Minimal residual disease assessment following CD19-targeted therapy in B-cell precursor acute lymphoblastic leukemia using standardized 12-color flow cytometry: A EuroFlow study
Graphical Abstract
Abstract
Detection of minimal/measurable residual disease (MRD) is a critical prognostic marker in B-cell precursor acute lymphoblastic leukemia (BCP-ALL). The EuroFlow Consortium previously developed an 8-color flow cytometric MRD protocol, effective for >98% of BCP-ALL patients treated with chemotherapy. This study aimed to enhance MRD detection, particularly for patients treated with CD19-targeted therapies, by expanding the EuroFlow protocol to a 12-color panel. This new panel incorporates additional B-cell markers and exclusion T/NK-cell markers (CD3 and CD7). Through an evaluation of 237 diagnostic BCP-ALL samples, CD22, CD24, and HLA-DR were selected as additional B-cell gating markers. Two 12-color tubes were technically optimized and clinically validated across 101 patient follow-up samples, demonstrating excellent concordance with molecular MRD levels (R2 = 0.88). The 12-color BCP-ALL MRD tubes were compatible with the previously developed 8-color automated gating and identification (AGI) tool and demonstrated good reproducibility. Our findings indicate that the 12-color panel performs comparably to the 8-color BCP-ALL MRD panel, including both CD19-positive and CD19-negative cases. However, it offers an improved definition of the B-cell lineage, particularly for expert-guided manual data analysis, and provides additional information on the expression of the targetable marker CD22.
INTRODUCTION
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common form of pediatric cancer with an incidence peak between 2 and 5 years of age,1 and the second most common acute leukemia in adults.2 Due to advancements in treatment strategies, the long-term survival rate for pediatric BCP-ALL nowadays exceeds 90%.3, 4 In adults, minimal/measurable residual disease (MRD)-oriented treatment strategies have also improved outcomes across all age groups.5 In these strategies, the presence of MRD is a crucial prognostic marker for treatment decisions.6, 7 MRD levels can be accessed via molecular analysis, examination of B- and T-cell receptor gene rearrangements or fusion genes, or by flow cytometry.8, 9 The EuroFlow Consortium has previously developed a standardized operating procedure (SOP) for flow cytometric MRD assessment in BCP-ALL patients.10 This SOP includes standardized instrument settings and sample processing and staining with two 8-color antibody tubes, allowing MRD assessment in >98% of BCP-ALL patients with a sensitivity down to 0.001%.10 However, this protocol was designed and optimized using patients treated with classical chemotherapy.
Since the development of the EuroFlow BCP-ALL MRD protocol, novel targeted therapies, such as Bi-specific T-cell engagers (BiTEs) and chimeric antigen receptor (CAR) T cells, have emerged for BCP-ALL treatment, including CD19-targeting therapies.11, 12 Despite the clinical advantages of these novel therapeutics, up to 50% of BCP-ALL patients relapse after CD19-targeted therapy.13-15 These relapses are partly caused by the outgrowth of CD19-negative ALL cells,16-18 implying that CD19 can no longer be used as the primary gating marker for BCP-ALL cells. Indeed, we observed a negative impact on inter-expert reproducibility from 90% (CD19+) to 81% (CD19− samples) when manual gating was used, with the 8-color EuroFlow BCP-ALL MRD panel.19
Therefore, the EuroFlow Consortium evaluated an alternative gating strategy without CD19 as a B-cell marker.19 Furthermore, an automated gating and identification (AGI) tool was developed to facilitate MRD assessment in BCP-ALL patients, including those undergoing CD19-targeted therapy.20 Since the validation of the EuroFlow 8-color BCP-ALL MRD protocol, new clinical flow cytometers with additional fluorescence channels have been launched. These flow cytometers allow the implementation of novel protocols with additional markers to identify B-cells. In addition, flow cytometry can be used to evaluate the surface expression of targetable antigens on MRD cells. For instance, high expression of CD22 is associated with favorable outcomes after inotuzumab ozogamicin treatment,21 while dim or low expression of CD22 is associated with poor therapy response.22
Hence, we evaluated whether extending the EuroFlow BCP-ALL MRD protocol with additional antibodies for identifying BCP-ALL cells would improve MRD detection, especially post-CD19-targeted therapy, with the use of semi-automated, expert-guided data analysis strategies. We first assessed the expression of potential additional B-cell gating markers on BCP-ALL cells at diagnosis. Several MRD panels were technically evaluated and optimized. Finally, the definitive panel was clinically evaluated using BCP-ALL patients treated with various therapies, including CD19-targeted therapies, and compared with the EuroFlow BCP-ALL MRD 2-tube 8-color approach.
