 19 Of particular relevance is a recent study in which sufficient amounts of liver biopsy protein (for example, 90 μg) supported analysis via 2-dimensional gel electrophoresis in combination with mass spectrometry but resulted in the identification of only 36 protein abundance changes associated with ischemia/reperfusion injury during liver transplantation.19 Taken together, these findings demonstrate that the ultra-high sensitivity of the AMT tag strategy provides a marked advantage for performing global quantitative protein expression profiling studies from low microgram amounts of starting material.
Global Quantitative Comparative Analysis of Liver Biopsy Specimens.
In an effort to identify those protein abundance changes most likely attributable to HCV-associated liver disease (rather than random biological variation), we performed a 1-way analysis of variance (ANOVA) in Elucidator. Those proteins exhibiting statistically significant differences (P < 0.05) between groups of biopsies from different fibrosis stages were then filtered and analyzed in Spotfire using a hierarchical clustering algorithm that allowed for the grouping of similar samples based on the pattern of fold change in protein abundance. A total of 210 proteins of differential abundance (summarized in Supplementary Table 3) define the clusters shown in Fig. 2. The resulting dendrogram showed 2 distinct groups of tissue samples. Biopsies representative of later fibrosis stages (for example, stages 3-4) tended to group together and exhibited a distinct protein expression profile, indicating an apparent decline in the relative abundance of many proteins when compared with samples representative of earlier fibrosis stages (for example, stages 0–2). A notable exception was observed for 2 samples (203 and 514), which had much less similarity to the remaining samples from patients with advanced liver disease. This may reflect the influence of several clinical variables (for example, gender, age, HCV genotype, length of disease) on the host response to HCV infection and points to the importance of considering interindividual variations when searching for disease-associated protein abundance changes.20 Additional possibilities include the occurrence of sampling errors that preclude accurate and reproducible histological assessment of liver disease.21, 22 Nevertheless, the data demonstrate the potential of proteomics to identify group-associated proteomic differences that occur along the continuum from virus infection to liver disease.
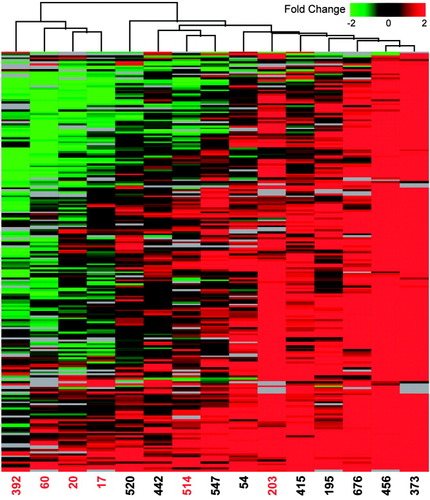
Clustering of proteins exhibiting statistically significant differences among fibrosis groups. An error-weighted 1-way ANOVA was performed in Elucidator using a Benjamini and Hochberg multiple test correction. Proteins exhibiting statistically significant (P < 0.05) differences between fibrotic groups (for example, stages 0–4) were then filtered to select proteins exhibiting a 1.5-fold or higher change in expression (P < 0.05) in at least 3 experiments for subsequent cluster analysis in Spotfire (Spotfire Inc., Somerville, MA). Before 2-dimensional hierarchical clustering with a weighted average method and Cosine correlation metric, the data were further filtered to include only those proteins detected in a minimum of 10 samples to minimize adverse effects of missing data points on the consistency of clustering. Each horizontal row represents an individual protein (210 total) and each vertical column an individual liver biopsy sample (represented by the corresponding patient number). Protein abundance ratios are colored according to the fold changes, and the color scale indicates the magnitude of fold change. Black indicates no change in protein abundance. Gray indicates missing data. Patients exhibiting fibrosis stages 3 or 4 are highlighted in red.
Functional Analysis of Stage-Specific Protein Abundance Changes in HCV-Associated Fibrosis.
We have further explored the biological significance of the altered protein abundance pattern described above by classifying the associated proteins within the context of functional pathways and networks using GeneGo's (St. Joseph, MI) MetaCore software analysis tool. This software leverages a manually curated database containing known molecular interactions (for example, protein–protein interactions), functional and disease interrelationships, and pathway interactions for biological interpretation of high-throughput microarray and proteomic data sets. We began by investigating the distribution of proteins exhibiting differential abundance among functional pathways. Those pathways containing a statistically significant enrichment of differentially expressed proteins were identified based on the probability that a random set of proteins the same size as the input list would give rise to a particular mapping by chance (P-value). Among the highest scoring of these pathways were several associated with amino acid, carbohydrate, and lipid metabolism. Most notably, we observed that several proteins functioning in fatty acid beta-oxidation (for example, 13 of 34 lipid metabolism–associated proteins constituting the ANOVA list) were adversely impacted in biopsies exhibiting advanced fibrosis (Fig. 3A).
