Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses†
Potential conflict of interest: Nothing to report.
Abstract
A timely, efficient, and coordinated activation of both CD4 and CD8 T cell subsets following HCV infection is believed to be essential for HCV control. However, to what extent a failure of the individual T cell subsets can contribute to the high propensity of HCV to persist is still largely undefined. To address this issue, we analyzed the breadth, vigor, and quality of CD4 and CD8 responses simultaneously with panels of peptides covering the entire HCV sequence or containing the HLA-A2–binding motif, and with recombinant HCV proteins in 16 patients with acute HCV infection by tetramer staining, ELISPOT, and intracellular cytokine staining for interferon γ, interleukin (IL)-2, IL-4, and IL-10. Our results indicate that at clinical onset, CD8 responses are similarly weak and narrowly focused in both self-limited and chronically evolving infections. At this stage, CD4 responses are deeply impaired in patients with a chronic outcome as they are weak and of narrow specificity, unlike the strong, broad and T helper 1–oriented CD4 responses associated with resolving infections. Only patients able to finally control infection show maturation of CD8 memory sustained by progressive expansion of CD127+ CD8 cells. Thus, a poor CD8 response in the acute stage of infection may enhance the overall probability of chronic viral persistence. In conclusion, the presence of functional CD4 responses represents one of the factors dictating the fate of infection by directly contributing to control of the virus and by promoting maturation of protective memory CD8 responses. (HEPATOLOGY 2006;44:126–139.)
Spontaneous clearance of hepatitis C virus infection occurs in a small percentage of acutely infected patients and is associated with a vigorous cellular immune response of a broad specificity.1-9 In contrast, chronic infection is characterized by less vigorous and narrowly focused CD4 and CD8 T cell responses. The mechanisms responsible for immune failure in patients who do not succeed in clearing the virus spontaneously are still unclear. Although there have been several reports of the hepatitis C virus (HCV)-specific cellular immune response during acute illness, a comprehensive study analyzing simultaneously the contribution of each T cell subsets to the mechanisms of clearance or persistence has never been performed. Most recent reports used wide panels of 10- to 15-mer HCV peptides that cannot distinguish the relative contribution of CD4 and CD8 cells to the overall antiviral T cell response.5-11 Alternatively, a small number of preselected epitopes of known HLA restriction or whole recombinant HCV proteins were tested.12-18 Moreover, only limited information is available about the ex vivo profile of cytokine production in the acute phase of infection, because ELISPOT and intracellular cytokine staining (ICS) analysis were based on the measure of interferon-γ (IFN-γ) only.12, 19
In the present study, we used panels of genotype-matched, overlapping peptides covering the entire HCV sequence to obtain a reliable representation of the overall T cell response to HCV, associated with control of infection or virus persistence. Breadth and vigor of CD4 and CD8 responses were then carefully analyzed ex vivo by ICS. The extent to which CD4 and CD8 cells contributed to the overall magnitude of the T cell response was confirmed through the generation of short-term T cell lines. CD8 responses were further analyzed with an additional panel of 199 8- to 10-mer peptides of optimal length for CD8 stimulation containing the HLA-A2–binding motif, while the CD4 profile of cytokine production was characterized by ex vivo ICS for IFN-γ, interleukin (IL)-2, IL-4, and IL-10 using recombinant structural and nonstructural HCV proteins to stimulate CD4 cells.
Our results indicate that CD8 T cell response is poor at the onset of disease, irrespective of the outcome. CD8 cells are allowed to complete their differentiation and acquire fully protective antiviral activity only in patients who can mount an efficient helper CD4 activity. Therefore, both CD4 and CD8 functions are required for HCV control; however, the type of CD4 response seems to be critical for driving evolution of infection to control or persistence of the virus.
Abbreviations
HCV, hepatitis C virus; ICS, intracellular cytokine staining; IFN-γ, interferon-γ; IL, interleukin; PBMC, peripheral blood mononuclear cell; Th1, T helper 1.
