PGE1-induced NO reduces apoptosis by D-galactosamine through attenuation of NF-κB and NOS-2 expression in rat hepatocytes
Abstract
Prostaglandin E1 (PGE1) reduces cell death in experimental and clinical liver dysfunction. We have previously shown that PGE1 preadministration protects against NO-dependent cell death induced by D-galactosamine (D-GalN) through a rapid increase of nuclear factor κB (NF-κB) activity, inducible NO synthase (NOS-2) expression, and NO production. The present study investigates whether PGE1-induced NO was able to abolish NF-κB activation, NOS-2 expression, and apoptosis elicited by D-GalN. Rat hepatocytes were isolated following the classical method of collagenase perfusion of liver. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) in primary culture rat hepatocytes. PGE1 reduced inhibitor κBα degradation, NF-κB activation, NOS-2 expression, and apoptosis induced by D-GalN. The administration of an inhibitor of NOS-2 abolished the inhibitory effect of PGE1 on NF-κB activation and NOS-2 expression in D-GalN–treated hepatocytes. Transfection studies using different plasmids corresponding to the NOS-2 promoter region showed that D-GalN and PGE1 regulate NOS-2 expression through NF-κB during the initial stage of hepatocyte treatment. PGE1 was able to reduce the promoter activity induced by D-GalN. In addition, a NO donor reduced NOS-2 promoter activity in transfected hepatocytes. In conclusion, administration of PGE1 to hepatocytes produces low levels of NO, which inhibits its own formation during D-GalN–induced cell death through the attenuation of NF-κB–dependent NOS-2 expression. Therefore, a dual role for NO in PGE1-treated D-GalN–induced toxicity in hepatocytes is characterized by a rapid NO release that attenuates the late and proapoptotic NOS-2 expression. Supplementary material for this article can be found on the HEPATOLOGY website (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html). (HEPATOLOGY 2004;40:1295–1303.)
Prostaglandins are biologically active polyunsaturated fatty acids derived from arachidonic acid present in most mammalian tissues.1 Prostaglandin E1 (PGE1) reduces liver injury induced experimentally2 and in fulminant viral hepatitis in humans.3 PGE1 exerts most of its effects by promoting membrane stabilization,4, hepatocyte proliferation,5 vasodilation,6 and inhibition of fibrogenesis.7 The preadministration of PGE1 also reduces liver injury through the enhancement of inducible NO synthase (NOS-2) expression in hepatocytes.8 Nevertheless, the detailed molecular mechanism through which PGE1 regulates NOS-2 expression is not completely understood. It is clearly established that the effects of PGE result from its binding to its receptors, EP1, EP2, and EP4, all of which can stimulate the production of the second messenger cyclic 3′,5′ adenosine monophosphate (cAMP). This messenger induces NOS-2 expression in numerous cell types, including vascular smooth muscle cells, cardiac myocytes, renal messangial cells, and macrophages,9-12 whereas it inhibits NOS-2 expression and nuclear factor κB (NF-κB)–binding activity in hepatocytes.13
NF-κB is a transcription factor involved in immune and inflammatory responses and in cellular defense mechanisms.14 The p50/p65 NF-κB heterodimer is retained in the cytoplasm by associating with inhibitor κB (IκB). The activation of cells with an appropriate stimulus results in the phosphorylation, ubiquitinylation, and degradation of IκB, which allows NF-κB to translocate into the nucleus and to trigger κB-dependent transcription. NF-κB activation has been shown to suppress tumor necrosis factor alpha–dependent induction of apoptosis15, 16 through mechanisms that involve the inhibition of c-Jun N-terminal mitogen activating kinase pathway17 and/or the increase of the antiapoptotic proteins c-IAP1, c-IAP2, TRAF2, and Bcl-2.18-20
NO has emerged as a regulatory molecule involved in the control of many different biological processes. It is produced by constitutive and inducible forms of NOS present in a variety of cell types.21 Hepatocytes are known to express NOS-2 upon stimulation with cytokines.22 In this regard, NF-κB has been shown to be the most relevant transcription factor regulating the expression of human and rat NOS-2 in hepatocytes,23, 24 and NO plays an important role in the outcome of liver injury,25 mediating26 or abolishing27, 28 cell death in primary culture of rat hepatocytes. Hatano et al.29 have shown that NF-κB–dependent expression of NOS-2 protects hepatocytes from tumor necrosis factor alpha– and Fas-mediated apoptosis.