MATERIALS AND METHODS
FCS files for marker selection
For the selection of possible additional B-cell markers, the expression of markers included in the EuroFlow Acute Leukemia Orientation Tube (ALOT) and the BCP-ALL diagnosis (Dx) panel23 was evaluated in 237 BCP-ALL samples at diagnosis. BCP-ALL cells were identified based on the expression of CD19 in combination with dim or negative expression of CD45. For each marker, we defined the percentage of BCP-ALL cells that had a fluorescence intensity value >1000, which we arbitrarily chose as the lower limit for positivity. In addition, we evaluated the percentage of BCP-ALL patients with their mean fluorescence intensity (MFI) value >1000 (referred to as marker-positive cases).
Design and optimization of the 12-color BCP-ALL MRD panel
For the design of the 12-color BCP-ALL MRD panel, we aimed to maintain the 8-color BCP-ALL MRD backbone and to add up to four markers mostly devoted to improved identification of CD19-negative BCP-ALL cells. Three combinations were initially designed based on the availability of antibody-fluorochrome combinations. To evaluate the 12-color BCP-ALL MRD panels, we stained diagnostic and/or follow-up samples from BCP-ALL patients and normal bone marrow obtained from patients without immunological or hematological disease. Samples were processed using the EuroFlow bulk lysis protocol, stained with the various antibody combinations, measured using FACSLyric flow cytometers (BD Biosciences, San José, CA), and analyzed using Infinicyt software (Cytognos SL, Salamanca, Spain). Based on the results, the antibody combinations were adapted and subsequently evaluated (see “Results” section and Supporting Information).
Validation of the 12-color BCP-ALL MRD panel
For further technical and clinical validation, follow-up samples from BCP-ALL were stained with the final antibody panel, manually analyzed, and compared with molecular MRD levels.24 To establish a standardized gating strategy, we processed BCP-ALL follow-up samples using the BCP-ALL MRD AGI tool.20 The MRD levels obtained with the AGI tool were compared to those obtained through manual analysis as previously described.19 In a subset of (CD19-negative) MRD samples, in which enough sample was available for reaching the maximum required MRD sensitivity, a comparison between flowcytometry and molecular MRD results was additionally performed. Statistical analysis was performed using GraphPad Prism (version 9.0.0). Correlations of MRD levels between different methods were calculated using Pearson's correlation on the log10-transformed MRD levels of double-positive data. MRD levels between both methods were considered concordant if both positive MRD levels were within a factor of three of each other or if MRD levels were negative by both methods.
RESULTS
Marker selection
In the initial phase, we aimed to select markers other than CD19 that could be used for the identification of (CD19-negative) BCP-ALL cells. Therefore, marker expression was evaluated on the leukemic cells of BCP-ALL patients stained at diagnosis (n = 237) with the EuroFlow ALOT and the BCP-ALL Dx panel. First, the percentage of positive BCP-ALL cells in each patient was evaluated for the different individual markers (Figure 1A). Second, we evaluated for each marker the percentage of marker-positive BCP-ALL patients (Figure 1B). Since nTdT requires intracellular staining, and CD58 is expressed in low levels on normal BCPs25 (Figure 1C), CD22 and CD24 emerged as the most promising candidates for extending the 8-color BCP-ALL MRD panel. However, combined analysis of CD22 and CD24 showed that eight patients were double negative for both markers, while 42 patients were negative for CD22 but positive for CD24 (Figure 1D). Additionally, both CD22 and CD24 are not exclusively expressed in normal B-cells and BCP-ALL cells. Therefore, we opted to include additional markers to better identify BCPs and BCP-ALL cells. As the remaining evaluated makers are not suitable for B-cell identification, we added HLA-DR in the panel as an additional, previously established pan-B-cell/BCP-ALL-cell marker.26 Given that HLA-DR is not specific for B-cells we also considered CD3 in combination with CD7 as exclusion markers to further distinguish BCP-ALL cells from T/NK cells, an approach that was successfully applied in chronic lymphocytic leukemia.27, 28