Patients and Methods
Patients and Virological Assessment
Sixteen patients with acute hepatitis C were enrolled at the Unit of Infectious Diseases and Hepatology of the University Hospital of Parma. The diagnosis of acute HCV infection was based on the following criteria: documented seroconversion to anti-HCV antibodies by RIBA assay, levels of serum alanine aminotransferase at least 10 times the upper limit of normal (50 U/L), detection of HCV RNA, and exclusion of other possible causes of acute hepatitis (i.e., viruses, toxins, alcohol, autoimmunity, metabolic factors). Anti-HCV antibodies were determined using commercial enzyme immunoassay kits (Ortho Diagnostic Systems, Raritan, NJ) and RIBA (RIBA II, Ortho Diagnostic Systems). Serum HCV RNA was quantified by branched DNA assay and is expressed as copies/mL of serum (Bayer System 340bDNA Analyzer, Bayer Corporation, Tarrytown, NY); it was also analyzed by qualitative polymerase chain reaction (Cobas Amplicor; Roche Molecular Systems, Inc., Branchburg, NJ). The lower limit of detection by the branched method and qualitative polymerase chain reaction is 2,500 copies/mL and 50 copies/mL, respectively. The study was approved by the ethical committee of the Azienda Ospedaliero-Universitaria di Parma, and all subjects gave written informed consent to participate in the study.
Synthetic Peptides, Recombinant Proteins, Peptide-HLA Class I Tetramers, and Antibodies
We used two distinct sets of peptides (Chiron Mimotopes, Victoria, Australia) in this study: (1) a panel of 601 15-mer peptides based on a genotype 1a sequence (HCV-1) covering all structural (core, E1, E2) and nonstructural (NS3, NS4, NS5) HCV regions and overlapping by 10 residues; and (2) a panel of 199 synthetic peptides (9, 10, or 11 amino acids in length) containing the HLA-A2–binding motif and selected among 962 HCV sequences (identified by scanning the entire HCV-1 genome for the presence of the HLA-A2–binding motif) based on the prediction of their high binding affinity to HLA-A2.20 The HCV antigens E1, E2, core, NS3, NS4, and NS5 were expressed as C-terminal fusion proteins with human superoxide dismutase in yeast and were kindly provided by Chiron Corporation (Victoria, Australia). Purity of the antigen preparations ranged from 85% to 95%. Phycoerythrin-labeled tetrameric peptide-HLA class I complexes were purchased from Proimmune LTD (Oxford, UK). HLA-A2 tetramers contained the HCV peptides NS3 1073-1081, NS3 1406-1415, NS4 1812-1820, NS4B 1992-2000, and NS5 2594-2602. Anti–IFN-γ FITC (conjugated fluorescein isothiocyanate) was purchased from Sigma Aldrich (St. Louis, MO). Anti-CD8 (APC), anti–CD-4 (phycoerythrin), anti-CD3 PerCP), anti–IL-4 (FITC), anti–IL-10 (APC), anti–IL-2 (APC), anti-CD127 (APC), and anti-CCR7 (FITC) were purchased from Becton Dickinson Biosciences (San Diego, CA).
Isolation of Peripheral Blood Mononuclear Cells and In Vitro Expansion of HCV-Specific T Cells
Peripheral blood mononuclear cells (PBMC) were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation and resuspended in RPMI 1640 supplemented with 25 mmol/L HEPES, 2 mmol/L L-glutamine, 50 μg/mL gentamycin, and 8% human serum (complete medium). For T cell expansion, PBMC were resuspended in 96-well plates at a concentration of 2 × 106/mL in complete medium and stimulated with HCV peptides at a final concentration of 1 μmol/L. Recombinant IL-2 was added on day 4 of culture (50 UI/mL), and the immunological assays were performed on day 10.
Cell Surface and Intracellular Staining
Staining With Tetramers and Other Surface Markers.
Next, 1 × 106 PBMC either freshly isolated or after in vitro expansion for 10 days were incubated for 30 minutes at 37°C with the phycoerythrin-labeled tetrameric complex in RPMI 1640 and 8% human serum. After washing, staining was performed for 15 minutes in the dark, using an anti-CD8 APC-conjugated antibody. The cells were then washed and analyzed immediately by flow cytometry (FACScalibur, Becton Dickinson) using CELLQuest software. For phenotypic analysis, tetramer-stained cells were incubated with CD127 (APC) and CCR7 (FITC) antibodies.
Intracellular Cytokine Staining.
PBMC or T cells lines were incubated either with medium alone (control) or with viral peptides (1 μmol/L) or HCV proteins (1 μmol/L) for 2 hours; brefeldin A (10 μg/mL) was then added for an overnight incubation. Stimulation with PMA (50 ng/mL) and ionomycin (0.5 μg/mL) lasted 4 hours in the presence of brefeldin A. After washing, the cells were stained with CD3, CD4, or CD8 monoclonal antibodies for 20 minutes at 4°C, and then fixed and permeabilized with a permeabilization buffer (Caltag, Burlingame, CA). Cells were finally stained with antibodies to IFN-γ, IL-10, IL-4, or IL-2 for 15 minutes at room temperature, washed again, and analyzed by flow cytometry. The cut-off value of 0.01% of cytokine-positive cells per total CD4 or CD8 cells was derived from 15 experiments performed with HCV antigens and peptides in uninfected control subjects (data not shown); controls never showed levels of cytokine-positive CD4 and CD8 cells >0.01%.