We have previously shown that the induction of cell death by D-galactosamine (D-GalN) is associated with an increase in NO production by hepatotoxins in cultured rat hepatocytes.30 Nevertheless, the inhibition of NOS-2 revealed that early NO production also mediates the protection by PGE1 preadministration against D-GalN–induced apoptosis.31 The aim of the present study was to elucidate the mechanisms through which PGE1 is able to regulate the cell death induced by D-GalN and whether initial synthesis of NO induced by PGE1 can exert a negative feedback regulation of NOS-2 expression and NO produced after D-GalN challenge.
Abbreviations
PGE1, prostaglandin E1; D-GalN, D-galactosamine; NF-κB, nuclear factor κB; NOS-2, inducible NO synthase; cAMP, cyclic 3′,5′ adenosine monophosphate; IκB, inhibitor κB; FBS, fetal bovine serum; HEPES, hydroxyethylpiperazine-N-2 ethanesulfonic acid; EDTA, ethylenediaminetetraacetic acid; RT-PCR, reverse-transcriptase polymerase chain reaction; mRNA, messenger RNA; PCR, polymerase chain reaction; LUC, luciferase; L-NAME, NG-nitro-L-arginine methyl ester; SNAP, S-nitroso-N-acetyl penicillamine.
Materials and Methods
Materials.
All reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. William's medium E was obtained from AppliChem (Darmstadt, Germany). Antibiotic–antimycotic solution and fetal bovine serum (FBS) were obtained from Invitrogen, Ltd. (Paisley, UK). Interleukin 1 and interferon gamma were obtained from Genzyme Diagnostic (Cambridge, MA). PGE1 was obtained from Pharmacia & Upjohn (Puurs, Belgium). All experimental animals received humane care, and the study protocols complied with the institutional guidelines for animal usage in research.
Isolation and Culture of Primary Rat Hepatocytes.
Male Wistar rats (200-250 g) were anesthetized with sodium thiopental via intraperitoneal administration. Hepatocytes were isolated by way of a nonrecirculating in situ collagenase perfusion of livers cannulated through portal vein following a protocol described by Seglen et al.32 Livers were perfused first with an oxygenated solution I (10 mmol/L hydroxyethylpiperazine-N-2 ethanesulfonic acid [HEPES], 6.7 mmol/L KCl, 145 mmol/L NaCl and 2.4 mmol/L egtazic acid), pH 7.4 at 37°C at a flow of 40 mL/min for 10 minutes, and then with solution II (100 mmol/L HEPES, 6.7 mmol/L KCl, 67 mmol/L NaCl, 10 g/L albumin, 4.8 mmol/L CaCl2, and 0.05 % collagenase A) (pH 7.4) at 37°C at a flow of 20 mL/min for 10 minutes. Hepatocytes were filtered through a nylon mesh, centrifuged, and washed three times in William's medium E (pH 7.4), supplemented with 1 μmol/L insulin, 0.6 μmol/L hydrocortisone, 15 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin, 2 mmol/L glutamine, and 26 mmol/L NaHCO3. Cell viability was consistently above 85% as determined via Trypan blue exclusion. Contamination of hepatocyte cultures with Kupffer cells was not detected morphologically, through latex bead ingestion (3 μm), or via fluorescein isothiocyanate–labeled ED-1 positivity (Serotec Ltd., Oxford, UK). Hepatocytes (150,000 cells/cm2) were plated in a Petri dish coated with collagen type I (Iwaki, Gyouda, Japan) and cultured in supplemented William's medium E (pH 7.4) containing 5% FBS. After 2 hours, the medium was removed and replaced with fresh supplemented medium without FBS, and the culture was maintained for 20 hours before prostanoid treatment (Supplementary Fig. 1). The 20-hour stabilization period was required because of the observation that collagenase digestion of the liver enhanced NOS-2 expression in hepatocytes and included the highest level of NOS-2 expression 14 hours after cell isolation (data not shown). The final concentration of ethanol, as PGE1 solvent, had no effect on the studied variables. Different parameters were assessed in cultures from the same cell isolation. The experiments related to cell death and NOS-2 expression were run together. Data related to NF-κB activation and NOS-2 promoter activity were independently obtained.
Preparation of Nuclear Extracts.
Nuclear extracts were prepared according to Schreiber et al.33 Briefly, hepatocytes (8 × 106) were recovered in 800 μL of lysis buffer (10 mmol/L HEPES [pH 7.9], 10 mmol/L KCl, 0.1 mmol/L ethylenediaminetetraacetic acid [EDTA], 0.1 mmol/L egtazic acid, 5 μg/mL aprotinin, 10 μg/mL leupeptin, 0.5 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol) and allowed to swell on ice for 15 minutes. Afterward, 50 μL of 10% Nonidet NP-40 was added, vortexed for 30 seconds, and centrifuged at 15,000g for 1 minute at 4°C. The supernatant, as a cytoplasmatic fraction, was recovered and frozen at −80°C. The pellet containing the nuclear fraction was resuspended in 75 μL of nuclear extraction buffer (20 mmol/L HEPES [pH 7.9], 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L egtazic acid, 5 μg/mL aprotinin, 10 μg/mL leupeptin, 0.5 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L dithiothreitol). The tube was incubated on ice for 20 minutes with continuous mixing and centrifuged at 15,000g for 5 minutes at 4°C. Aliquots of the nuclear lysate were stored at −80°C.
