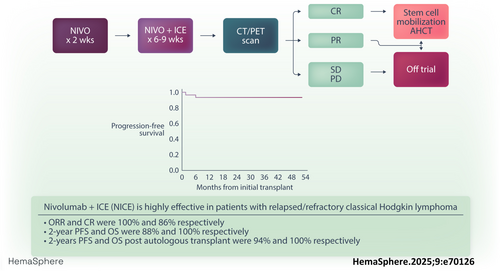
Nivolumab plus ifosfamide, carboplatin, and etoposide are a highly effective first salvage regimen in high-risk relapsed/refractory Hodgkin lymphoma
Graphical Abstract
Abstract
Nivolumab is an anti-PD-1 antibody that is effective in patients with relapsed/refractory (RR) classic Hodgkin lymphoma (cHL). We previously showed PET-adapted sequential nivolumab ± ifosfamide, carboplatin, and etoposide (ICE) chemotherapy as the first salvage in RR cHL was a safe and effective bridge to autologous stem cell transplant (ASCT) (cohort A). We then tested a non-PET-adapted schema where all patients received nivolumab + ICE (cohort B). In this study, we present results from cohort B. Patients with high-risk RR cHL after frontline treatment received 240 mg nivolumab followed by 2–3 cycles of NICE (240 mg nivolumab day 1, standard doses of ICE). High-risk disease was defined as having one of the following: primary refractory cHL, relapse within 1 year of completing frontline therapy, B symptoms at relapse, extranodal disease at relapse, or frontline brentuximab vedotin use. PET/CT was performed after nivolumab × 1 and NICE × 2. Responding patients (complete response [CR] or partial response) were intended to proceed to ASCT. The primary endpoint was CR rate per 2014 Lugano classification. A total of 35 patients were enrolled, all of whom were evaluable for safety and efficacy. Overall response rate and CR were 100% and 86%, respectively; 2-year progression-free survival (PFS) and overall survival (OS) were 88% and 100%, respectively. Thirty-two patients proceeded to ASCT directly after NICE; 2-year post-ASCT PFS and OS were 94% and 100%, respectively. Immune-related toxicities were all grades 1–2, and no patient discontinued treatment for toxicity. Nivolumab/NICE is a highly effective salvage regimen and bridges patients effectively to ASCT.
INTRODUCTION
While the majority of patients with classical Hodgkin lymphoma (cHL) are cured by frontline combination chemotherapy, 10%–30% of patients will have primary refractory or relapsed (RR) disease. The standard treatment approach for fit RR cHL patients consists of salvage therapy, with the intent to achieve a deep response, followed by autologous hematopoietic cell transplantation (ASCT).1, 2 Historically, salvage therapy has consisted of multiagent cytotoxic chemotherapy, although multiple trials have shown that regimens which include the anti-CD30 antibody drug conjugate brentuximab vedotin (BV) with or without anti-PD-1 therapy (either nivolumab or pembrolizumab) are also highly effective.3-5
We conducted a phase 2 trial evaluating sequential nivolumab followed by nivolumab combined with ifosfamide, carboplatin, and etoposide (NICE) as the first salvage therapy in RR cHL. We previously published results from an initial cohort (cohort A) which featured a PET-adapted scheme with patients receiving nivolumab monotherapy for six cycles followed by ASCT for those in complete response (CR) or NICE in those who did not achieve CR. Outcomes were excellent in cohort A with 91% of patients achieving CR at the end of protocol therapy; however, of 43 patients enrolled, only nine patients received NICE.6 Therefore, we expanded the trial design to include cohort B where all patients would receive NICE as salvage before ASCT to evaluate the safety and efficacy of this combination in a high-risk RR cHL cohort.