ELISPOT Assay
The 601 15-mer peptides overlapping by 10 residues and covering the overall HCV-1 sequence were pooled in 60 mixtures, each containing 10 synthetic peptides. HCV-specific T cell responses were analyzed upon overnight stimulation with individual peptide mixtures (1 μmol/L of each peptide). Briefly, 96-well plates (Multiscreen-IP-Millipore S.A.S., Molsheim, France) were coated overnight at 4°C, as recommended by the manufacturer, with 5 μg/mL capture mouse anti-human IFN-γ monoclonal antibody (1 DIK, Mabtech, Nacka Strand, Sweden). Plates were then washed 7 times with phosphate-buffered saline/0.05% Tween 20 and blocked with RPMI/10% fetal calf serum for 2 hours at 37°C; 2 × 105 PBMC were seeded per well. Plates were incubated for 18 hours at 37°C in the presence or absence of peptides. After washing with phosphate-buffered saline/0.05%Tween 20, a biotinylated secondary mouse anti-human IFN-γ monoclonal antibody (1 μg/mL; 7B6-1, Mabtech) was added. After 3 hours of incubation at room temperature, plates were washed 4 times and 100 μL goat alkaline phosphatase anti-biotin antibody (Vector Laboratories Inc., Burlingame, CA) was added to wells, and the plates were incubated for a further 2 hours at room temperature. Plates were then washed 4 times, and 75 μL of alkaline phosphatase conjugate substrate (5-bromo-4-chloro-3-indolyl phosphate; Bio-Rad Laboratories, Hercules, CA) was added. After 4-7 minutes, the colorimetric reaction was stopped by washing with distilled water. Plates were air-dried and spots were counted using an automated ELISPOT reader (AID Elispot Reader System; Autoimmune Diagnostika Gmbh, Strassberg, Germany). IFN-γ–producing cells were expressed as spot-forming cells per 2 × 105 cells. The number of specific IFN-γ–secreting cells was calculated by subtracting the unstimulated control value from the stimulated sample. Unstimulated wells never exceeded 5-7 spots/well. Positive controls consisted of PBMC stimulated with PHA. Wells were considered positive if they were at least 2 times above background.
Results
Antigen-Specific T Cell Responses Are Broader and Stronger in Self-Limited Than in Persistent HCV Infection.
Breadth and magnitude of the anti-HCV–specific T cell response was analyzed ex vivo in PBMC of 16 acutely infected subjects to define the role of adaptive T cell immunity in determining the course of HCV infection. PBMC were tested by ex vivo IFN-γ ELISPOT assay using a panel of 601 15-mer peptides overlapping by 10 residues and spanning the entire HCV sequence of genotype 1, pooled in 60 mixtures of 10 peptides each. All patients were enrolled at the time of clinical presentation and were infected by genotype 1 (Table 1); a self-limited outcome was observed in six of these patients, and ten developed a chronic disease as indicated by persistently elevated HCV RNA levels (Fig. 1). A sharp difference was observed in the global profile of the HCV-specific cellular immune response in the acute phase of infection, depending on the outcome of the disease. Patients with a self-limiting evolution displayed a multispecific T cell response, targeting structural and nonstructural proteins and recognizing most HCV antigens. In contrast, individuals with chronic evolution showed a narrower cellular immune response, targeting a limited number of peptides, predominantly located in nonstructural HCV regions (Fig. 2A).