Electrophoretic Mobility Shift Assay.
Electrophoretic mobility shift assays were performed using the NF-κB consensus oligonucleotides 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and 3′-TCAACTCCCCTGAAAGGGTCCG-5′ (Promega, Madison, WI). Probes were labeled at the 5′ end with T4 kinase (Promega) and [g-32P]ATP (Amersham Biosciences, Uppsala, Sweden). Excess unreacted adenosine triphosphate was separated from the labeled probe using Sephadex G-25 column (Amersham Biosciences). The binding reaction contained 15 mg nuclear protein, 5 mL incubation buffer (50 mmol/L Tris-HCl [pH 7.5], 200 mmol/L NaCl, 5 mmol/L EDTA, 5 mmol/L b-mercaptoethanol, and 20% glycerol), and 1 mg poly(dI-dC) (Promega). Samples were incubated for 15 minutes on ice and added the corresponding amount of [32P]-labeled probe. Samples were incubated for 15 minutes at room temperature and loaded on a 6% native polyacrylamide gel electrophoresis. Gels were fixed (30% methanol, 10% glacial acetic acid) and dried at 80°C under a vacuum. Dried gels were visualized via autoradiography (Hyperfilm, Amersham Biosciences).
DNA Fragmentation.
The whole hepatocyte population, including the floating cells obtained from collected culture medium, was treated with 1 mL of lysis buffer (100 mmol/L Tris-HCl, 5 mmol/L EDTA, 150 mmol/L NaCl, and 0.5% sarkosyl) (pH 8.0) at 4°C for 10 minutes. Samples were incubated in ribonuclease (50 μg/mL) at 37°C for 2 hours and proteinase K (100 μg/mL) at 48°C for 45 minutes. DNA was obtained via phenol/chloroform/isoamyl alcohol (25:24:1) extraction and precipitated with cold isopropanol (1:1) at −20°C for 12 hours. DNA was recovered via centrifugation at 20,800g at 4°C for 10 minutes. The precipitate was washed with 70% ethanol, dried, and resuspended in Tris-EDTA (pH 8.0). Samples were analyzed on 1.5% agarose gel.
Total RNA Isolation.
Total RNA from the entire hepatocyte population was extracted using Trizol reagent according to the manufacturer's recommendations (Invitrogen Ltd.). Following extraction, RNA was precipitated using ice-cold isopropanol, washed with 75% ethanol, and resuspended in ribonuclease-free water (Promega), and its integrity was verified following separation via electrophoresis on 0.8% agarose gel containing ethidium bromide.
Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time RT-PCR.
The expression of messenger RNA (mRNA) for NOS-2 was first examined via RT-PCR using a commercial kit (Promega). The reaction was performed with a PE Gene Amp PCR System 2400 (Perkin-Elmer, Inc., Wellesley, MA). First-strand complementary DNA was synthesized using 2 μg of total RNA diluted in reaction mixture including specific sets of primers for rat NOS-2 and β-actin (Table 1). Polymerase chain reaction (PCR) was cycled as follows after initial denaturation for 3 minutes at 94°C for 35 cycles: 94°C for 30 seconds, 61°C for 45 seconds, and 72°C for 90 seconds. A final extension step of 10 minutes was performed at 72°C. PCR products were electrophoresed in a 2% agarose gel stained with ethidium bromide. Quantitative real-time RT-PCR was measured using the LightCycler thermal cycler system (Roche Diagnostics, Indianapolis, IN). The primers designed for this study and the theoretical size of the PCR products are given in Table 1. RT-PCR was performed in one step using the QuantiTect SYBR Green RT-PCR kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's protocol. To confirm amplification specificity, the PCR products were subjected to a melting curve analysis, and negative controls containing water instead of RNA were run to confirm that the samples were not cross-contaminated. Quantitation of relative expression was determined using the 2−Δ(ΔCT) method.34
| Target | Primer 5′→3′ | Size Products |
|---|---|---|
| β-Actin | ||
| Sense primer | TGAGAGGGAAATCGTGCGT | 216 bp |
| Antisense primer | TCATGGATGCCACAGGATTCC | |
| NOS-2 | ||
| Sense primer | CATTGAGATCCCGAAACGCTAC | 138 bp |
| Antisense primer | AGCCTCATGGTGAACAGTTCT |
IκBα Expression.