METHODS
Patients
This was a multicenter, two cohort (A and B), phase 2 trial in patients with initial RR cHL. Eligible patients were ≥18 years, weighing >40 kg. Patients were required to have histologically confirmed CD30+ cHL that was either primary refractory or had relapsed after initial therapy. Results for patients treated in cohort A, which used a response-adapted scheme with sequential nivolumab/NICE, were previously described.7 In cohort B, patients needed to have at least one of the following high-risk features: receipt of BV as part of frontline therapy, disease relapse within 1 year of completion of frontline therapy, primary refractory disease, B symptoms at the time of relapse, or extranodal disease at the time of relapse. Primary refractory cHL was defined as less than CR following completion of initial therapy. Other enrollment criteria for the study were identical to those previously described for cohort A. All patients provided informed consent for participation in the clinical trial. The study was approved by the Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki.
Study treatment
Patients received 240 mg nivolumab administered IV over ~30 min on cycle 1, day 1 as an outpatient. Patients then received two cycles of NICE (21 days per cycle) starting 2 weeks after cycle 1: nivolumab 240 mg day 1, etoposide 100 mg/m2 IV on days 1–3, carboplatin area under the curve 5 (750 mg maximum) IV on day 2, ifosfamide IV 5000 mg/m2 on day 2 (inpatient), or divided as 1670 mg/m2 daily on days 1–3 (outpatient). Positron emission tomography–computed tomography (PET/CT) was performed to assess disease response after three cycles of therapy (nivolumab × 1 and NICE × 2). Patients who achieved a CR could proceed directly to ASCT, with patients in PR proceeding at the discretion of the treating physician. An optional third cycle of NICE was also allowed per physician discretion. Routine antiemetic prophylaxis was administered with each cycle of NICE per institutional standards and included 12 mg oral or IV dexamethasone on day 1, and 8 mg on days 2 and 3. Patients received granulocyte colony-stimulating factor support and antimicrobial prophylaxis per institution guidelines following each cycle of NICE. Stem cell mobilization, ASCT, and post-ASCT consolidation were performed at the discretion of the treating investigator according to institutional practices after the completion of study therapy.
Study assessments and endpoints
Safety was monitored continuously with toxicities assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Unacceptable toxicity (UT) was defined as any hematologic or non-hematologic grade 3/4 toxicity that did not resolve to a grade 1/2 within 14 days and was considered at least possibly related to nivolumab and/or NICE. Any other regimen-related death was also classified as a UT. Response assessment was performed by investigators according to the 2014 Lugano Classification. The co-primary endpoints were UT and CR rate at the end of protocol therapy. Secondary endpoints included estimates of overall response rate (ORR), response duration, overall and event-free survival (OS and PFS), CD34+ yield, and proportion of patients who collected ≥2 × 106 CD34+ cells/kg. PFS is defined as time from start of treatment to disease progression or death (from any cause), whichever occurs first. For patients who underwent ASCT, time to engraftment, non-relapse mortality, and relapse-progression incidence post-ASCT were further secondary endpoints.
Trial design and statistical considerations
Cohort B utilized a Simon two-stage minimax design to evaluate the anti-lymphoma activity of the two-agent combination as assessed by CR rate in patients with high-risk HL and was expected to treat 19 (minimum) to 35 (maximum) patients. The sample size was based on a promising CR rate of 74% and disappointing CR rate of 50% using a type I error rate of 0.05 and power of 90%. Interim analysis took place after 19 patients were treated, with study termination planned if ≤9 patients achieved a CR. At stage 2, 16 additional patients would be treated, for a total of 35. Ultimately, if ≥23 patients achieved CR, the combination would be deemed worthy of further study.
Analysis plan
Baseline characteristics and toxicities were summarized using descriptive statistics. Response rates were calculated as the percentage of evaluable patients that had objective response by radiographic imaging and 95% Clopper Pearson confidence limits were calculated. Survival estimates were calculated based on the Kaplan–Meier product-limit method; 95% confidence intervals were calculated using the log transformation and the Greenwood variance estimate. All calculations were performed using SAS® version 9.4 (SAS Institute, Cary, NC) or R v3.6.3. Trial data were locked for analysis on October 22, 2024.