| Genotype | Outcome | ALT | HLA-A2 | |
|---|---|---|---|---|
| 1S | 1b | Self-limited | 876 | + |
| 2S | 1a | Self-limited | 1,078 | − |
| 3S | 1b | Self-limited | 747 | − |
| 4S | 1b | Self-limited | 903 | + |
| 5S | 1a | Self-limited | 503 | − |
| 6S | 1a | Self-limited | 429 | + |
| 1C | 1a | Chronic | 1,029 | + |
| 2C | 1a | Chronic | 2,100 | − |
| 3C | 1a | Chronic | 688 | − |
| 4C | 1a | Chronic | 1,805 | + |
| 5C | 1b | Chronic | 2,607 | + |
| 6C | 1a | Chronic | 580 | − |
| 7C | 1a | Chronic | 336 | + |
| 8C | 1a | Chronic | 2,753 | + |
| 9C | 1a | Chronic | 587 | − |
| 10C | 1a | Chronic | 1,060 | + |
| 11C | 1b | Chronic | 192 | + |
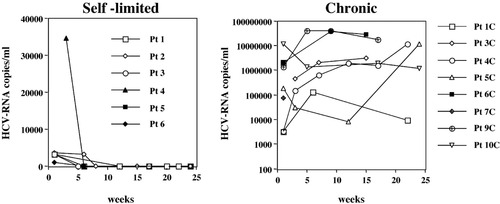
Longitudinal analysis of viremia in patients with acute hepatitis C and different evolution of infection. Time 0 corresponds to clinical presentation. Negative results were confirmed via qualitative polymerase chain reaction. HCV, hepatitis C virus; Pt, patient.
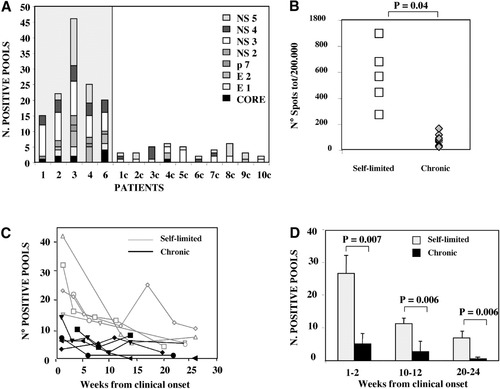
Breadth and intensity of overall T cell response analyzed in patients with acute HCV infection via ex vivo IFN-γ ELISPOT performed with a panel of 601 15-mer peptides spanning the entire HCV sequence of genotype 1 and pooled in 60 mixtures. (A) Number of peptide pools able to induce IFN-γ production in patients with self-limited (shaded area) or chronic evolution of infection analyzed at the onset of the disease. (B) Intensity of the T cell response expressed as the total number of spots detected in each individual patient (sum of the spots from each positive well after subtraction of the background value) at the time of the clinical presentation with all peptide mixtures (each symbol corresponds to a single patient). (C) Longitudinal analysis of the breadth of the T cell response from the onset of the disease throughout a follow-up period ranging from 20 to 25 weeks in patients with self-limited (grey line) or chronic (black line) evolution of hepatitis. (D) Average number of positive peptide pools able to induce IFN-γ production in patients with self-limited (grey bars) or chronic (black bars) evolution of hepatitis calculated at 3 different time points ranging from weeks 1 to 2, 10 to 12, and 20 to 22. Statistical analysis was performed using the Student t test.
The overall number of spots, calculated per 2 × 105 cells in each single patient at the time of the onset, was significantly greater among patients with a self-limited evolution of infection, showing that their T cell response was not only wider but also stronger compared to patients with persistent infection (Fig. 2B).
The breadth of the cellular immune response was analyzed longitudinally ex vivo from the onset of disease throughout a follow-up ranging from 20 to 24 weeks (Fig. 2C). Individuals with self-limited hepatitis showed a broader T cell response that persisted throughout follow-up.
In patients with chronic evolution of disease, the cellular immune response was already narrower during the acute phase of illness and declined further at later time points; in some patients the response was totally undetectable a few weeks from onset. Thus, the difference in the breadth of the T cell response between patients with self-limited or persistent infections was maintained throughout the follow-up, and this difference was statistically significant not only during the acute phase of infection but also at weeks 10-12 and weeks 20-24 from the onset of disease (Fig. 2D).
A Different Breadth and Strength of the CD4 But Not of the CD8 Response Distinguishes the Acute Phase of Self-Limited and Persistent HCV Infections.