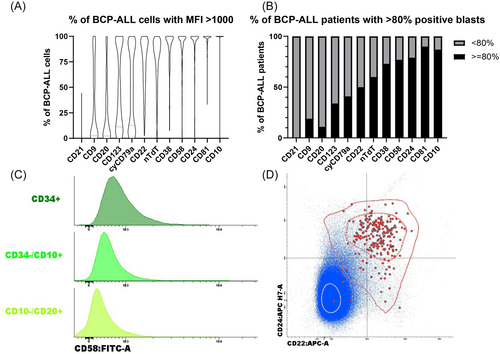
Design and optimization of the 12-color BCP-ALL MRD panel
Next, we designed and optimized a 12-color BCP-ALL MRD panel using the existing EuroFlow 8-color tube 1 (with CD66c/CD123 in the PE channel) supplemented with CD22, CD24, HLA-DR, and CD3/CD7. Using the FACSLyric flow cytometer, the additional markers were positioned in the 720/30, 606/36, 715/50, and >755 nm channels. In three phases, we developed and technically evaluated several 12-color BCP-ALL MRD tubes (see details in Table 1 and Supporting Information). Finally, two 14-marker 12-color tubes were technically approved and selected, which include the CD3/CD7/CD10/CD19/CD20/CD22/CD24/CD34/CD38/CD45/CD66c/CD123 backbone markers plus HLA-DR in tube 1 and CD73/CD304 in tube 2 (Table 1).
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | BV605 | BV711 | APCR700 | Stain buffer+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First phase | |||||||||||||
| Tube 1 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | HLA-DR | CD38 | CD20 | CD45 | CD22 | CD24 | CD3/CD7 | − |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | G46-6 | LS198-4-3 | 2H7 | HI30 | HIB22 | ML5 | SK7/M-T701 | ||
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | BV605 | BV711 | APCR700 | ||
| Tube 2 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | CD3/CD7 | CD38 | CD20 | CD45 | CD22 | CD24 | HLA-DR | − |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | SK7/M-T701 | LS198-4-3 | 2H7 | HI30 | HIB22 | ML5 | G46-6 | ||
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | SB600 | BV711 | APCR700 | ||
| Tube 3 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | CD3/CD7 | CD38 | CD20 | CD45 | CD22 | CD24 | HLA-DR | − |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | SK7/M-T701 | LS198-4-3 | 2H7 | HI30 | 4KB128 | ML5 | G46-6 | ||
| Second phase | FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | SB600 | BV711 | APCR700 | |
| Tube 1 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | HLA-DR | CD38 | CD20 | CD45 | CD22 | CD24 | CD3/CD7 | +/− |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | G46-6 | LS198-4-3 | 2H7 | HI30 | 4KB128 | ML5 | SK7/M-T701 | ||
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | SB600 | BV711 | APCR700 | ||
| Tube 2 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | CD3/CD7 | CD38 | CD20 | CD45 | CD22 | CD24 | HLA-DR | +/− |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | SK7/M-T701 | LS198-4-3 | 2H7 | HI30 | 4KB128 | ML5 | G46-6 | ||
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-AF750 | PB | PO | SB600 | BV711 | R718 | ||
| Tube 3 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | HLA-DR | CD38 | CD20 | CD45 | CD22 | CD24 | CD3/CD7 | +/− |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | G46-6 | LS198-4-3 | 2H7 | HI30 | 4KB128 | ML5 | SK7/M-T701 | ||
| Final tubes | FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-H7 | PB | V500c | SB600 | BV711 | R718 | |
| Tube 1 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | HLA-DR | CD38 | CD20 | CD45 | CD22 | CD24 | CD3/CD7 | + |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | G46-6 | HB7 | 2H7 | 2D1 | 4KB128 | ML5 | SK7/M-T701 | ||
| FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | BV786 | APC-H7 | PB | V500c | SB600 | BV711 | R718 | ||
| Tube 2 | CD81 | CD66c/CD123 | CD34 | CD19 | CD10 | CD73/CD304 | CD38 | CD20 | CD45 | CD22 | CD24 | CD3/CD7 | + |
| JS-81 | KOR-SA3544/AC145 | 8G12 | J3-119 | HI10a | AD2/U21-1283 | HB7 | 2H7 | 2D1 | 4KB128 | ML5 | SK7/M-T701 |
- Note: Bold text indicates changes in the tube compared to previous phases.