RESULTS
A total of 35 patients were enrolled and received the planned study therapy. Baseline characteristics are listed in Table 1. Twenty-one (21) patients were male with a median age of 30 years (range: 19–70). Thirty patients had primary refractory disease or disease relapse within 1 year of completion of frontline therapy, 10 patients had extra nodal disease at baseline, and seven patients had B symptoms at baseline. Four patients had bulky disease at the time of study enrollment. Six patients had prior BV, all of whom had at least one other high-risk feature.
| Characteristic (n = 35) | N (%) or median (range) |
|---|---|
| Male gender | 21 (60) |
| Age (median, range) in years | 30 (19–70) |
| Stage at diagnosis | |
| I | 0 (0) |
| II | 17 (49) |
| III | 6 (17) |
| IV | 12 (34) |
| Stage at enrollment | |
| I | 2 (6) |
| II | 17 (49) |
| III | 3 (8) |
| IV | 13 (37) |
| Extranodal disease at enrollment | 10 (29) |
| Bulky disease at enrollment | 4 (11) |
| Primary refractory disease | 21 (60) |
| Frontline BV use | 6 (17) |
- Abbreviation: BV, brentuximab vedotin.
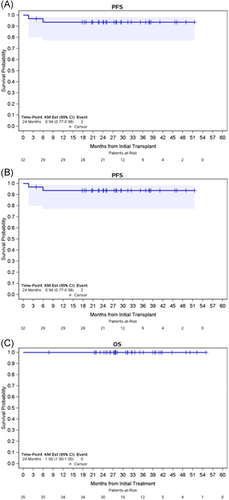
All 35 patients were evaluable for safety and response; 28 patients received two cycles of NICE, while seven patients received three cycles of NICE. The median follow-up for the overall cohort was 31.2 months (range: 7.8–55). ORR and CR rates for the overall cohort were 100% (95% CI: 90–100) and 86% (95% CI: 73–97), respectively. Responses stratified by primary refractory status are listed in Table 2. Thirty-two patients (91%) proceeded to ASCT directly after protocol therapy. One patient did not proceed to ASCT while in response due to personal preference, while two patients experienced disease progression before planned ASCT. All patients mobilized at least 2 × 106/kg CD34+ cells/kg and among 31 patients with available data regarding stem cell apheresis, the median number of CD34+ cells/kg collected was 5.23 × 106/kg (range: 2.51–32). Median number of days to neutrophil and platelet engraftment were 11 (range: 9–14) and 12 (range: 9–22), directly. The 2-year PFS and OS for the overall cohort were 88% and 100%, respectively (Figure 1). Among the 32 patients who proceeded directly to ASCT, with a median post-ASCT follow-up time of 27.6 months, the 2-year post-ASCT PFS and OS were 94% and 100%, respectively. Twenty-five percent (n = 8) patients received post-ASCT maintenance; five patients received BV maintenance, two received nivolumab, and one received maintenance with lenalidomide. Details regarding ASCT are provided in Table 3.
| Variable | All (%) | Primary refractory | Relapsed disease |
|---|---|---|---|
| Response after final cycle | N = 35 | N = 22 | N = 13 |
| ORR | 35 (100) | 22 (100) | 13 (100) |
| CR | 30 (86) | 19 (86) | 12 (92) |
| PR | 5 (14) | 3 (14) | 1 (8) |
| 2-year PFS | 88% | ||
| 2-year OS | 100% |
- Abbreviations: CR, complete response; ORR, overall response rate; PR, partial response.
| Variable | Median (range) N (%) |
|---|---|
| Number of apheresis sessions | 1 (1–9) |
| Number of CD34+ cells x 106/kg | 5.23 (2.51–32) |
| Post-transplant maintenance | |
| No | 24 (75) |
| Yes | 8 (25) |
| BV | 5 |
| Nivolumab | 2 |
| Revlimid/ipilimumab | 1 |
| Time to neutrophil engraftment | 11 (9–14) |
| Time to platelet engraftment | 12 (9–22) |
- Abbreviation: BV, brentuximab vedotin.
