Development of myeloid neoplasia associated with prolonged immune cell-associated hematotoxicity after CAR T-cell treatment of B-cell lymphoma: Should we surveille for pre-existing myeloid mutations?
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the outcome of patients with B-cell malignancies as recently exemplified by the Swedish cohort.1 Although the development of secondary cancers such as T-cell lymphomas following viral transduction has been a major concern from the very beginning,2 relatively few such cases have been reported. Instead, there is accumulating evidence that myeloid malignancies such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) can occur in up to 5%–7% of patients, which, together with a smaller proportion of other secondary malignancies, constitutes the second most common cause of non-relapse mortality after CAR T-cell therapy.3-6 However, so far, we do not understand the biology behind therapy-related myeloid neoplasia (tMN), nor can we predict who is at risk. To better understand this, we have launched an ex vivo correlative study (Ethical Review Board Dnr 2021-04692) to which we enroll our patients undergoing CAR T-cell treatment.
In this study, we present two patients with B-cell lymphoma who developed tMN after receiving CD19-directed CAR T-cell therapy that triggered long-lasting immune effector cell-associated hematotoxicity (ICAHT)7 (Table 1 and Figure 1). Both patients were males diagnosed with follicular lymphoma (FL) for which they received Rituximab–Bendamustine as the first line. Both patients transformed to large B-cell lymphoma (LBCL) and were primary refractory to Obinutuzumab–Cyclophosphamide–Vincristine–Doxorubicine–Dexamethasone (HyperCVAD), therefore qualifying for CAR T-cell therapy with Axi-cel as standard of care. Patient A received bridging therapy with Ifosfamide–Carboplatin–Etoposide (ICE) combined with radiotherapy, and patient B received Rituximab–Gemcitabine–Oxaliplatin (GemOx), resulting in a partial response in both cases. Axi-cel was administrated after lymphodepletion with Fludarabine and Cyclophosphamide. Patient A had no CRS or ICANS, while patient B developed a grade I CRS and received Tocilizumab. The course of treatment for each patient is summarized in Figure 1.
| Patient A | Patient B | |
|---|---|---|
| Sex | Male | Male |
| Age | 67 y.a. | 60 y.a. |
| Diagnosis | FL transformed to DLBCL | FL transformed to DLBCL |
| 1st line treatment | R-Bendamustine | R-Bendamustine |
| 2nd line treatment | O-HyperCVAD | O-HyperCVAD |
| 3rd line treatment | Axi-cel | Axi-cel |
| Bridging therapy | ICE-Rdx | R-GemOx |
| CAR-Hematotox score | High | High |
| ICAHT grading | Early grade IV Late grades II–III |
Early grade III Late grade III |
| ICAHT duration | 16 moths | 6 months |
| G-CSF | 1–3/week | 1–2/week |
| Mutations pre-CAR T | PPM1D (VAF 3%) | TP53 p.Tyr205Cys (VAF 7%) |
| Mutations post-CAR T | PPM1D p.Cys478* (VAF 35%) RUNX1 p.Trp106Leu (VAF 56%) NRAS p.Gly13Arg (VAF 11%) |
DNMT3A p.Tyr735Thrfs*44 (VAF 8%) PPM1D p.Trp427* (VAF 7%) PPM1D p.Arg458* (VAF 6%) TP53 p.Gln331His (VAF 5%) RUNX1 p.Ser303* (frameshift) (VAF 4%) CSF3R p.Cys142Phe (VAF 5%) NOTCH1 p.Gly1765Cys (VAF 5%) |
| Karyotype | 46,XY,−7,idic(21)(p11.2)[18]/46,XY[2] | 46,XY[20] |
| IPSS-R | Not applicable | Low |
| IPSS-M | Very high | Moderate low |
- Abbreviations: G-CSF, granulocyte colony-stimulating factor; ICAHT, immune effector cell–associated hematotoxicity; IPSS-M, Molecular International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System.
