Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study†‡
Potential conflict of interest: Nothing to report.
Presented in part at the 39th Annual Meeting of the European Association for the Study of the Liver and appeared in abstract form (J. Hepatol. 2004; 40 (Suppl 1):29).
Abstract
The prevalence of and the risk factors for fatty liver have not undergone a formal evaluation in a representative sample of the general population. We therefore performed a cross-sectional study in the town of Campogalliano (Modena, Italy), within the context of the Dionysos Project. Of 5,780 eligible persons aged 18 to 75 years, 3,345 (58%) agreed to participate in the study. Subjects with suspected liver disease (SLD), defined on the basis of elevated serum alanine aminotransferase (ALT) and γ-glutamyl-transferase (GGT) activity, hepatitis B surface antigen (HBsAg), or hepatitis C virus (HCV)-RNA positivity, were matched with randomly selected subjects of the same age and sex without SLD. A total of 311 subjects with and 287 without SLD underwent a detailed clinical, laboratory, and anthropometrical evaluation. Fatty liver was diagnosed by ultrasonography, and alcohol intake was assessed by using a 7-day diary. Multinomial logistic regression was used to detect risk factors for normal liver versus nonalcoholic fatty liver disease (NAFLD) and for alcoholic fatty liver (AFLD) versus NAFLD. The prevalence of NAFLD was similar in subjects with and without SLD (25 vs. 20%, P = .203). At multivariable analysis, normal liver was more likely than NAFLD in older subjects and less likely in the presence of obesity, hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and systolic hypertension; AFLD was more likely than NAFLD in older subjects, males, and in the presence of elevated GGT and hypertriglyceridemia, and less likely in the presence of obesity and hyperglycemia. In conclusion, NAFLD is highly prevalent in the general population, is not associated with SLD, but is associated with many features of the metabolic syndrome. (HEPATOLOGY 2005.)
In the last two decades, nonalcoholic fatty liver disease (NAFLD) has been increasingly recognized as the most common liver disease in Western countries. Estimates obtained from clinical series, autopsy studies, and convenience samples of the general population suggest that 20% to 30% of individuals in Western countries have NAFLD.1, 2 These estimates, however, need to be confirmed in representative samples of the general population.2
The risk factors for NAFLD have been extensively investigated. The association with sex is controversial: whereas in old studies NAFLD was more frequent in women, the opposite was found in recent series.3 The prevalence of NAFLD increases with age, type 2 diabetes, obesity, and hypertriglyceridemia.3, 4 Visceral obesity, as operationally defined by a large waist circumference,5 is also considered a risk factor for NAFLD.3 After exclusion of other risk factors for liver disease, an elevated serum activity of alanine aminotransferase (ALT) has been suggested as the hallmark of NAFLD in the general population.3 However, the prevalence of NAFLD is substantially higher than that predicted on the basis of elevated ALT.3
In the absence of obesity and diabetes, hyperinsulinemia and insulin resistance are associated with NAFLD and ALT activity.6, 7 The presence of hyperinsulinemia or insulin resistance and the association with some of the features of the metabolic syndrome suggest that NAFLD might be the liver component of the metabolic syndrome.6 In clinical series,6 individuals with the metabolic syndrome are at greater risk for NAFLD, but no data are available at the population level, and the relative contribution of each component of the metabolic syndrome to the risk of NAFLD is unknown.
We took advantage of the Dionysos Study on the prevalence, incidence, and natural history of chronic liver disease (CLD) to perform a cross-sectional study aimed to establish the prevalence of and risk factors for NAFLD in a representative sample of the general population.
Abbreviations
NAFLD, nonalcoholic fatty liver disease; ALT, alanine aminotransferase; CLD, chronic liver disease; SLD, suspected liver disease; GGT, γ- glutamyltransferase; HBsAg, hepatitis B virus surface antigen; HCV, hepatitis C virus; HOMA, homeostasis model assessment; AFLD, alcoholic fatty liver disease; BMI, body mass index; AST, aspartate aminotransferase; HDL, high-density lipoprotein.
Patients and Methods
Study Design.
In 1991 to 1992, our group started the Dionysos Study, aimed to assess the prevalence, incidence, and natural history of CLD in the residents aged 12 to 65 years of 2 towns of Northern Italy: Campogalliano (province of Modena) and Cormons (province of Gorizia).8 The first phase of the Dionysos Study provided important and novel information on the prevalence of CLD in a representative sample of apparently healthy subjects from the general population and allowed determination of the threshold of alcohol intake for liver damage.8-11 Ten years later (2001-2002), all of the residents of the same towns were invited by letter to participate in the second phase of the Dionysos study, aimed to define the incidence and natural history of CLD.