Safety
The most common adverse events (AEs) attributable to nivolumab monotherapy were fatigue (26%), hypertension (23%), nausea (14%), pruritis (14%), headache (9%), and rash (9%); all were grade 1 except for one instance each of grade 2 fatigue, hypertension, and pruritis. The most common AEs attributable to NICE were anemia (69%), nausea (69%), fatigue (60%), and hypertension (51%) (Tables 4 and 5). Specific AEs judged by the investigator to be possibly immune-related included transaminitis (26%), rash (20%), pruritis (14%), hyperthyroidism (6%), and hypothyroidism (3%); all were grade 1 except two grade 2 pruritis events. No patient discontinued therapy due to toxicity. Of patients, 14% (n = 4) developed engraftment syndrome, defined as at least two symptoms within 48 h of engraftment not attributable to other causes, including fever in combination with diarrhea, rash, or pulmonary infiltrates at a median of 10 days post-ASCT (range: 9–10).
| Grade 1 | Grade 2 | All grades | Percentage (%) | |
|---|---|---|---|---|
| Fatigue | 8 | 1 | 9 | 26 |
| Hypertension | 7 | 1 | 8 | 23 |
| Nausea | 5 | 0 | 5 | 14 |
| Pruritus | 4 | 1 | 5 | 14 |
| Headache | 3 | 0 | 3 | 9 |
| Rash maculopapular | 3 | 0 | 3 | 9 |
| Diarrhea | 2 | 0 | 2 | 6 |
| Fever | 2 | 0 | 2 | 6 |
| Increased alanine aminotransferase | 2 | 0 | 2 | 6 |
| Anorexia | 2 | 0 | 2 | 6 |
| Back pain | 2 | 0 | 2 | 6 |
| Dyspnea | 2 | 0 | 2 | 6 |
| Anemia | 1 | 0 | 1 | 3 |
| Palpitations | 1 | 0 | 1 | 3 |
| Sinus tachycardia | 1 | 0 | 1 | 3 |
| Hypothyroidism | 1 | 0 | 1 | 3 |
| Blurred vision | 1 | 0 | 1 | 3 |
| Dry mouth | 1 | 0 | 1 | 3 |
| Oral mucositis | 1 | 0 | 1 | 3 |
| Chills | 1 | 0 | 1 | 3 |
| Injection site reaction | 1 | 0 | 1 | 3 |
| Papulopustular rash | 1 | 0 | 1 | 3 |
| Increased aspartate aminotransferase | 1 | 0 | 1 | 3 |
| Neutrophil count decreased | 1 | 0 | 1 | 3 |
| Myalgia | 1 | 0 | 1 | 3 |
| Bacteriuria | 1 | 0 | 1 | 3 |
| Dry skin | 1 | 0 | 1 | 3 |
| Rash acneiform | 1 | 0 | 1 | 3 |
| Hot flashes | 1 | 0 | 1 | 3 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | All grades | Percentage (%) | |
|---|---|---|---|---|---|---|
| Anemia | 12 | 8 | 4 | 0 | 24 | 69 |
| Nausea | 21 | 3 | 0 | 0 | 24 | 69 |
| Fatigue | 17 | 4 | 0 | 0 | 21 | 60 |
| Hypertension | 9 | 9 | 0 | 0 | 18 | 51 |
| Sinus tachycardia | 17 | 0 | 0 | 0 | 17 | 49 |
| Vomiting | 13 | 2 | 1 | 0 | 16 | 46 |
| Constipation | 14 | 1 | 0 | 0 | 15 | 43 |
| Neutrophil count decreased | 5 | 1 | 3 | 2 | 11 | 31 |
| Platelet count decreased | 7 | 1 | 0 | 3 | 11 | 31 |
| White blood cell decreased | 3 | 2 | 6 | 0 | 11 | 31 |
| Dysuria | 9 | 0 | 0 | 0 | 9 | 26 |
| Alanine/aspartate aminotransfere increased | 9 | 0 | 0 | 0 | 9 | 26 |
| Lymphocyte count decreased | 0 | 0 | 5 | 2 | 7 | 20 |
| Rash | 7 | 0 | 0 | 0 | 0 | 20 |
| Anorexia | 6 | 0 | 0 | 0 | 6 | 17 |
| Dysgeusia | 5 | 0 | 0 | 0 | 5 | 14 |
| Hematuria | 5 | 0 | 0 | 0 | 5 | 14 |
| Dyspnea | 5 | 0 | 0 | 0 | 5 | 14 |
| Pruritis | 3 | 2 | 0 | 0 | 5 | 14 |
| Hyper/hypo-thyroidism | 4 | 0 | 0 | 0 | 4 | 11 |
| Hypokalemia | 2 | 2 | 0 | 0 | 4 | 11 |
| Arthralgia | 4 | 0 | 0 | 0 | 4 | 11 |
| Headache | 4 | 0 | 0 | 0 | 4 | 11 |
| Peripheral sensory neuropathy | 4 | 0 | 0 | 0 | 4 | 11 |
| Proteinuria | 2 | 2 | 0 | 0 | 4 | 11 |
DISCUSSION
The incorporation of PD-1 blockade into cHL therapy has been a major therapeutic advance for patients with cHL. PD-1 blockade has demonstrated efficacy in multiple lines of treatment including newly diagnosed, initial R/R disease, and multiply R/R disease. One important setting where PD-1 blockade has been tested with results that are favorable compared to historical control data is as first salvage therapy in ASCT-eligible patients with R/R disease. Nivolumab combined with BV as first salvage therapy in ASCT-eligible patients yielded a CR rate of nearly 70%, results similar to historical controls albeit with an entirely chemotherapy-free regimen.5 Subsequently, we tested PET-adapted nivolumab-based therapy in ASCT-eligible patients in the initial cohort of the present trial with over 90% of patients achieving CR at the end of protocol therapy.6 Because few patients in cohort A received NICE, cohort B was added to specifically study efficacy and toxicity of NICE and was restricted to a high-risk population.