The cytoplasmatic fraction obtained as described in the Preparation of Nuclear Extracts section was used for the measurement of IκBα levels via Western blot analysis. Proteins (100 mg) were separated via 14% SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose (BioTrace NT; PALL Life Sciences, Pensacola, FL). The membrane was incubated with rabbit polyclonal primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Anti–rabbit IgG horseradish peroxidase antibodies (Santa Cruz Biotechnology, Inc.) were used as secondary antibodies revealing protein content via electrochemiluminescence.
NOS-2 Expression and Caspase-3 Processing.
The whole hepatocyte population was treated with 1 mL of lysis solution (50 mmol/L Tris-HCl [pH 7.5], 2 mmol/L EDTA, 100 mmol/L NaCl, 1% nonidet NP-40, 1 mmol/L phenylmethylsulfonyl fluoride, 20 mg/mL aprotinin, 20 mg/mL leupeptin, and 20 mg/mL pepstatin A) at 4°C for 10 minutes and centrifuged at 20,800g at 4°C for 5 minutes. Proteins (100 mg) were separated via 5% (NOS-2) or 12% (caspase-3) SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose. The membranes were incubated with mouse monoclonal (BD Transduction Laboratories, Erembodegem, Belgium) or rabbit polyclonal (Santa Cruz Biotechnology, Inc.) primary antibodies for NOS-2 protein or caspase-3, respectively. Afterward, membranes were incubated with anti–mouse or anti–rabbit IgG horseradish peroxidase (Santa Cruz Biotechnology, Inc.) respectively, as secondary antibodies; protein content was revealed via electrochemiluminescence.
Caspase-3–Associated Activity.
Caspase-3–associated activity was measured in cell lysate via a colorimetric assay using the peptide-based substrate ac-N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA) (Bachem AG, Bubendorf, Switzerland). The increase in absorbance of enzymatically released p-nitroanilide was measured at 405 nm for 1 hour in a DU 640 Spectrophotometer (Beckman Coulter, Inc., CA).
Plasmids.
The 980-bp fragment corresponding to the 5′-flanking region of the mouse NOS-2 gene was fused to a luciferase (LUC)-reported gene (pNOS-2-LUC+/+). Mutated κB sequences of the promoter (nucleotides −980 to +165) were generated via PCR using oligonucleotide primers in which two GG bases of the distal (position −971 to −961) and proximal (position −85 to −75) κB motif were replaced with a CC pair (pNOS-2-LUC−/−). These vectors were sequenced to ascertain their fidelity. Both pNOS-2-LUC constructions included an activator protein 1 site located at −725 bp. A plasmid that contained three copies of the κB motif from the human immunodeficiency virus long terminal repeat enhancer coupled to the conalbumin A promoter [(κB)3ConA.LUC] was used to evaluate κB activity. The plasmid ConA.LUC was used as control.
Transient Transfection Assays.
Plasmids were purified using an Endo-free plasmid kit (Qiagen). Hepatocytes (15,000 cells) were plated in 6-well plates and transfected for 12 hours with 1 μg of plasmid containing constructions and 0.5 μg pβ-galactosidase vector as internal control using the FUGENE transfection reagent following the manufacturer's instructions (Boehringer Mannheim, Germany). After transfection, cells were washed with PBS and maintained with culture medium without FBS for 2 hours before stimulation. LUC and β-galactosidase activities were determined in cell lysates using a commercial LUC cell lysis buffer (Promega). Results were normalized to β-galactosidase activity.
Statistics.
Results are expressed as the mean ± SE. Differences between groups were assessed via one-way ANOVA. If the variances between groups were homogenous (Levene's test), groups were subjected to the multiple comparison least significant differences test.
Results
Protection of PGE1 Against D-GalN–Induced Apoptosis.
We have previously shown that PGE1 reduces D-GalN–induced apoptosis in primary culture of rat hepatocytes.30, 36 In the present study, D-GalN induced the maximum proapoptotic effect 8 hours after its administration in cultured hepatocytes. At this time point, PGE1 significantly reduced DNA fragmentation (Fig. 1A), caspase-3–associated activity (Fig. 1B), and caspase-3 processing (Fig. 1C).