Between January 2002 and August 2003, within the context of the second phase of the Dionysos study, we performed the Dionysos Nutrition and Liver Study, a cross-sectional study aimed to define the prevalence of and the risk factors for NALFD in the general population of the town of Campogalliano. Of 5,780 eligible subjects aged 18 to 75 years identified from the local municipal archives, 3,345 (58%) agreed to participate. Nonresponders were younger (42 ± 15 vs. 47 ± 15 years, mean ± standard deviation, P < .0001, unpaired Student t test) and more frequently males (male:female ratio 1.2 vs. 0.9, P < .0001, Fisher's exact test) than responders. Second-level sociodemographic data required by the Dionysos Project could not be retrieved from the municipal archives for 16 of these 3,345 subjects (0.5%); these subjects were not considered for further analysis. The remaining 3,329 subjects were examined following the same procedures employed for the first phase of the Dionysos Project.8 Four hundred ninety-seven (15%) had suspected liver disease (SLD) according to at least one of the following criteria: (1) ALT > 30 U/L; (2) elevated γ-glutamyltransferase (GGT) (>35 U/L); (3) presence of hepatitis B surface antigen (HBsAg); and (4) presence of antibodies against hepatitis C virus (anti-HCV). The proportion of individuals with SLD was virtually the same as that observed during the first phase of the Dionysos Study.8, 11 The 497 subjects with SLD were matched with an equal number of subjects of the same age and sex but without SLD randomly selected among the remaining 2,832 subjects. Selection on the basis of SLD status was performed to test the hypothesis that NAFLD is more prevalent in individuals with than in those without SLD.
Methods.
Besides a clinical and laboratory evaluation,8 each subject underwent a liver ultrasonography, an anthropometric assessment, and a 7-day diary of food intake. The clinical examination included a detailed interview aimed to exclude the use, in the last 6 months, of drugs able to induce fatty liver (e.g., amiodarone). Surgical interventions able to induce fatty liver (e.g., bilio-pancreatic diversion) were also excluded by this means. Systolic and diastolic blood pressure was measured in triplicate on the same day, and the mean value of the three measurements was used for analysis.12 HBsAg and anti-HCV antibodies were assessed as described elsewhere, and subjects with anti-HCV antibodies underwent an HCV-RNA assessment to confirm HCV infection.9 Measurements of fasting glucose, triglycerides, cholesterol, and high-density lipoprotein (HDL) cholesterol were performed by standard laboratory methods. Insulin was measured by radioimmunoassay (ADVIA Insulin Ready Pack 100, Bayer Diagnostics, Milan, Italy), with intra- and inter-assay coefficients of variation <5%. Insulin sensitivity was estimated using the homeostasis model assessment (HOMA) method ([glucose (mmol/L) / insulin (mU/L)]/22.5).1, 13 The diagnosis of fatty liver was performed by ultrasonography, using standardized criteria.14 Liver ultrasonography was performed in all subjects by the same operator, who was unaware of the clinical and laboratory data. NAFLD was operationally defined as fatty liver in a subject drinking ≤20 g/day of ethanol, and alcoholic fatty liver disease (AFLD) as fatty liver in a subject drinking > 20 g/day of ethanol, in the absence of both HBsAg and HCV-RNA positivity.1 The anthropometric assessment included measurements of weight, stature, and waist circumference.15 Body mass index (BMI) was calculated as weight (kg)/stature (m2). The 7-day diary of food intake was administered to the subjects by 2 trained dietitians, who discussed it with the subject when he or she returned it 1 week later. To avoid the possible confounding effect of seasonality on food intake, the 7-day diary was administered to a similar number of cases and controls each month. Mean daily ethanol intake was calculated as the mean value of ethanol intake as assessed by the 7-day diary, which is the recognized gold standard for the assessment of food intake16 and is more accurate than conventional methods for the evaluation of alcohol intake.17 For the present study, the 7-day diary was used only to obtain alcohol consumption data. The study protocol was approved and supervised by the Scientific Committee of the Fondo per lo Studio delle Malattie del Fegato-ONLUS (Trieste, Italy), and all subjects gave their written informed consent to participate to the study.
Statistical Analysis.