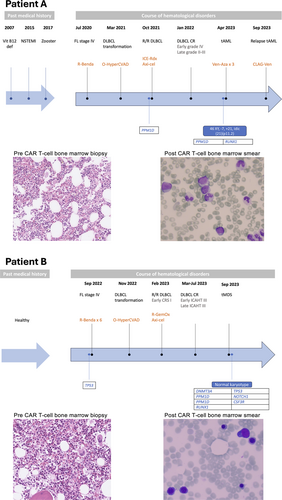
After cell infusion, patient A developed early ICAHT grade III that resulted in primary aplastic bone marrow while patient B developed biphasic ICAHT grade III in both its early and late stages. Both patients were promptly treated with G-CSF with suboptimal responses. The schedules for G-CSF administration slightly differed between the patients; while patient A was treated with 1-3 doses/week, patient B received 1-2 doses/week. The CAR/Hematotox8 score was retrospectively calculated for both patients and determined high, underscoring the relevance of this tool on predicting later complications. In particular patient B was allocated to the same group even if lacking ferritin levels since a total score of 5 was achieved. Of note, patient A had several CMV reactivations both before and after CAR T-cell infusion and for which he was treated with multiple cycles of either Valganciclovir or Foscarnet and eventually started Letermovir prophylaxis, while patient B had multiple viral infections but also bacteremia with Acinetobacter that required antibiotic treatment. Routine evaluation of blood status and viral load was performed according to hospital policies. This underlines how infections represent the most common complication seen in these patients.
Seventeen months after CAR T-cell treatment, Patient A rapidly developed dyspnea and fatigue. Blood work-up showed leukocytosis, triggering further diagnostics. Instead, Patient B had persistent neutropenia six months after CAR T-cell therapy and was therefore subject to control for bone marrow pathology. Bone marrow samples were hence collected from both patients for morphology, flow cytometry, karyotyping and assessment of mutations per the standard new generation sequencing (NGS) myeloid panel used at Karolinska University Hospital. Based on these results and the results from the blood work-up, the patients were evaluated according to WHO criteria and 2022 ELN guidelines.9, 10 Patient A was diagnosed with therapy-related acute myeloid leukemia (tAML), characterized by 24% blasts in the bone marrow. Following induction and consolidation chemotherapy, the patient presented with and later succumbed to a fungal pneumonia and therefore never reached an allogeneic stem cell transplantation (SCT). Patient B was diagnosed with therapy-related myelodysplastic syndrome (tMDS) (Figure 1, Table 1) and underwent a haplo-identical SCT. While being free from both tMDS and lymphoma, the patient died 5 months after the transplantation due to multiple viral complications. Importantly, at the time of diagnosis of tMN, both patients were in complete metabolic remission for LBCL with ongoing B-cell aplasia and CAR-T cell persistence by flow and PCR.
Morphological re-evaluation of pre-CAR T-cell bone marrow samples revealed signs of mild grade dysplasia in Patient A, while no dysplasia was found for Patient B. The NGS analyses revealed that both patients had mutations prior to CAR T-cell treatment that can be associated with myeloid disease. While Patient A had a PPM1D mutation, known as a CHIP mutation and associated with chemotherapy resistance,11, 12 Patient B had a TP53 mutation. Of note, the TP53 mutation found in the bone marrow reanalysis of Patient B differed from that found at tMDS diagnosis, indicating that the former might be associated with the primary lymphoproliferative disease. At the time of tMN diagnosis, both patients had additional mutations and interestingly both had mutations in the PPM1D and RUNX1 genes. Notably, Patient B had a frameshift mutation in the RUNX1 gene at the time of tMDS diagnosis, whereas this was not the case for Patient A. Patient B also had two mutations in PPM1D, while Patient A had one point mutation in this gene. Further details on the mutations, including variant allele frequency (VAF), are represented in Table 1.