The excellent results of cohort B of this trial are consistent with the other published trials of combination PD-1 blockade with cytotoxic chemotherapy in R/R cHL. Pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin (GVD), and pembrolizumab with ICE had CR rates of 95% and 87%, respectively, as second-line treatment.8, 9 Another trial of tislelizumab (an anti-PD1 monoclonal antibody approved for use in China in R/R cHL) with gemcitabine and oxaliplatin (GEMOX) had a 97% CR rate in a mixed cohort of patients with initial relapse and who received more than one prior line of therapy.10 Collectively, these studies suggest excellent outcomes with PD-1 blockade with chemotherapy irrespective of the specific anti-PD-1 agent or chemotherapy backbone, and post-ASCT outcomes in patients who received PD-1 based salvage are better than historical controls, possibly due to chemosensitization.11, 12 To that end, ASCT done after PD-1 blockade appears to be effective, even in patients with previously chemoresistant disease.13 While maintenance therapy was allowed in our study, only 25% of patients received maintenance of any type, and only 16% specifically received BV maintenance; furthermore, recent data suggest that BV maintenance may not result in clinical benefit for patients previously exposed to novel therapies before ASCT.14
Multiple questions remain unanswered, chief among them being whether or not PD-1 plus cytotoxic chemotherapy should be the standard of care in the salvage setting. While there are no randomized prospective trials comparing PD-1-based to non-PD-1 based salvage, the efficacy observed in this study as well as the multiple other published studies have led to widespread use of the combination of PD-1 blockade and chemotherapy as a standard option in the relapsed setting. Another question pertains to patients who receive PD-1 blockade in the frontline setting. Nivolumab with adriamycin, vinblastine, and dacarbazine was recently shown to result in superior efficacy while decreasing toxicity compared to BV, adriamycin, vinblastine, and dacarbazine in a large randomized phase 3 trial. Optimal salvage for patients who received frontline PD-1 blockade (which will almost certainly represent a growing proportion of patients with RR cHL in the future) remains undefined.15 Nonetheless, PD-1 based salvage options will still remain important for patients who have not had frontline PD-1 blockade including for patients treated with BrECADD (BV, etoposide, cyclophosphamide, adriamycin, dexamethasone, dacarbazine), which has recently emerged as an important option for newly diagnosed advanced stage cHL.16 Finally, whether all R/R cHL patients should undergo ASCT is also an open question as ASCT originally was done in an era where salvage therapy consisted of cytotoxic chemotherapy alone. As ASCT has long been the standard of care in eligible patients with RR cHL, most published data of PD-1 blockade with chemotherapy in the salvage setting have included an intent for ASCT. Given the uniformly high CR rates seen with salvage PD-1 blockade and chemotherapy, however, it is entirely conceivable that some patients may in fact be cured without consolidative ASCT. To that end, trials with pembrolizumab with GVD (clinicaltrials.gov: NCT03618550), and BV with nivolumab (NCT04561206) without intent to proceed to ASCT are underway, and results are eagerly awaited.