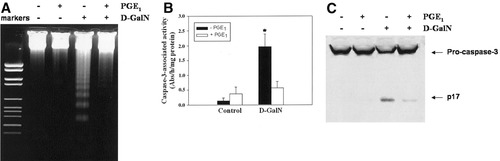
Cytoprotection of PGE1 against D-GalN–induced apoptosis in primary culture of rat hepatocytes. (A) DNA fragmentation, (B) caspase-3–associated activity, and (C) procaspase-3 processing. Apoptosis was measured in hepatocytes treated for 8 hours with D-GalN (5 mmol/L). The cytoprotective effect of PGE1 (1 μmol/L) preadministered to D-GalN–treated hepatocytes was evaluated. PGE1 reduced all the parameters related to apoptosis induced by D-GalN. The blots presented in panels A and C are representative of 5 independent experiments. Data from panel B are the mean ± SE from 5 independent experiments. *P ≤ .02 versus control and PGE1-treated hepatocytes. PGE1, prostaglandin E1; D-GalN, D-galactosamine.
Regulation of NF-κB Activation by PGE1 in D-GalN–Treated Hepatocytes.
Recently, we reported the role of NF-κB in D-GalN–induced hepatotoxicity and its protection by PGE1.31, 35 In these studies, we observed that although NF-κB expression in the nuclear fraction of control hepatocytes varied among cultures, the profile of NF-κB activation did not change. PGE1 administration to hepatocytes induced a rapid NF-κB translocation (0-1 hour). In the present study, PGE1 cytoprotection was also associated with a reduction of NF-κB activation induced by D-GalN at 6 to 24 hours. PGE1 reduced NF-κB translocation to the nucleus (Fig. 2A) and recovered cytoplasmatic IκBα expression (Fig. 2B) in D-GalN–treated hepatocytes. Both observations strongly support the inhibitory effect of PGE1 against NF-κB activation induced by D-GalN.
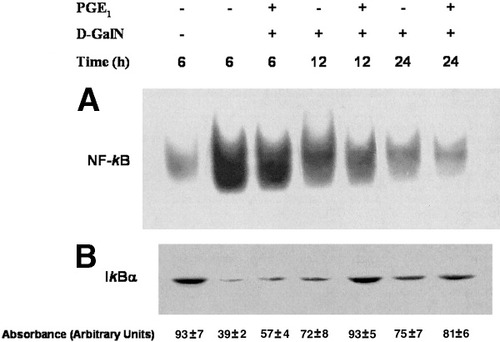
Kinetic study of (A) NF-κB activation and (B) IκBα degradation induced by PGE1 in D-GalN–treated hepatocytes. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) in cultured hepatocytes. NF-κB activation was evaluated in the nuclear fraction and IκBα expression in the cytoplasmatic fraction of hepatocytes. The blots are representative of 5 independent experiments. PGE1, prostaglandin E1; D-GalN, D-galactosamine; NF-κB, nuclear factor κB; IκB, inhibitor κB.
Regulation of NOS-2 Expression by PGE1.
NF-κB is the most relevant transcription factor that regulates NOS-2 expression in hepatocytes.23, 24 We have previously observed that PGE1 modulates NOS-2 protein expression in control and D-GalN–treated hepatocytes.30 PGE1 enhanced by itself the expression of mRNA (Figs. 3A and 5) and protein (Fig. 3B) of NOS-2 in control hepatocytes. Under our conditions, PGE1 significantly raised the expression of NOS-2 protein content compared with the value observed at 1 hour in control hepatocytes. The prostanoid was also able to significantly reduce the induction of mRNA (Fig. 4A; see Fig. 5) and protein levels (Fig. 4B) of NOS-2 induced by D-GalN.
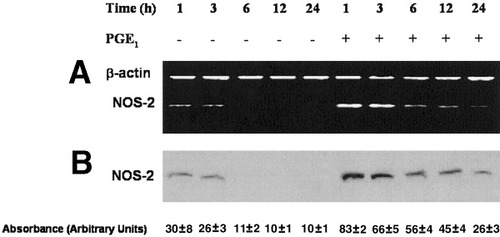
Kinetic study of induction of NOS-2 (A) mRNA and (B) protein expression by PGE1 in hepatocytes. Measurement of NOS-2 mRNA and protein expression was performed using RT-PCR and Western blot analysis, respectively. In the RT-PCR assay, two bands of 216 bp and 138 bp corresponding to β-actin and NOS-2 mRNA, respectively, were observed in agarose gels. PGE1 enhanced NOS-2 mRNA and protein expression in cultured hepatocytes. The blots and agarose gels are representative of 4 independent experiments. PGE1, prostaglandin E1; NOS-2, inducible NO synthase.