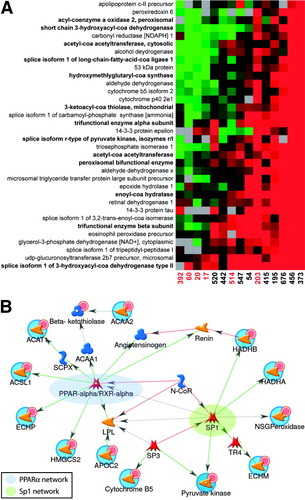
Differential protein abundance and network-oriented analysis of a subset of proteins related to lipid metabolism. (A) The fold change in relative protein abundance is shown for the 34 lipid metabolism-associated proteins constituting the ANOVA cluster in Fig. 2. Legend of Fig. 2 gives details on the applied color scheme and experiment representation. A total of 16 proteins functioning in fatty acid metabolic processes are represented in this list, including 13 associated with fatty acid oxidation (indicated by bold lettering). (B) Network analysis of the 16 differentially regulated fatty acid metabolism proteins using the auto-expand algorithm available in Metacore. This algorithm uses the input list of proteins to build sub-networks consisting of nearest neighbors. Because the expansion stops when sub-networks intersect, only 10 proteins from the input list (indicated by red circles) connected to the intersecting sub-networks at the time expansion terminated. The differently colored nodes represent transcription factors (red shapes), enzymes (yellow arrows) and other proteins (blue shapes). Colored hexagons on the vectors between nodes describe the type of interaction where TR = transcriptional regulation, CN = competition, B = binding, C = cleavage, and CR indicates that an object belongs to a group of related proteins. The green color indicates a positive effect, red indicates a negative effect, and black corresponds to an unspecified effect. Transcriptional regulatory networks centered around peroxisome proliferator-activated receptor alpha/RXRα (highlighted in blue) and Sp1 (highlighted in green) were detected.
We further explored those fatty acid metabolism proteins exhibiting differential abundances within the context of biological networks of signaling, regulatory and biochemical interactions using MetaCore. Consistent with the literature, the network shown in Fig. 3B demonstrates that several of the fatty acid metabolism proteins exhibiting altered abundances in this study are regulated by peroxisome proliferator-activated receptor alpha, a transcriptional activator of fatty acid oxidation genes whose expression is down-regulated by HCV.23, 24 Additionally, we identified a transcriptional regulatory subnetwork centered around specificity protein 1 (Sp1), a ubiquitous mammalian transcription factor whose DNA binding activity can either be inhibited25 or stimulated26 in the presence of HCV viral proteins. Whether HCV-mediated perturbations in Sp1 DNA-binding capacity occur that could contribute to the decline in abundance of fatty acid metabolism proteins remains undetermined. The network further demonstrates that the activity of both peroxisome proliferator-activated receptor alpha and Sp1 can be inhibited by nuclear receptor corepressor (Fig. 3B). Another possibility is that HCV disrupts the relative abundance and/or balance between binding of transcriptional coactivators and repressors, thus altering lipid homeostasis. Taken together, these findings offer “proof of principle” demonstrating the utility of proteomics for assisting in the determination of proteins/pathways affected by HCV infection and suggest future avenues of research aimed at further understanding the role of transcriptional regulatory mechanisms in the impaired β-oxidation observed during advanced fibrosis.
Additional down-regulated proteins included mitochondrial proteins of the oxidative phosphorylation system and proteins responding to oxidative stress and reactive oxygen species (Fig. 4). This contrasts with the general up-regulation, albeit to variable extents, of various endoplasmic reticulum (ER) and cytosolic proteins constituting the cluster shown in Fig. 2 (for example, glucose-regulated protein, 78 kDa, calreticulin, ER protein 29, heat shock 70-kDa protein 1B, protein disulfide-isomerase A3 precursor, and ubiquitin-activating enzyme E1). The increased abundance of candidates functioning in protein folding and degradation is not surprising because HCV induces ER stress.27 More importantly, the up-regulation of these proteins across all samples suggests that the opposing down-regulation of mitochondrial and antioxidant proteins during advanced fibrosis is not likely to result from a widespread decline in viable hepatocytes. Further support for this idea comes from the observation that all individuals with cirrhosis were well compensated [for example, none had a Child-Turcotte-Pugh score over 6 (Class A) or model for end-stage liver disease score over 6], thus indicating maintenance of normal liver function and a lack of grossly measurable decreases in hepatocyte mass.