Continuous variables are given as medians and interquartile ranges (IQR) because of skewed distributions. Comparisons of continuous variables between subjects with and without SLD were performed with the Mann-Whitney U test and those of nominal variables with the Fisher' s exact test. The analysis of risk factors for NAFLD was performed in the pooled sample by comparing subjects with NAFLD with those with AFLD and those with normal liver. Comparisons of continuous variables between subjects with NAFLD, AFLD, and normal liver were performed with the Kruskal-Wallis H test and those of nominal variables with the Pearson's chi-square test. When a significant difference was detected with the Kruskal-Wallis H test, the Mann-Whitney U test with Bonferroni' s correction for three groups (NAFLD, AFLD, and normal liver) was used to identify the source of the difference.18 Potential predictors were evaluated for their ability to distinguish NAFLD from AFLD and normal liver. To this aim, a multinomial logistic regression model was used,19 with the outcome variable coded as 0 = NAFLD, 1 = normal liver, and 2 = AFLD, and with logit 1/0 and logit 2/0 as the logits of interest. The Wald test statistic of each logit was used to obtain an indication of the importance of the predictor and the likelihood ratio test was used to assess overall significance. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Significant predictors of the outcome at univariable analysis were evaluated at multivariable analysis. Age was analyzed as quartiles. The cutoffs for liver enzymes were based on the upper limit of normal of our laboratory.8 ALT was thus classified as ≤30 and >30 U/L (“elevated ALT”), aspartate aminotransferase (AST) as ≤30 and >30 U/L (“elevated AST”), and GGT as ≤35 and >35 U/L (“elevated GGT”). Obesity was defined as BMI ≥ 30.0 kg/m2.20 The cutoffs for waist circumference (>88 cm in women and >102 cm in men or “large waist”), glucose (≥110 mg/dL or “hyperglycemia”), triglycerides (≥150 mg/dL or “hypertriglyceridemia”), HDL-cholesterol (<40 mg/dL in men and <50 mg/dL in women or “low HDL”), and systolic (≥130 mm Hg or “systolic hypertension”) and diastolic blood pressure (≥ 85 mm Hg or “diastolic hypertension”) were those used by the National Cholesterol Education Program to define the metabolic syndrome.5 Insulin and HOMA were analyzed as quartiles. Statistical significance was set to a P value of less than .01 for comparisons involving three groups and to a P value of less than .05 for all other tests. Statistical analysis was performed using STATA 8.2 (STATA Corporation, College Station, TX).
Results
Characteristics of Subjects With and Without SLD.
Three hundred twenty-four of the subjects with SLD (65%) and 335 of those without SLD (67%) agreed to participate to the Nutrition and Liver Study. The variables of interest for the analysis were available for 311 subjects with (96%) and 287 (86%) without SLD. Sixty-five percent of the subjects with SLD had elevated ALT, and 54% elevated GGT, 8% were HBsAg positive and 20% HCV-RNA positive, and 40% of them had two or more entry criteria. Ninety-eight percent of the study subjects were white. Figure 1 shows the flow of subjects across the study, and Table 1 reports the characteristics of the subjects with and without SLD. There were no between-group differences in age, sex, ethanol intake, stature, cholesterol, triglycerides, systolic blood pressure, and diastolic blood pressure. On the contrary, ALT (P < .0001), AST (P < .0001), GGT (P < .0001), weight (P = .024), BMI (P = .002), waist circumference (P = .001), glucose (P = .007), insulin (P < .0001), HOMA (P < .0001), and HDL-cholesterol (P < p < .0001) were higher in subjects with SLD than in those without it.
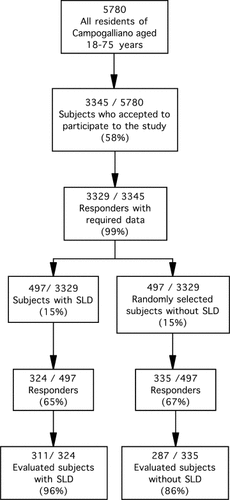
Flow of the subjects across the study. SLD, suspected liver disease.
| SLD (n = 311) | No SLD (n = 287) | P | |
|---|---|---|---|
| Age (years), n (%) | 58 (22) | 60 (21) | .194 |
| Gender (male/female, n) | 194/117 | 156/131 | .056 |
| Ethanol (g/day) | 9 (34) | 11 (31) | .644 |
| ALT (U/L)§ | 35 (23) | 17 (9) | <.0001 |
| AST (U/L) | 27 (13) | 18 (5) | <.0001 |
| GGT (U/L) | 38 (44) | 18 (11) | <.0001 |
| Weight (kg) | 75.5 (18.7) | 73.3 (16.5) | .024 |
| Stature (m) | 1.65 (.15) | 1.65 (.14) | .857 |
| BMI (kg/m2) | 27.8 (6.0) | 26.7 (4.7) | .002 |
| Waist circumference (cm) | 93 (16) | 90 (14) | .001 |
| Glucose (mg/dL)† | 94 (16) | 91 (13) | .007 |
| Insulin (mU/L)†† | 8 (8) | 6 (4) | <.0001 |
| HOMA | 1.9 (2.0) | 1.3 (1.2) | <.0001 |
| Cholesterol (mg/dL)††† | 213 (58) | 211 (53) | .689 |
| HDL-cholesterol (mg/dL)††† | 37 (17) | 32 (11) | <.0001 |
| Triglycerides (mg/dL)†††† | 105 (86) | 100 (74) | .081 |
| Systolic blood pressure (mm Hg) | 130 (20) | 130 (20) | .872 |
| Diastolic blood pressure (mm Hg) | 80 (5) | 80 (5) | .752 |
- NOTE. Continuous variables are given as median and interquartile ranges (in brackets) and nominal variables as the number of subjects with the feature of interest. Between-group comparisons were performed with Mann-Whitney U test for continuous variables and with Fisher's exact test for nominal variables.