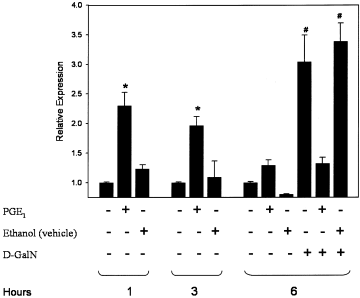
Relative quantification of NOS-2 gene expression in hepatocytes stimulated with PGE1 and/or D-GalN. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) in primary culture of rat hepatocytes. The quantitative measurement of NOS-2 mRNA expression was performed using real-time RT-PCR. Each sample of RNA was analyzed in triplicate, the threshold cycle (CT) was determined, and the mean was obtained (variation coefficient ≤ 3%). Efficiencies for both β-actin and NOS-2 genes were calculated from the slope of the curves generated from the dilution series of RNA using the formula: PCR efficiency = 10−1/slope. Quantification of relative expression was determined using the 2−Δ(ΔCT) method.34 Data are the mean ± SE from 3 independent experiments. *P ≤ .01 versus non–PGE1-treated hepatocytes; #P ≤ .01 versus control and PGE1-treated hepatocytes. PGE1, prostaglandin E1; D-GalN, D-galactosamine.
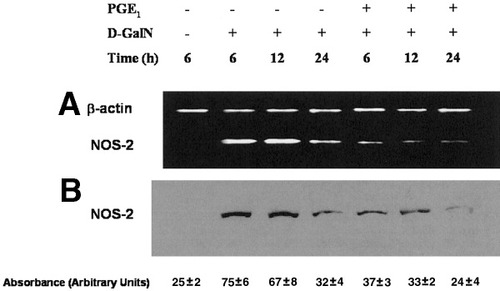
Reduction of NOS-2 (A) mRNA and (B) protein expression by PGE1 in D-GalN–treated hepatocytes. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) in primary culture of rat hepatocytes. The measurement of NOS-2 mRNA and protein expression was performed using RT-PCR and Western blot analysis, respectively. In the RT-PCR assay, two bands of 216 bp and 138 bp corresponding to β-actin and NOS-2 mRNA, respectively, were observed in agarose gels. The blots and agarose gels are representative of 4 independent experiments. PGE1, prostaglandin E1; D-GalN, D-galactosamine; NOS-2, inducible NO synthase.
Effect of NOS-2 Inhibition on IκBα and NOS-2 Expression During PGE1 Cytoprotection Against D-GalN–Induced Apoptosis in Hepatocytes.
The administration of the NOS-2 inhibitor NG-nitro-L-arginine methyl ester (L-NAME) showed that NO mediates the protection by PGE1 against D-GalN–induced apoptosis.31 In this sense, NOS-2 inhibition by L-NAME should abolish the inhibition of D-GalN–induced NOS-2 expression by the prostanoid. According to this suggestion, L-NAME abolished the effect of PGE1 on NOS-2 expression in hepatocytes treated with a hepatotoxin (Fig. 6). In concordance with the preceding data (see Figs. 2B and 4B), the recovery of IκBα by PGE1 in D-GalN–treated hepatocytes correlated with a reduction in NOS-2 expression (see Fig. 6). L-NAME significantly reduced IκBα and enhanced NOS-2 expression in PGE1 + D-GalN–treated hepatocytes. These results suggest that PGE1-derived NO was mediating the attenuation of NF-κB activation and NOS-2 expression obtained by the prostanoid in hepatocytes treated with a hepatotoxin.
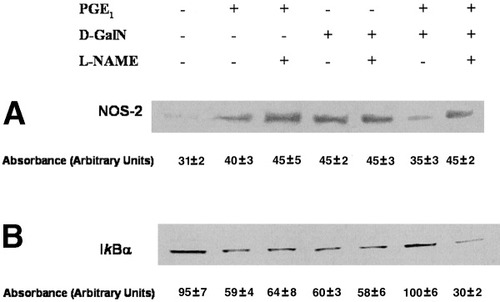
Effect of L-NAME on (A) NOS-2 and (B) IκBα protein expression in PGE1 and/or D-GalN–treated hepatocytes. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) and L-NAME (0.5 mmol/L) 2 hours before PGE1 or 4 hours before D-GalN in cultured hepatocytes. The measurement of NOS-2 and IκBα expression was made via Western blot analysis. The blots are representative of 3 independent experiments. PGE1, prostaglandin E1; D-GalN, D-galactosamine; L-NAME, NG-nitro-L-arginine methyl ester; NOS-2, inducible NO synthase; IκB, inhibitor κB.
Effect of PGE1 and/or D-GalN on NOS-2 Promoter Activity.