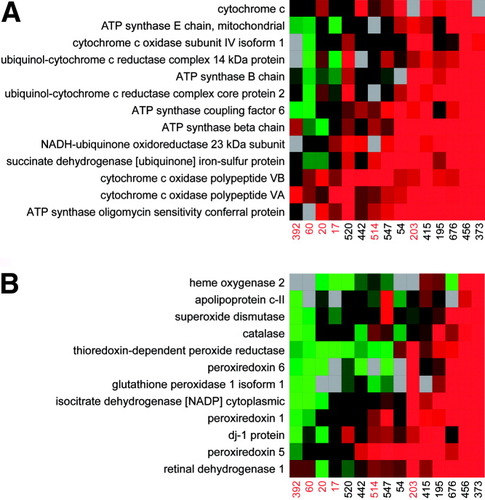
Differential abundance of proteins functioning in (A) oxidative phosphorylation and (B) response to oxidative stress and reactive oxygen species. The fold change in relative protein abundance is shown for the proteins associated with these functional categories and constituting the ANOVA cluster in Fig. 2. Fig. 2 gives details on the applied color scheme and experiment representation.
The protein abundance changes reported here are in good agreement with the role of oxidative stress and altered mitochondrial function in the pathogenesis of HCV.23, 28, 29 A decline in antioxidant proteins would impair the capacity to counteract the elevation in reactive oxygen species and ensuing liver damage associated with inflammatory immune responses, virus-mediated perturbations in ER, mitochondrial homeostasis, and so forth. The accompanying down-regulation of proteins functioning in mitochondrial β-oxidation would favor an increase in hepatocellular lipid content (for example, steatosis), a common occurrence in hepatitis C,23, 30 and histological feature observed here among 4 of 6 patients with advanced fibrosis (for example, patients 392, 60, 514, and 203 showed evidence of grade 1 steatosis with fat accumulation in 5%–33% of hepatocytes). In accordance with the “2-hit” hypothesis, this would provide the added fuel for further amplifying the generation of reactive oxygen species and lipid peroxidation products that trigger hepatic stellate cell activation and fibrogenesis.
One limitation to the current study was our inability to identify protein abundance changes associated with innate antiviral signaling pathways, an area of considerable interest given the ability of HCV to antagonize host defense responses and establish a persistent infection.29, 31, 32 This may be explained in part by the limited representation of immune response proteins in our current liver protein database. Although our human liver AMT tag database represents 1 of the most comprehensive liver compendiums reported, the complexity and high dynamic range of protein abundances in mammalian tissues presents a significant challenge for the effective detection of low-abundance proteins (for example, transcription factors and cytokines). Thus, that the deregulated pathways identified in this study reflect the more readily detectable higher abundance proteins associated with key metabolic functions of the liver is not surprising. This remains a common theme among studies of the liver proteome and emphasizes the importance of implementing additional strategies (for example, subcellular fractionation, glycoproteome, or cysteinyl subproteome enrichment) to reduce sample complexity, improve proteome coverage, and enhance the detection of low-abundance proteins important to the study of liver function and disease.33-35
In conclusion, we describe herein the application of an ultrasensitive “nano-proteomics” platform for performing to our knowledge, the first global quantitative proteome analysis of HCV infection in human liver biopsy specimens. Our findings suggest that profound modifications in the liver proteome may contribute to HCV-induced liver damage and lay the foundation for future proteomic efforts aimed at better understanding the molecular mechanisms underlying the interplay between oxidative stress, steatosis (for example, virally induced versus metabolic), and liver disease progression in a clinically relevant setting.
Acknowledgements
We thank Zachary Caldwell for his technical assistance and Dr. Kathie Walters for critically reading the manuscript. We thank the National Institute on Drug Abuse (grant 1P30DA01562501 to M.G.K.) for support of this research, and the Environmental Molecular Sciences Laboratory at PNNL for use of the instrumentation applied in this research. Portions of this research were supported by the NIH National Center for Research Resources (RR 18522). Work was performed in the Environmental Molecular Science Laboratory, a U.S. Department of Energy (DOE) national scientific user facility located on the campus of Pacific Northwest National Laboratory (PNNL) in Richland, WA. PNNL is a multiprogram national laboratory operated by Battelle Memorial Institute for the DOE under contract DE-AC05-76RLO-1830.