- To convert in SI units multiply †by .05551, ††by 7.175, †††by .02586, and ††††by .01129.
- Abbreviations: SLD, suspected liver disease; ALT, alanine aminotransferase; AST, aspartate-aminotransferase; GGT, γ-glutamyltransferase; BMI, body mass index; HOMA, homeostasis model assessment; HDL-cholesterol, cholesterol bound to high-density lipoproteins.
- § Normal values for laboratory tests: ALT, 0–30 U/L; AST, 0–30 U/L; GGT, 0–35 U/L; glucose, 70–110 mg/dL; cholesterol <200 mg/dL (NCEP5); HDL-cholesterol, ≥40 mg/dL in males; and ≥50 mg/dL in females (NCEP5), triglycerides <150 mg/dL (NCEP5).
Prevalence of Fatty Liver and NAFLD in Subjects With and Without SLD.
The number of cases of fatty liver is given in Fig. 2 for subjects with and without SLD. After exclusion of subjects with fatty liver and either HBsAg (n = 4) or HCV-RNA positivity (n = 19), fatty liver was more prevalent in subjects with SLD (44 vs. 35%, P < .0001) but NAFLD was similarly prevalent in subjects with and without SLD (25 vs. 20%; P = .203). Twenty-one HBsAg-positive and 43 HCV-RNA-positive subjects had normal liver. The frequency of fatty liver was therefore 16% (4/25) in HBsAg-positive subjects and 31% (19/62) in HCV-RNA–positive subjects.
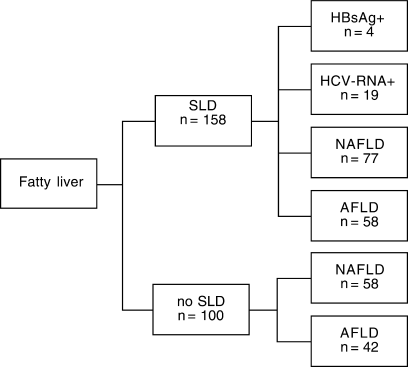
Cases of fatty liver among subjects with and without suspected liver disease. SLD, suspected liver disease; HBsAg+, subjects with HBsAg positivity; HCV-RNA+, subjects with HCV-RNA positivity; NAFLD, nonalcoholic fatty liver disease; AFLD, alcoholic fatty liver disease.
Comparison of Subjects With NAFLD, AFLD, and Normal Liver.
Because the prevalence of NAFLD was similar in subjects with and without SLD, individuals with NAFLD (n = 135) were compared with those with AFLD (n = 100) and those with normal liver and without HBsAg or HCV-RNA positivity (n = 276). Table 2 reports the characteristics of these three groups. Age and cholesterol were similar in all groups. Males accounted for 56%, 94%, and 53% of the individuals with NAFLD, AFLD, and normal liver, respectively (P < .0001 for all comparisons). The median (interquartile range) ethanol intake was 1 (9) g/day in subjects with NAFLD, 43 (36) in subjects with AFLD, and 11 (31) in subjects with normal liver (P < .01 for all between-group comparisons). Subjects with AFLD were taller than those with NAFLD or normal liver (P < .01 for all between-group comparisons) because of the different sex distribution. ALT, AST, weight, BMI, waist circumference, glucose, insulin, HOMA, HDL-cholesterol, triglycerides, and systolic and diastolic blood pressure were similar in subjects with NAFLD and AFLD but higher than in those with normal liver (P < .01 for all between-group comparisons). GGT activity was increased in subjects with AFLD as compared with those with NAFLD, and in these latter than in those with normal liver (P < .01 for all between-group comparisons).
