Reassessment of the algorithm for prediction of liver fibrosis in patients with features of the metabolic syndrome†
Potential conflict of interest: Nothing to report.
We previously published in HEPATOLOGY an algorithm including a serum hyaluronate and carbohydrate-deficient transferrin/transferrin (CDT/Tf) ratio that allowed for the exclusion of clinically relevant hepatic fibrosis in patients with increased serum aminotransferase levels associated with features of the metabolic syndrome, regardless of current or past alcohol consumption.1 In this study, serum hyaluronate levels had been determined using a hyaluronate radioimmunoassay (H-RIA) kit (Pharmacia HA test) that is no longer available from Pharmacia & Upjohn (St. Quentin-en-Yvelines, France) or—to our best knowledge—any other supplier. We then tested whether our algorithm remained valid when measuring serum hyaluronate via ELISA (H-ELISA) (Corgénix Laboratories, Peterborough, UK) in the same 173 patients using sera stored at −20°C.
Univariate analysis showed that H-ELISA levels were also significantly associated with the presence of significant fibrosis defined as METAVIR2 fibrosis stages 2, 3, and 4 (26 ± 20 μg/L in nonfibrotic patients versus 167 ± 342 μg/L in fibrotic patients; P < .0001). A new logistic regression analysis then demonstrated that the best model for discriminating patients with and without significant fibrosis was still based on H-ELISA and serum CDT/Tf levels. According to receiver-operating characteristic curve analysis, the best compromise between sensitivity (90.5%; 95% CI 77–97) and specificity (84.7%; 95% CI 77–90) was found when using a H-ELISA threshold of 41 μg/L (vs. 49 μg/L for H-RIA). The threshold that allowed for 100% sensitivity (i.e., identification of all patients with fibrosis stages 2, 3, and 4) was 15 μg/L (35 μg/L for H-RIA), with a lower specificity than for H-RIA (35% vs. 53%). Analysis of the CDT/Tf receiver-operating characteristic curve performed in the 128 remaining patients in whom biopsy would have been indicated because they had serum H-ELISA levels higher than 15 μg/L showed that the CDT/Tf threshold was still 0.9, provided the misclassification of a few patients with fibrosis stage 2 was accepted (Fig. 1).
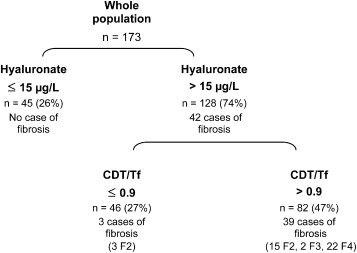
Results of the application of the algorithm to the 173 patients: specificity 67% (95% CI 60–74); sensitivity 93% (95% CI 89–97); positive predictive value 48% (95% CI 41–55); negative predictive value 97% (95% CI 94.5–99.5); and diagnostic accuracy 73% (95% CI 66–80). CDT, serum carbohydrate-deficient transferrin; Tf, serum transferrin.
As a whole, changing the method of measurement of serum hyaluronate did not significantly modify the clinical relevance of our algorithm, because liver biopsy would have been spared in 53% of patientsusing H-ELISA versus 60% using H-RIA. Otherwise, we observed 9 cases of cirrhosis in which H-ELISA levels comprised between 15 and 75 μg/L when the manufacturer gave 75 μg/L as the upper limit of normal for H-ELISA levels.
In conclusion, our algorithm remains valid when using ELISA to determine serum hyaluronate levels if a threshold of 15 μg/L is applied. The upper limit of normal for H-ELISA as provided by Corgénix Laboratories is too high for patients with chronic liver disease.
References
Fabrice Lainé* , Claude Bendavid , Jeff Morcet*, Michéle Perrin*, Catherine Massart , Yves Deugnier* , * Centre d'Investigation Clinique, France, Service des Maladies du Foie, France, Laboratoire de Génétique Moléculaire et d'Hormonologie Rennes, France.