The capacity of D-GalN and/or PGE1 to stimulate activity of the NOS-2 promoter and the participation of NF-κB was assessed using pNOS-2-LUC+/+ and (κB)3ConA.LUC plasmids, respectively, in cultured rat hepatocytes (Fig. 7A). PGE1 significantly enhanced LUC activity using both constructions. A reduction of D-GalN–induced LUC prostanoid activity was also observed. To further demonstrate the role of NO on NOS-2 expression, different low concentrations of S-nitroso-N-acetyl penicillamine (SNAP), as a NO donor, were added to transfected hepatocytes. The concentrations of SNAP used in the present study (0.0012-0.03 μmol/L) have been previously shown to reduce cell death induced by D-GalN in cultured rat hepatocytes.31 It was observed that 0.03 μmol/L SNAP was able to reduce LUC activity using pNOS-2-LUC+/+ and (κB)3ConA.LUC (Fig. 7B).
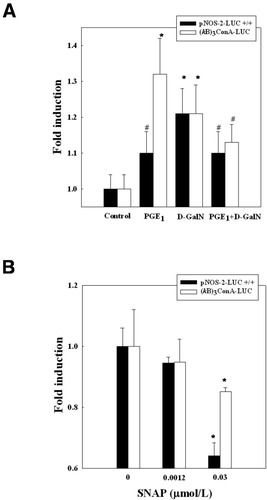
Regulation of pNOS-2-LUC+/+ and (κB)3ConA-LUC reporter activities (A) 6 hours after PGE1 and/or D-GalN treatment and (B) 12 hours after treatment with a NO donor. Hepatocytes were transfected with 1 mg of plasmids and 0.5 mg of pβgalactosidase/control vector. PGE1 (1 μmol/L) was administered 2 hours before D-GalN (5 mmol/L) to primary culture of rat hepatocytes. SNAP, as a NO donor, was added at different concentrations (0-0.03 μmol/L) to primary culture of rat hepatocytes. LUC and β-galactosidase activities were determined, and the results were expressed as fold induction versus control hepatocytes after normalization of β-galactosidase activity. Data are the mean ± SE from 3 independent experiments. *P < .05 versus control hepatocytes; #P < .05 versus D-GalN–treated hepatocytes. PGE1, prostaglandin E1; D-GalN, D-galactosamine; SNAP, S-nitroso-N-acetyl penicillamine.
Regulation of Cytokine-Induced NOS-2 Expression by PGE1 in Hepatotoxic Conditions.
PGE1 has been shown to prevent the expression of NOS-2 induced by lipopolysaccharide in hepatocytes.37 Therefore, we investigated whether PGE1 was also able to regulate NOS-2 expression in hepatocytes stimulated with proinflammatory cytokines during cytotoxicity induced by a hepatotoxin. The stimulation with cytokines during a 12-hour period produced high levels of NOS-2 mRNA despite D-GalN treatment (Supplementary Fig. 2). The stimulatory effect of cytokines on NOS-2 expression was higher than that observed with the prostanoid. Interestingly, PGE1 was able to reduce the expression of NOS-2 mRNA induced by cytokines in the presence or absence of D-GalN.
Discussion
PGE1 reduces cell death in experimental and clinical liver dysfunction. NO plays an important role in the outcome of liver injury.25 We have previously shown that PGE1 preadministration protects against NO-dependent cell death induced by D-GalN through a rapid increase of NF-κB activation, NOS-2 expression, and NO production. Data reported in the literature show that prostaglandins either enhance or reduce NOS-2 expression in relation to the cell type used. Prostaglandins inhibit NOS-2 expression in Kupffer cells, hepatocytes, macrophages, and islet cells.37, 38 In contrast, the present study shows that NO mediated the prostanoid inhibition of NF-κB activation, NOS-2 expression, and cell death induced by D-GalN in cultured hepatocytes. Accordingly, the inhibition of NOS-2 by L-NAME blocked the effect of PGE1 on the regulation of D-GalN–induced NOS-2 expression, revealing a role for NOS-2 activity and NO production by PGE1 preadministration in the reduction of D-GalN–induced NOS-2 protein expression and cytoprotection against D-GalN–induced apoptosis.31 The observation that NOS-2 expression was induced or inhibited by prostaglandins depending on the cell type suggests the existence of suppressors and activators of NOS-2 expression. Different authors have observed specific transcriptional regulation of the NOS-2 promoter gene region. Gavrilyuk et al.39 showed that a cAMP response element site located at the −187- to −160-bp region of the NOS-2 promoter is critical for cAMP-dependent inhibition of gene expression in glial cells. To elucidate which transcription factor may mediate the regulation of NOS-2 transcription by PGE1 and/or D-GalN, a selected nonmutated region and distal and proximal mutated κB sites (−980 bp) of the NOS-2 promoter (pNOS-2-LUC+/+ and pNOS-2-LUC−/−, respectively), as well as a plasmids containing none or three NF-κB sites (ConA.LuC and (κB)3ConA.LUC, respectively) were transfected to cultured hepatocytes. PGE1 and D-GalN enhanced the reporter activity encoded by pNOS-2-LUC+/+ and (κB)3ConA.LUC plasmids. Interestingly, PGE1 was unable to regulate pNOS-2-LUC−/− activity. These results indicate that κB sites are essential for regulation of PGE1-dependent NOS-2 expression, but this activation is not prevented by a cAMP response element site present in pNOS-2-LUC+/+. Therefore, cAMP does not seem to play an important role during PGE1 regulation of NOS-2 in D-GalN–induced cell death in cultured hepatocytes.