| NAFLD (n = 135) | AFLD (n = 100) | Normal Liver (n = 276) | P | |
|---|---|---|---|---|
| Age (years), n (%) | 57 (19) | 58 (15) | 57 (25) | .511 |
| Gender (male/female, n) | 76/59 | 94/6 | 145/131 | <.0001 |
| Ethanol (g/day) | 1 (9)a | 43 (36)b | 11 (31)c | <.0001 |
| ALT (U/L)* | 28 (26)a | 27 (17)a | 19 (14)b | <.0001 |
| AST (U/L) | 22 (11)a | 23 (8)a | 20 (8)b | <.0001 |
| GGT (U/L) | 27 (25)a | 36 (46)b | 19 (14)c | <.0001 |
| Weight (kg) | 81.0 (2.0)a | 81.7 (15.6)a | 70.2 (15.5)b | <.0001 |
| Stature (m) | 1.65 (.15)a | 1.69 (.09)b | 1.65 (.15)a | <.0001 |
| BMI (kg/m2) | 30.2 (7.2)a | 28.6 (4.0)a | 25.8 (4.1)b | <.0001 |
| Waist circumference (cm) | 98 (15)a | 98 (14)a | 86 (14)b | <.0001 |
| Glucose (mg/dL)† | 96 (22)a | 96 (14)a | 89 (13)b | <.0001 |
| Insulin (mU/L)†† | 10 (10)a | 8 (8)a | 5 (4)b | <.0001 |
| HOMA | 2.3 (2.5)a | 1.9 (1.9)a | 1.2 (.9)b | <.0001 |
| Cholesterol (mg/dL)††† | 216 (59) | 225 (44) | 212 (52) | .168 |
| HDL-cholesterol (mg/dL)††† | 41 (16)a | 36 (16)a | 29 (12)b | <.0001 |
| Triglycerides (mg/dL)†††† | 125 (82)a | 148 (118)a | 91 (60)b | <.0001 |
| Systolic blood pressure (mm Hg) | 135 (20)a | 135 (14)a | 130 (20)b | <.0001 |
| Diastolic blood pressure (mm Hg) | 85 (5)a | 85 (10)a | 80 (5)b | <.0001 |
- NOTE. Continuous variables are given as median and interquartile ranges (in brackets) and nominal variables as the number of subjects with the feature of interest. Between-group comparisons were performed with Kruskal-Wallis H test for continuous variables and with Pearson chi-square test for nominal variables. When a significant difference was detected by the Kruskal-Wallis H test, Bonferroni correction for 3 groups was used to identify the source of the difference.
- To convert in SI units, multiply †by .05551, ††by 7.175, †††by .02586, and ††††by .01129.
- Abbreviations: SLD, suspected liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; BMI, body mass index; HOMA, homeostasis model assessment; HDL-cholesterol, cholesterol bound to high-density lipoproteins.
- * Normal values for laboratory tests: ALT, 0–30 U/L; AST, 0–30 U/L; GGT, 0–35 U/L; glucose, 70–110 mg/dL; cholesterol, <200 mg/dL (NCEP5); HDL-cholesterol, ≥40 mg/dL in males and ≥50 mg/dL in females (NCEP5); triglycerides, <150 mg/dL (NCEP5).
- a, b, c Values not sharing the same superscript are significantly different at a P < .01.
Univariable Analysis of Risk Factors for NAFLD.
Table 3 gives the results of the univariable analysis of risk factors for NAFLD. As detected by the likelihood ratio test, age was significantly associated with the outcome (P = .017), even if none of the interquartile differences was detected as significant by the Wald test. Normal liver was less likely than NAFLD in subjects with elevated ALT (OR = 0.36), elevated GGT (OR = 0.41), obesity (OR = 0.11), large waist (OR = 0.17), hyperglycemia (OR = 0.11), low HDL-cholesterol (OR = 0.16), hypertriglyceridemia (OR = 0.29), systolic hypertension (OR = 0.50), and diastolic hypertension (OR = 0.58). The odds of normal liver versus NAFLD decreased for increasing levels of insulin (OR = 0.24, 0.17, and 0.05 for the 2nd, 3rd, and 4th quartiles, respectively) and HOMA (OR = 0.43, 0.23, and 0.06 for the 2nd, 3rd and 4th quartiles, respectively). The odds of normal liver versus NAFLD were similar in males and females and in subjects with and without elevated AST. AFLD was more likely than NAFLD in males (OR = 12.16), in individuals with elevated GGT (OR = 2.17), and in those with hypertriglyceridemia (OR = 1.76), whereas it was less likely than NAFLD in individuals with obesity (OR = 0.46) and in those with a large waist (OR = 0.52). The odds of AFLD versus NAFLD were similar for all quartiles of insulin and HOMA and in individuals with or without elevated ALT, elevated AST, hyperglycemia, low HDL, and systolic and diastolic hypertension.