Although virus elimination and control of HCV replication have been reported to be associated with a strong and multispecific cellular immune response, the real contribution of CD4 and CD8 T cell subsets is still controversial. To address this issue, the pools of peptides responsible for the detectable responses in ELISPOT were selected and used to stimulate PBMC of the earliest time point (clinical presentation). Through ex vivo intracellular cytokine staining performed with anti-CD4, anti-CD8, and anti–IFN-γ antibodies, the T cell subset responsible for IFN-γ production upon stimulation with each single pool was identified. As illustrated in Fig. 3, the overall number of peptides able to induce a T cell response detectable by ICS was higher in patients with self-limited outcome of infection (Fig. 3A) than in patients with chronic evolution (Fig. 3B). However, the cellular immune response was not similarly sustained by CD4 and CD8 T cell subsets in the two categories of patients. In self-limiting hepatitis, IFN-γ production was sustained predominantly by the CD4 T cell subset (Fig. 3A), whereas in patients with chronic evolution of disease (Fig. 3B), CD4-mediated responses were undetectable and IFN-γ production was induced by a small number of peptide pools and sustained exclusively by the CD8+ subset in all individuals, with the exception of a single pool in patient 1c (Fig. 3B). These results were confirmed with short-term T cell lines derived by 10-day stimulation of the same early PBMC samples with the 60 pools of peptides. T cell lines were first tested by IFN-γ ELISPOT analysis (data not shown) and T cell reactivity was confirmed byICS to define the contribution of the different T cell subsets. The results shown in Fig. 3A indicate that in patients with self-limited infection, a strong expansion of HCV-specific CD4 cells was detectable, whereas proliferation of CD8 cells was less vigorous and was induced by a more limited number of peptide pools. In patients with chronic evolution of the disease, neither CD4 nor CD8 cell subsets expanded efficiently upon peptide stimulation (Fig. 3B).
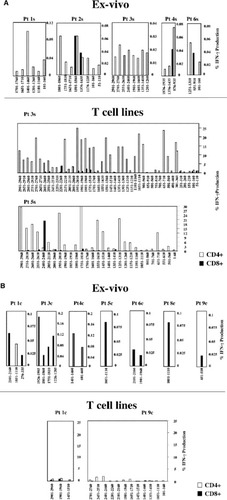
Percentage of CD4 and CD8 cells able to produce IFN-γ. Analysis was performed by ICS ex vivo (upper) or upon 10 days stimulation (bottom) in patients with (A) self-limited or (B) chronic evolution of disease with the indicated peptides previously shown to be positive in ELISpot at the time of acute illness. Results are expressed as the percentage of IFN-γ–positive CD4 or CD8 cells among the total CD3 population. Pt, patient; IFN-γ, interferon-γ.
The Acute Phase Impairment of the CD8 Function Is Transient in Self-Limited But Not in Persistent HCV Infection.
To further investigate the behavior of the HCV-specific CD8 T cell response during the acute and the recovery phase of HCV infection, different complementary approaches were used simultaneously. In HLA-A2–positive patients, the analysis of the cytotoxic T lymphocyte response was also performed with a panel of 199 synthetic peptides containing the HLA-A2 binding motif pooled in 20 mixtures, assessing IFN-γ production by ex vivo ELISPOT analysis. These peptides were selected by scanning the entire HCV sequence (HCV-1 strain) for the presence of the HLA-A2–binding motif and selecting those sequences that were predicted to bind HLA-A2 more efficiently. Also through this approach, all patients tested during the acute phase of infection displayed a weak and narrowly focused CD8 response, irrespective of the outcome of disease (Fig. 4A). Defective IFN-γ production at this stage of infection was also confirmed by analyzing tetramer-positive CD8 cells specific for five epitopes widely recognized by HLA-A2–positive patients with acute HCV infection. Whereas a relevant frequency of tetramer-positive CD8 cells was present in the circulation, no IFN-γ tetramer-positive CD8 cells were detectable ex vivo upon stimulation with either specific peptide (Fig. 4A) or PMA and ionomycin (Fig. 4B).
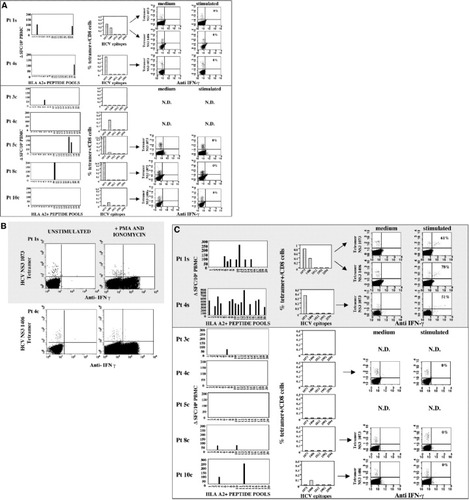
(Pages 134-135.) Frequency and function of HCV-specific CD8 cells (A,B) at clinical presentation and (C) 6 months later in patients with self-limited (shaded area) and chronically evolving acute hepatitis (white area). In panels A and C, left histograms illustrate the frequency of IFN-γ–positive cells detected via ELISPOT ex vivo with a panel of 199 synthetic peptides containing the HLA-A2–binding motif, pooled in 20 mixtures. Middle histograms represent the frequency of tetramer-positive cells specific for the 5 indicated epitopes analyzed ex vivo. Right dot plots illustrate the percentage of tetramer-positive cells able to produce IFN-γ ex vivo before and after peptide stimulation. Panel B illustrates IFN-γ production by HCV-specific CD8 cells from 2 representative patients with different evolution of the disease tested by ICS ex vivo upon stimulation with PMA and ionomycin. Pt, patient; PBMC, peripheral blood mononuclear cells; HCV, hepatitis C virus; IFN-γ, interferon-γ; SFC, spot-forming cells; N.D., not determined.