The development of tMN is typically correlated to previous exposure to alkylating agents and radiotherapy. The incidence is particularly high (8,2-10%) in patients with primary non-Hodgkin lymphomas and commonly peaks within 5 years from treatment.13 The long-term follow-up of the original studies14 that lead to CAR T-cell approval for B-cell lymphoma do not report the incidence of tMN.15 However, the risk of developing tMN after CD19 CAR T-cell treatment is still not fully understood and follow-up data are hence greatly warranted. Factors driving secondary malignant transformation may include previously acquired mutations after exposure to chemo- and radiotherapy as well as inflammatory challenges to the blood stem cell niche associated with prolonged hematotoxicity triggered by the CAR T-cells. Therefore, evaluation of myeloid disease-associated mutations prior to CAR-T therapy may be needed. This may be particularly important in aged individuals as they have increased presence of CHIP and/or those treated with multiple lines prior to CAR T-cell therapy. Moreover, tools such as the CAR/Hematotox score and ICAHT grading could potentially help identify patients with a higher risk and a prompt recognition post CAR T-cell treatment could lead to earlier diagnosis of tMN. An understanding of the immune cell compartment may also be valuable to help determine the risk of developing a tMN as both T cell and NK cell dysfunction have been associated with increased risk of myeloid malignancy.16 In line with this, chronic inflammation has already been associated with occurrence of Immune Effector Cell HLH-like syndrome (IEC-HS)17 and thus might be linked also to ICAHT due to a dysregulated immune compartment and ineffective hematopoiesis. This may theoretically be associated with or even directly stimulate the development of certain subtypes of MDS,18 including those carrying mutation in genes such as PPM1D.
Here, we are adding on to the reports highlighting an association between long-lasting cytopenia and secondary myeloid malignant transformation. Indeed, cases of tMDS after CAR T-cell therapy have recently been reported, but few have yet described that predisposing myeloid disease-associated mutations can be found in the bone marrow prior to treatment with CAR T-cells.6 We believe that there is a value to keep track of pre-existing myeloid mutations with respect to ICAHT development. Such analyses could also help bring clarity to the need for assessing this factor in the work-up for CAR T-cell treatment, at least for selected patients. This is particularly important as the field currently lacks validated strong tools for proper risk assessment. This study also highlights that careful assessments, including bone marrow sampling to control for tMN as a differential diagnostic to ICAHT, may be needed in patients with prolonged cytopenia. As part of this, the use of G-CSF in patients with long-standing ICAHT needs to be discussed both as a potential risk of promoting the development of a tMN and more importantly as an indicator of need for full bone marrow assessment including genetics. More clinical data, a better understanding of how our currently available tools could be used and deeper biological insights on the mechanisms influencing the development of tMN are needed to establish the role of pre-CAR T-cell bone marrow screening along other pre- and post-treatment assessments. A structured analysis would facilitate our understanding and hopefully lead to a consensus in the emerging field of CAR T-cell therapy for malignant and more recently also non-malignant indications. In this context, risk for tMN will likely vary between the underlying diseases treated with different CAR T-cell constructs and the inflammatory conditions. At best, we will be able to limit the inflammatory stress on the stem cell niche by optimal patient selection and preparation, thereby reducing the likelihood of ICAHT development and consequent risk of secondary myeloid transformation. Such information can also be of value in other settings, such as the risk of tMN after treatment with bispecific antibodies or autologous stem cell transplantation.19, 20
AUTHOR CONTRIBUTIONS
Mattias Carlsten: Conceptualization; investigation; writing—original draft; methodology; writing—review and editing; formal analysis; project administration; visualization; data curation; supervision. Elisa Linnea Lindfors Rossi: Conceptualization; writing—original draft; methodology; investigation; writing—review and editing; formal analysis; project administration; visualization; data curation. Martin Jädersten: Validation; investigation. Bianca Tesi: Validation; investigation. Sulaf Abd Own: Validation; investigation. Brigitta Sander: Investigation; validation. Stefan Deneberg: Investigation; validation. Anne Ivinskiy: Investigation. Kristina Sonnevi: Investigation. Hanna Sjölund: Data curation. Gunilla Enblad: Resources. Björn Wahlin: Investigation. Stephan Mielke: Conceptualization; writing—original draft; supervision; resources; project administration; funding acquisition; validation; methodology; formal analysis; investigation.
CONFLICT OF INTEREST STATEMENT
S. M. has received speaker fees via his institution from Celgene/BMS, Novartis, Janssen, Kite/Gilead, and Pfizer for DSMB activities from Immunicum/Mendes and Miltenyi, also via his institution, and Research Funding/CRADA from Kite/Gilead. He is the founder of SWECARNET.
FUNDING
This work was funded by the Swedish Innovation Authority VINNOVA and by the VINNOVA sponsored initiative Swelife aimed at strengthening Life Science in Sweden.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