| Normal Liver vs. NAFLD | AFLD vs. NAFLD | Model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P-Wald | OR (95% CI) | P-Wald | P-LR | |
| Age (years) | |||||
| 2nd quartile (45–56) | .79 (.45–1.41) | .428 | 1.88 (.88–4.04) | .103 | |
| 3rd quartile (57–65) | .67 (.38–1.18) | .163 | 1.64 (.77–3.50) | .200 | .017 |
| 4th quartile (≥66) | 1.54 (.84–2.82) | .166 | 2.10 (.92–4.77) | .078 | |
| Gender | |||||
| Male | .86 (.57–1.30) | .473 | 12.16 (4.98–29.69) | <.0001 | <.0001 |
| ALT (U/L) | |||||
| >30 | .36 (.23–.55) | <.0001 | .65 (.38–1.11) | .116 | <.0001 |
| AST (U/L) | |||||
| >30 | .56 (.31–1.00) | .050 | .81 (.4–1.64) | .555 | .137 |
| GGT (U/L) | |||||
| >35 | .41 (.26–.66) | <.0001 | 2.17 (1.27–3.68) | .004 | <.0001 |
| BMI (kg/m2) | |||||
| ≥30 | .11 (.06–.18) | <.0001 | .46 (.27–.78) | .004 | <.0001 |
| Waist (cm) | |||||
| >102 in males or >88 in females | .17 (.11–.27) | <.0001 | .52 (.31–.88) | .015 | <.0001 |
| Glucose (mg/dL) | |||||
| ≥110 | .11 (.05–.23) | <.0001 | .52 (.27–1.03) | .060 | <.0001 |
| Insulin (mU/L) | |||||
| 2nd quartile (4–6) | .24 (.11–.52) | <.0001 | .61 (.22–1.67) | .336 | |
| 3rd quartile (7–9) | .17 (.08–.36) | <.0001 | .74 (.28–1.94) | .539 | <.0001 |
| 4th quartile (≥10) | .05 (.02–.11) | <.0001 | .49 (.19–1.24) | .131 | |
| HOMA | |||||
| 2nd quartile (1.1–1.4) | .43 (.20–.89) | .023 | .99 (.37–2.63) | .989 | |
| 3rd quartile (1.5–2.4) | .23 (.11–.46) | <.0001 | .93 (.37–2.34) | .875 | <.0001 |
| 4th quartile (≥2.5) | .06 (.03–.12) | <.0001 | .62 (.26–1.50) | .288 | |
| HDL-cholesterol (mg/dL) | |||||
| <40 in males or <50 in females | .16 (.09–.27) | <.0001 | .90 (.53–1.53) | .693 | <.0001 |
| Triglycerides (mg/dL) | |||||
| ≥150 | .29 (.18–.48) | <.0001 | 1.76 (1.04–2.97) | .036 | <.0001 |
| Systolic blood pressure (mm Hg) | |||||
| ≥130 | .50 (.32–.77) | .002 | 1.49 (.83–2.70) | .185 | <.0001 |
| Diastolic blood pressure (mm Hg) | |||||
| ≥85 | .58 (.39–.88) | .011 | 1.53 (.90–2.60) | .116 | <.0001 |
- NOTE. Data are given as odds ratios and 95% confidence intervals. The Wald test was used to assess the significance of each logit and the likelihood ratio test to assess overall significance.
- Abbreviations: NAFLD, nonalcoholic fatty liver disease; AFLD, alcoholic fatty liver disease; P-Wald, value of P associated with the Wald test; P-LR, value of P associated with the likelihood ratio test; SLD, suspected liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; BMI, body mass index; HOMA, homeostasis model assessment; HDL-cholesterol, cholesterol bound to high-density lipoproteins.
Multivariable Analysis of Risk Factors for NAFLD.
Table 4 gives the results of the multivariable analysis of risk factors for NAFLD. Normal liver was more likely than NAFLD in individuals aged 66 years and older (OR = 3.10) and less likely than NAFLD in those with obesity (OR = 0.26), hyperglycemia (OR = 0.33), hypertriglyceridemia (OR = 0.54), and systolic hypertension (OR = 0.50). Normal liver was also less likely than NAFLD in individuals with insulin levels in the 2nd, 3rd, and 4th quartiles (OR = 0.19, 0.33, and 0.22, respectively) but the interquartile trend observed at univariable analysis was lost. The odds of normal liver versus NAFLD were similar in males and females, in individuals with or without elevated ALT or GGT, large waist, low HDL, and diastolic hypertension. AFLD was more likely than NAFLD in individuals aged 66 years and older (OR = 3.36), in males (OR = 16.24), in subjects with elevated GGT (OR = 2.61), and in those with hypertriglyceridemia (OR = 1.99). AFLD was less likely than NAFLD in individuals with obesity (OR = 0.43) and hyperglycemia (OR = 0.30). The odds of AFLD versus NAFLD were similar in individuals with insulin levels in the 2nd, 3rd, and 4th quartiles of insulin and in individuals with or without elevated ALT, large waist, low HDL, systolic hypertension, and diastolic hypertension. When HOMA was added to the multivariable model in place of glucose and insulin, it contributed significantly to the outcome of interest (P = .045, likelihood ratio test; model not shown), albeit to a lesser degree than either glucose and insulin.