Six months later (Fig. 4C), the CD8 response analyzed by ex vivo ELISPOT with HLA-A2 binding peptides became multispecific and directed toward different HCV proteins in patients who resolved the infection. In contrast, CD8 responses remained very weak or totally undetectable in patients with chronic evolution of infection. In this latter category of patients, frequencies of HCV-specific CD8 cells detectable by tetramer staining declined over time and, when detectable, CD8 cells remained unable to produce cytokines upon ex vivo peptide stimulation (Fig. 4C). In contrast, tetramer-positive CD8 cells were still detectable at this later time point in recovered patients and they acquired the capacity to produce IFN-γ upon peptide stimulation (Fig. 4C).
Memory CD8 Cells Are Generated in Self-Limited But Not in Persistent HCV Infection.
Acquisition of proliferative potential and capacity to secrete effector cytokines upon antigen exposure by CD8 cells of patients with a self-limited infection may reflect the generation of long-lived, IL-7 receptor–positive (CD127+) memory CD8 cells able to survive the contraction phase of the immune response that follows acute infection and clonal expansion. Lack of their generation in patients with chronic evolution of hepatitis may contribute to inefficient control of the infection, as proposed in different animal models.
To investigate the capacity of developing memory CD8 T cells in relation to the outcome of acute HCV infection, we examined in HLA-A2–positive patients the cell surface expression on antigen-specific CD8 cells of CD127 and CCR7, a lymph node homing receptor. Viremia and percentage of CD127/HCV-specific tetramer-positive cells were analyzed in parallel in patients with different evolution of the disease during a 15- to 34-week follow-up starting from clinical onset (Fig. 5). At the earliest time point analyzed, corresponding to the acute phase of infection, an elevated percentage of virus-specific CD8 cells were CD127−, irrespective of the outcome (Fig. 5A-B). With the resolution of the infection and in the absence of measurable HCV RNA, a diffferentiation to a CD127+/CCR7+ central memory phenotype occurred (Fig. 5A). In contrast, persistence of high levels of viremia were associated with a long term-maintenance of a CD127−/CCR7− effector phenotype by HCV-specific CD8 cells (Fig. 5B).
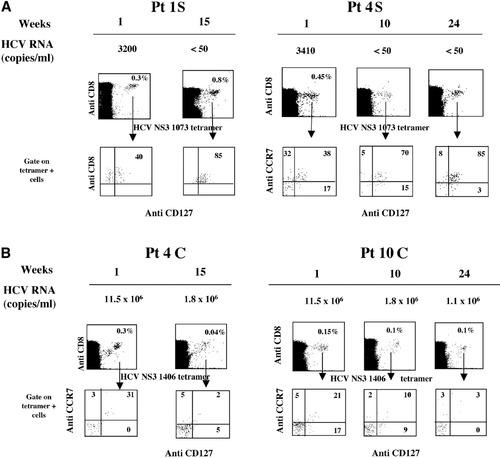
Frequency and phenotype of tetramer-positive/CD127+ CD8 cells in relation to viremia levels in patients with (A) self-limited and (B) chronic evolution of acute hepatitis. In the upper part of each panel, viremia is represented at the indicated weeks after clinical onset. The lower section illustrates the expression of CD127 and CCR7 surface activation markers in gated HCV-specific tetramer-positive CD8 cells. Pt, patient; HCV, hepatitis C virus.
The results obtained by costaining with CD127 and CCR7 indicate that during the acute phase of infection, a progressive memory CD8 T cell differentiation occurs in patients who spontaneously recover, whereas an altered memory differentiation is associated with the persistence of infection.
A T Helper 1–Oriented CD4 Response Is Associated With Virus Control But Not Persistence of Infection.