D-GalN–induced liver injury is a suitable experimental model based on its capacity to reduce the intracellular pool of uracil nucleotides in hepatocytes, thus inhibiting the synthesis of RNA and proteins.40 D-GalN induces hepatocyte cell death in vivo41-43 and in vitro.36, 44, 45 In our conditions, the maximum degree of apoptosis induced by D-GalN was observed at 8 hours in cultured hepatocytes. The induction of cell death by D-GalN paralleled the intracellular hydrogen peroxide production in cultured rat hepatocytes.36 In addition to intracellular oxidative stress, NO plays a key role during the induction of liver injury.25 In this sense, NO has been shown to mediate26 or prevent27, 28 cell death in rat hepatocytes. The induction of cell death by D-GalN was related to an increase in NOS-2 expression and NO production by hepatotoxins in hepatocytes.30 In addition, the inhibition of NOS-2 reduced D-GalN–induced apoptosis in cultured hepatocytes.31 In the present study, we observed that IκBα degradation, NF-κB activation, and NOS-2 expression were enhanced during D-GalN–induced cell death in hepatocytes. Taylor et al.45 have found that NO downregulates NOS-2 expression induced by cytokines through the inhibition of NF-κB activation in cultured rat hepatocytes. Our study showed that NO abolished IκBα degradation induced by D-GalN in hepatocytes. In addition, the administration of SNAP showed that NO was exerting an inhibitory effect on pNOS-2-LUC+/+ and (κB)3ConA.LUC activities in nonstimulated hepatocytes. We have previously shown that NF-κB is essential for the regulation of NOS-2 expression by PGE1 and D-GalN in cultured rat hepatocytes.31, 35 NF-κB is the most relevant transcription factor regulating the expression of human and rat NOS-2 by cytokines in hepatocytes.23, 24 Under our conditions, transfections with a plasmid containing the NOS-2 promoter with mutated NF-κB sites (pNOS-2-LUC−/−) showed a very low activity without differences between control and treated hepatocytes. In this sense, activator protein 1 does not seem to play an important role in our experimental conditions.
Our findings are consistent with results obtained by other groups. Harbrecht et al.37 showed that PGE2 inhibited cytokine-stimulated NOS-2 expression in hepatocytes but had no effect on unstimulated hepatocytes after 4 hours of stimulation. In our study, we detected mRNA and protein expression 1 and 3 hours after PGE1 administration that were maintained at low levels until the latest time points. In addition, we have found a similar effect of PGE1 on hepatocytes stimulated with cytokines. Griffon et al.46 found a reduction by PGE2 of lipopolysaccharide + interferon gamma–induced NO synthesis in hepatocytes induced by NO from stimulated macrophages. In this work, NO-dependent PGE2 synthesis in macrophages occurs during lipopolysaccharide + interferon gamma stimulation of NOS-2 in hepatocytes. In our study, PGE1 was added before the stimulation of hepatocytes with D-GalN and/or cytokines. In line with this finding, Harbrecht et al.37 also reported that PGE2 had to be present at the time of cytokine exposure to produce maximal inhibition of NO synthesis. We suggest a negative feedback mechanism of PGE1-derived NO on NOS-2 expression induced by D-GalN or cytokines (Supplementary Fig. 3). Preadministration of prostaglandins in hepatocytes produces low levels of NO, which inhibits the latter's own formation induced further by cytokines and/or D-GalN through the attenuation of NF-κB–dependent NOS-2 expression. This mechanism is supported by previous findings that a preexisting low dose of NO protects against cytotoxicity induced by a high dose of NO.45, 47, 48
In summary, this report shows that PGE1 induces NOS-2 expression through NF-κB activation in cultured hepatocytes. In addition to this, rapid PGE1-derived NO production reduces NF-κB activation, NOS-2 expression, and apoptosis induced by D-GalN. Because the induction of NOS-2 expression in chronic liver diseases and acute liver failure is associated with exacerbation of inflammatory reaction and tissue damage,49 PGE1 may be a useful treatment in the prevention of inflammatory processes during hepatocyte cell death and in liver diseases.