| Normal Liver vs. NAFLD | AFLD vs. NAFLD | Model | |||
|---|---|---|---|---|---|
| OR (95% CI) | P-Wald | OR (95% CI) | P-Wald | P-LR | |
| Age (years) | |||||
| 2nd quartile (45–56) | .83 (.40–1.74) | .630 | 1.90 (.79–4.60) | .153 | |
| 3rd quartile (57–65) | 1.00 (.47–2.16) | .992 | 2.11 (.83–5.34) | .117 | .009 |
| 4th quartile (≥66) | 3.10 (1.32–7.30) | .010 | 3.36 (1.20–9.45) | .021 | |
| Gender | |||||
| Male | .83 (.44–1.55) | .560 | 16.24 (5.28–49.91) | <.0001 | <.0001 |
| ALT (U/L) | |||||
| >30 | .73 (.40–1.32) | .294 | .51 (.26–1.03) | .061 | .162 |
| GGT (U/L) | |||||
| >35 | .78 (.41–1.49) | .452 | 2.61 (1.32–5.16) | .006 | .001 |
| BMI (kg/m2) | |||||
| ≥30 | .26 (.12–.57) | .001 | .43 (.18–1.00) | .049 | .002 |
| Waist (cm) | |||||
| >102 in males or >88 in females | .48 (.22–1.03) | .060 | 1.15 (.49–2.72) | .750 | .090 |
| Glucose (mg/dL) | |||||
| ≥110 | .33 (.13–.84) | .020 | .30 (.13–.71) | .006 | .006 |
| Insulin (mU/L) | |||||
| 2nd quartile (4–6) | .19 (.06–.61) | .005 | .74 (.25–2.21) | .587 | |
| 3rd quartile (7–9) | .33 (.14–.79) | .012 | 1.15 (.38–3.46) | .803 | .006 |
| 4th quartile (≥10) | .22 (.07–.69) | .010 | 1.37 (.27–7.11) | .706 | |
| HDL-cholesterol (mg/dL) | |||||
| <40 in males or <50 in females | .67 (.24–1.91) | .458 | .54 (.14–2.07) | .368 | .610 |
| Triglycerides (mg/dL) | |||||
| ≥150 | .54 (.29–.98) | .044 | 1.99 (1.06–3.77) | .033 | <.0001 |
| Systolic blood pressure (mm Hg) | |||||
| ≥130 | .50 (.27–.92) | .026 | 1.47 (.69–3.15) | .287 | .006 |
| Diastolic blood pressure (mm Hg) | |||||
| ≥85 | 1.14 (.64–2.04) | .661 | 1.25 (.63–2.48) | .531 | .812 |
- NOTE. Data are given as odds ratios and 95% confidence intervals. The Wald test was used to assess the significance of each logit and the likelihood ratio test to assess overall significance.
- P-LR for the full model = 1.9/10−45.
- Abbreviations: NAFLD, nonalcoholic fatty liver disease; AFLD, alcoholic fatty liver disease; P-Wald, value of P associated with the Wald test; P-LR, value of P associated with the likelihood ratio test; SLD, suspected liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; BMI, body mass index; HOMA, homeostasis model assessment; HDL-cholesterol, cholesterol bound to high-density lipoproteins.
Discussion
The Dionysos Nutrition and Liver Study is the first study specifically aimed at establishing the prevalence of and the risk factors for NAFLD in the general population. In Campogalliano, a town of Northern Italy, the prevalence of NAFLD was similar in that of individuals with and without SLD (25% vs. 20%, P = .203) and within the range (20%-30%) hypothesized for Western countries on the basis of clinical series, autopsy studies, and convenience samples of the general population.1, 2
Several risk factors for NAFLD have been identified in previous studies.3 Although age increases the risk of obesity and of the metabolic syndrome, NAFLD is not systematically associated with age.21 In our population, an age ≥ 66 years was an independent predictor of normal liver and AFLD as compared with NAFLD, suggesting that age may be a protective factor for NAFLD. Although NAFLD was originally described to be more prevalent in females,3 most recent series report a higher prevalence in males.22-24 These studies are biased, however, by their selection criteria and especially by the use of an elevated ALT as an entry criterion. We found that male sex was a strong and independent risk factor for AFLD but that the odds of normal liver versus NAFLD were similar in males and females. Thus, our study does not support the hypothesis that any specific gender is a risk factor for NAFLD in the general population. This conclusion applies to Caucasian subjects and might not be valid for individuals of other ethnic background.25, 26
An elevated ALT did not discriminate NAFLD either from normal liver or from AFLD, indicating that ALT is not an independent predictor of NAFLD. In addition, only 54% of NAFLD cases were observed in subjects with elevated ALT. This finding has relevant clinical implications because, as shown by biopsy studies, liver disease in subjects with NAFLD but without elevated ALT may be severe.27 The optimum cutoff of ALT for the diagnosis of liver disease has been recently reevaluated, and lower values are probably needed to increase the negative predictive value.28 Our findings confirm nonetheless that there is a high prevalence of NAFLD in subjects without elevated ALT in the general population and that the use of elevated ALT as a marker of NAFLD has to be discouraged. An elevated GGT was an independent risk factor for AFLD versus NAFLD, in agreement with the specific role of GGT as marker of alcohol abuse,29 but the odds of normal liver versus NAFLD were the same in subjects with and without elevated GGT.