Induction and maintenance of antiviral CD8+ responses generally depend on the presence of functionally efficient CD4 T cells. Moreover, the CD4 function may play a crucial role in virus control, irrespective of the effect on CD8 differentiation. To investigate the function of HCV-specific CD4 cells during the acute phase of infection and their possible role in HCV control, the cytokine secretion pattern of CD4+ cells in patients with different outcome of disease was analyzed by ex vivo ICS. PBMC were stimulated overnight with HCV recombinant proteins and then stained with anti–IFN-γ, IL-2, IL-10, and IL-4 antibodies. At the peak of alanine aminotransferase, the CD4 T cell response to HCV antigens was predominantly characterized by a T helper 1 (Th1) cytokine profile in all patients with self-limited evolution of the disease (Fig. 6A-B). In contrast, patients with chronically evolving disease showed impaired production of Th1 cytokines (Fig. 6C-D). No significant differences were detected between patients with different evolution of infection in IL-4 and IL-10 production.
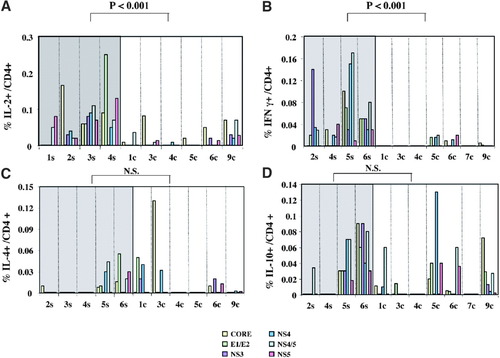
Ex vivo cytokine production by CD4 cells after stimulation with recombinant HCV proteins during the acute phase of infection in patients with self-limited (shaded area) and chronic (white area) evolution of the disease. PBMC were stimulated overnight with 6 recombinant proteins corresponding to structural and nonstructural HCV antigens and tested for (A) IL-2, (B) IFN-γ, (C) IL-4, and (D) IL-10 production via ICS. Results are expressed as the percentage of cytokine-positive CD4 cells among the total CD4 population. IL, interleukin; IFN-γ, interferon-γ; N.S., not significant.
Thus, CD4 T cells from patients with self-limited evolution of infection produced relevant amounts of effector Th1 cytokines, such as IL-2, that can help sustain cytotoxic T lymphocyte function and to generate stable and protective CD8 memory cells. In contrast, persistent viremia and persistent impairment of the CD8 function were associated with an almost complete lack of Th1 cytokine production by CD4 cells.
Discussion
The overall analysis of the T cell response during the acute phase of HCV infection shows a sharp difference between patients with different outcome of infection. Patients with a favorable evolution displayed a strong and multispecific cellular immune response targeting structural and nonstructural HCV proteins, whereas patients with chronic evolution showed weaker and narrower cellular immune responses. A more detailed analysis indicated that this difference was not determined by the behavior of the CD8 T cell subset, because the CD8-mediated T cell response was weak and narrowly focused in all patients, both ex vivo and following 10 days of peptide stimulation in vitro, irrespective of the outcome. Moreover, functional analysis showed that the CD8 response was not only weak and restricted in breadth but also inefficient in cytokine production and expansion upon antigen stimulation. Although a functional impairment of the HCV-specific CD8 response during the acute phase of hepatitis has already been reported,1-4, 21 our ICS and ELISPOT analysis with wide panels of overlapping peptides covering the overall HCV sequence or representing HLA-A2–binding sequences of optimal length for CD8 recognition further supports the concept that at this time of infection the CD8 T cell subset functions poorly.
It is widely accepted that the cytotoxic T lymphocyte response is a critical defense mechanism that the immune system employs against viruses.22 A complex differentiation process occurs during a CD8 T cell response to a viral infection, comprising three different phases that correspond to an initial expansion followed by contraction and final generation of long-lived memory if virus replication is successfully controlled.23, 24 In HCV infection, virus replication and spread start rapidly after exposure to HCV and precede the alanine aminotransferase peak, with a lag of several weeks between the time of infection and the acute phase of disease.2 Therefore, a first possible interpretation of the poor CD8 response at the time of clinical presentation is that the expansion phase occurred before immunological analysis was started so that CD8 cells were already in the contraction phase when they were first analyzed. Because of this, self-limited and chronically evolving infections may have displayed a similar behavior. However, because earlier time points were not available for functional analysis, we cannot formally exclude the alternative possibility that fully functional CD8 cells were not generated after infection. Inhibition by rapidly increasing concentrations of virus and antigen—as a result of the kinetics of HCV replication and spread, direct inhibitory effect of viral proteins on CD8, or accessory cell function—might affect the initial expansion phase, resulting in functionally defective CD8 responses of limited breadth, vigor, and quality. This may affect the efficiency of antiviral protection and may also translate into an indolent nature of disease, as is generally observed in HCV infection. Consistent with this interpretation, longitudinal ex vivo analysis of the CD8 response from the time of infection by tetramer staining indicates that CD8 cells may be undetectable for some weeks after infection, despite active viral replication, and may be functionally impaired for some additional weeks after their initial detection by tetramers.2-7, 25, 26 Thus, poor CD8 responses in the peripheral blood of patients at onset of acute disease may either reflect the physiological contraction phase of the CD8 response that normally follows initial expansion (which may have not been documented by our study because of the lack of earlier analysis) or may reflect a lack of induction of a fully functional CD8 response by HCV infection. Alternatively, the weakness of the peripheral blood responses and the lack of detection of functional HCV-specific CD8 cells in the periphery could be due to their compartmentalization in the liver. This latter hypothesis cannot be tested, however, because liver tissue samples cannot be obtained for ethical reasons at the acute stage of infection.