An important strength of this study is that efficacy appeared similar to published data with pembrolizumab with GVD and pembrolizumab with ICE. While those studies were open to all patients with RR cHL requiring first salvage therapy, we restricted enrollment to high risk patients alone. As such, traditional high risk features validated in the era of cytotoxic chemotherapy alone and used in the randomized AETHERA trial of BV consolidation post-ASCT may not retain their prognostic significance with PD-1 + chemotherapy salvage.17 In general, the rate of some toxicities (e.g., cytopenias) in our trial appeared to lower than what was reported with other anti-PD1 + chemotherapy salvage regimens including pembrolizumab with ICE or pembrolizumab with GVD. As the study protocol only mandated toxicity assessment on day 1 of each cycle with further lab collection per institutional standard, this difference likely reflects timing and frequency of assessment as opposed to a substantial difference between the regimens. As far as immune-related AEs, the incidence of thyroid disorders was 11% as opposed to none reported with pembrolizumab with ICE and 13% in pembrolizumab with GVD. Rash was seen in 20% (31% with pembrolizumab with ICE and 49% with pembrolizumab with GVD) and transaminitis in 26% (67% with pembrolizumab with ICE and 41% with pembrolizumab with GVD). Again, these differences may reflect differences in timing of toxicity capture and small sample size. Finally, while we independently designated frontline use of BV as a high-risk feature, all patients with frontline BV exposure had at least one other high-risk feature.
In summary, NICE salvage therapy in high-risk RR cHL was tolerable and resulted in a high CR rate with excellent post-ASCT PFS. Future studies evaluating the utility of NICE and similar PD1 based salvage regimens in the setting of prior frontline PD1 blockade are warranted. For patients treated with standard frontline chemotherapy (±BV), our results suggest that NICE is a highly effective salvage regimen.
AUTHOR CONTRIBUTIONS
Robert Chen, Joycelynne Palmer, and Alex Herrera conceived the study design. Matthew Mei, Alex Herrera, Joycelynne Palmer, and Ni-Chun Tsai analyzed the data. Matthew Mei, Alex Herrera, Joycelynne Palmer, and Ni-Chun Tsai prepared the first draft. All authors collected and assembled the data and revised the manuscript.
CONFLICT OF INTEREST STATEMENT
A. F. H. reports research funding from BMS, Merck, Genentech, Inc/F. Hoffmann-La Roche Ltd, Gilead Sciences, SeaGen, AstraZeneca, and ADC Therapeutics, and consultancy for BMS, Merck, Genentech, Inc/F. Hoffmann-La Roche Ltd, Kite Pharma/Gilead, SeaGen, Karyopharm, Takeda, Tubulis, AstraZeneca, Genmab, ADC Therapeutics, and Regeneron. M. G. M. reports research funding from BMS, Genentech, BeiGene, and Incyte; consultancy for Novartis, SeaGen, CTI, ADC therapeutics, AstraZeneca, and Synthekine; and speakers' bureau with SeaGen and Incyte. S. K. reports research funding from Genentech, ADC Therapeutics, Genmab, AbbVie, and Ipsen, and cosnultancy for Ipsen, Kite, and AbbVie. The remaining authors declare no competing financial interests.
FUNDING
This research was supported by the National Institutes of Health, National Cancer Institute (grant P50 CA107399-11A1), the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award (A. F. H.), and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award (A. F. H.). Bristol-Myers Squibb provided drug and funding support for this clinical trial.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.