Both normal liver and AFLD were less likely than NAFLD in obese subjects, confirming that BMI is an independent predictor of NAFLD.4, 30 By contrast, a large waist as identified by the operational definition of the metabolic syndrome,5 although associated with NAFLD at univariable analysis, was not an independent predictor of NAFLD. Waist circumference is a surrogate marker of visceral adiposity and a risk factor for cardiovascular and metabolic disease.20, 31 Visceral adiposity is supposed to play a central role in the pathogenesis of fatty liver by increasing the flux of fatty acids to the liver through the portal vein.32 In line with this hypothesis, one can speculate that, at the population level, the contribution of a large waist to fatty liver may be decreased by the inclusion of triglycerides into the model. Ethnic differences,5 potentially accounting for different visceral fat accumulation, do not play a role in our population because it is of almost uniformly Caucasian origin. Another confounding factor may be the use of thiazolidinediones, which may cause a redistribution of body fat.33 However, none of our subjects was under therapy with thiazolidinediones at the time the study was performed. Although waist circumference was not an independent predictor of NAFLD at the cutoff adopted by the definition of the metabolic syndrome,5 this does not imply that waist may not be a predictor of NAFLD at different cutoffs.
Normal liver was less likely and AFLD more likely than NAFLD in subjects with hypertriglyceridemia, in line with previous studies performed in selected groups of patients.6 Both normal liver and AFLD were less likely than NAFLD in individuals with hyperglycemia, confirming that an altered glucose metabolism is a risk factor for NAFLD.3 Importantly, hyperglycemia was associated with a greater risk of NAFLD independently from hypertriglyceridemia. On the contrary, insulin was identified as an independent predictor of NAFLD versus normal liver, but it did not discriminate NAFLD from AFLD. HOMA, a measure of insulin resistance, was less associated with NAFLD than its two individual components (glucose and insulin). It should be pointed out that HOMA is only a surrogate marker of insulin resistance, although extensively validated for epidemiological studies,34 and increasingly used as a marker of insulin resistance in NAFLD studies.1, 6, 35, 36 The clinical significance of fasting insulin as a marker of insulin resistance is also well documented.37 Our data show that insulin resistance is a risk factor for NAFLD but also indicate that in the general population, the two components of HOMA are independent and better predictors of NAFLD. HOMA is known to lose accuracy as a marker of insulin resistance in the presence of diabetes mellitus, when insulin production drops.38, 39 An analysis of the data after exclusion of subjects with diabetes (n = 29; 15 with NAFLD, 8 with AFLD and 6 with normal liver, as diagnosed by a value of blood glucose ≥ 126 mg/dL or the use of anti-diabetic drugs5) showed no change in the predictive power of HOMA both at univariable and multivariable analysis (data not shown).
Lastly, systolic, but not diastolic, hypertension was associated with a greater risk of NAFLD. The association of hypertension with NAFLD is well documented,40 independently of ALT elevation, and is confirmed and expanded by the current study at the population level.
NAFLD and AFLD were separated by using the currently suggested cutoff of 20 g/day of ethanol intake.1 Using a cutoff of 30 g/day, the prevalence of NAFLD would be 3% higher in subjects with (28%) and 4% higher (24%) in those without SLD. This difference is not very large, but, having being detected by a 7-day diary of alcohol intake, it points to the importance of an accurate assessment of ethanol consumption,17 besides the need of common diagnostic criteria for NAFLD.1 It is of some interest that ethanol intake was lower and less variable in subjects with NAFLD than in those with normal liver. Moreover, the percentage of abstainers was higher among NAFLD (48%) than normal liver (31%) subjects (P = .0015), giving partial support to the hypothesis that moderate alcohol consumption may be associated with a lower risk of NAFLD.41
Although performed in the general population, our study is not without limitations. The most important limitation is the suboptimal respondent rate. Only 58% of potential subjects agreed to participate to the study. Nonresponders were younger and more frequently males than responders, a pattern similar to that observed in the first phase of the Dionysos study.8, 11 The second limitation is the use of ultrasonography to diagnose liver steatosis. Even if ultrasonography is reasonably accurate as compared with nuclear magnetic resonance spectroscopy and liver biopsy, it cannot identify fatty infiltration of the liver below a threshold of 30%.2, 14, 42 Accordingly, an undefined number of cases of fatty liver is missed in subjects both with and without SLD. Ultrasonography also does not provide any information about the histological features more closely associated with disease progression, such as inflammation and fibrosis.1 This information can be obtained only by liver biopsy, but histological series are neither feasible nor ethically justified in a condition with low risk of progression and no definite treatment.3
In conclusion, our study shows that in the general population of a Northern Italian town, NAFLD is similarly prevalent in individuals with and without SLD and is associated with most of the features of the metabolic syndrome, strongly suggesting that NAFLD is the hepatic component of this syndrome.
Acknowledgements
The authors thank the dietitians Marilena Passalacqua and Francesca Cortesi for their help, and Drs. Deanna Bulgarelli, Enrico Zirilli and Nino Battistini for their collaboration and suggestions.