In all patients—regardless of outcome—CD8 cells showed a predominant CD127− phenotype at the first time point analyzed corresponding to clinical presentation, indicating that these cells were in a differentiation phase that may precede the maturation toward a memory phenotype.27-29 Memory HCV-specific CD8 cells were progressively generated beginning a few weeks after the clinical onset in self-limited but not chronic infections. Indeed, HCV-specific CD8 cells acquired the capacity to proliferate and to secrete effector cytokines upon antigen exposure and expressed high levels of IL-7 receptor (CD127+) exclusively in patients who recovered from infection.
This effect may have contributed the decline of viremia and the presence of functionally efficient helper CD4 cells. It is well documented that CD4 help is required for the generation of stable and protective CD8 memory,30-35 whereas it is less crucial during the primary expansion phase of CD8 cells. Cytokines secreted by CD4 cells become involved in this process by promoting proliferation and survival of memory cells.32-34 In particular, an important role seems to be played by IL-2, which is largely produced by the CD4 T cell subset, is required for sustained expansion of CD8 T cells, and is directly associated with the expression of CD127 and the acquisition of a memory phenotype.36 Furthermore, recent studies indicate that the absence of adequate CD4+ T cell help in the acute phase of infection can lead to the emergence of viral escape mutations in class I major histocompatibility complex–restricted epitopes and failure to resolve HCV infection.37
Our analysis of the CD4-mediated HCV-specific response in the acute phase of infection indicated that in patients who succeeded in controlling HCV, a strong and multispecific CD4+ T cell response was detectable ex vivo and that CD4 cells expanded vigorously upon peptide stimulation. In contrast, in patients with chronic evolution of the disease, the HCV-specific helper response was barely detectable ex vivo and had a limited capacity of expansion. CD4 responses at the peak of alanine aminotransferase were predominantly characterized by a Th1 profile in individuals with a favorable outcome and were associated with the production of elevated amounts of both IFN-γ and IL-2. In contrast, patients with a chronically evolving disease displayed an impaired production of Th1 cytokines. An impaired CD4-mediated proliferative response to HCV proteins in the acute phase of chronically evolving HCV infections was previously documented by long-term in vitro stimulation with recombinant HCV antigens and by ex vivo ELISPOT with overlapping synthetic peptides.12, 13, 38 Moreover, a similar failure in IL-2 production was observed in patients with an established condition of long-lasting chronic infection.39 In addition, rapid inactivation of antigen-specific CD4 cells with impairment of IL-2 or tumor necrosis factor α production and concurrent exhaustion of the CD8 response has recently been observed in persistent viral infections.40 Thus, early functional inactivation of CD4 cells and failure to produce effector cytokines in the acute phase of HCV infection may affect the generation of an efficient antiviral immune response. In this condition, HCV-specific CD8 cells may fail to complete their differentiation program and may remain dysfunctional, thereby precluding the possibility of final and persistent virus control.
In conclusion, our study shows that in the acute stage of infection, HCV-specific CD8 responses detectable in the peripheral blood are weak, restricted in breadth, and partially dysfunctional, regardless of the final outcome. Although information about the intrahepatic compartment is lacking, our findings may be consistent with the concept that an impaired CD8 response can diminish the possibility of the infected host eliminating the virus, thereby enhancing the likelihood of HCV persistence. In this condition, an efficient CD4 response may be a crucial determinant of resolution of infection, possibly by allowing CD8 cells to complete their maturation process.
Acknowledgements
The authors thank M. Houghton and Chiron Corporation for providing recombinant HCV proteins.