Validation of the 12-color BCP-ALL MRD panel
To evaluate whether the 12-color BCP-ALL MRD panel performed well, we validated the two technically approved tubes on follow-up samples of 101 BCP-ALL patients for whom molecular MRD data were available. Samples were stained with the two MRD tubes, and both generated FCS files were merged in the Infinicyt software and subsequently analyzed.
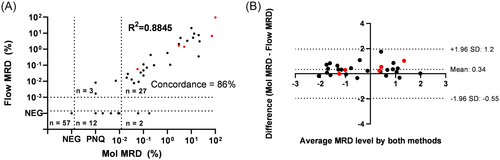
Among the 101 samples, 57 were found to be MRD-negative by both molecular and flow cytometric MRD analysis, and 30 were MRD-positive by both methods (including three samples positive by flow cytometry and positive below quantifiable range [<QR] by RQ-PCR), resulting in an overall concordance of 86% (Figure 2). Twelve samples were positive <QR by molecular MRD analysis and negative by flow cytometry. However, it should be noted that according to the recently published revised EuroMRD guidelines,29 9 out of the 12 samples positive below quantifiable range (<QR) by RQ-PCR would be classified as “MRD of uncertain significance” and therefore may well be false positives. Additionally, two samples showed quantitative molecular MRD levels but were reported negative by flow cytometry. For the samples determined MRD positive by both methods, log10-transformed MRD levels showed an excellent correlation (R2 = 0.8845) (Figure 2A) with limited bias as assessed by Blant–Altman analysis (Figure 2B). Of note, 5 out of 30 double-positive samples had CD19-negative BCP-ALL cells by flow cytometry (at relatively high levels) and showed good concordance with the molecular data. Thus, we found highly comparable results between molecular and flow cytometric MRD levels, confirming our previous results using the 8-color tubes,10 and indicating that the addition of five extra markers did not have a negative impact on the MRD tubes.
Data analysis strategies
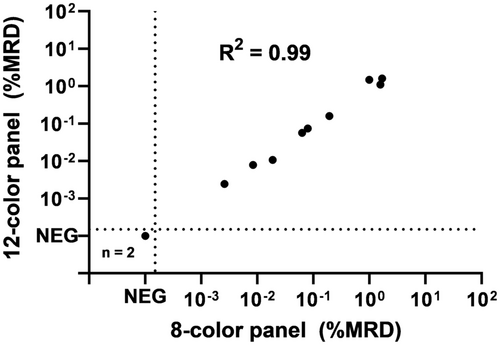
To establish a standardized gating strategy, we evaluated whether the previously developed 8-color BCP-ALL MRD AGI tool20 can be used for the semi-automated analysis of BCP-ALL follow-up samples, stained with the 12-color BCP-ALL MRD panel. This 12-color panel includes the 8-color backbone of the standardized EuroFlow BCP-ALL MRD approach. In the AGI tool-based analysis, the first eight markers are used for the AGI, while the additional markers (9–12) can be used by the expert when evaluating the check populations. For this evaluation, 12 BCP-ALL MRD samples were stained with both the 8-color and 12-color BCP-ALL MRD panels and analyzed using the AGI tool by the same expert. Two samples were identified as MRD-negative by both panels and 10 samples were identified as MRD-positive, containing CD19-positive BCP-ALL cells (Figure 3). Log10-transformed MRD levels of the double-positive samples showed an excellent correlation (R2 = 0.99) between the 8-color and 12-color BCP-ALL MRD panels, indicating that the 8-color AGI tool is technically suitable for analyzing (CD19-positive) BCP-ALL MRD samples stained with the 12-color tubes. Of note, BCP-ALL cells were always included in the checks population. Application of the 8-color AGI tool was further evaluated in a multicenter approach, including 90 (CD19-positive) BCP-ALL follow-up samples. Concordant results were obtained between manual and AGI-supported analysis in 89% of cases (see details in Supporting Information), similar to the reproducibility observed for CD19-positive cases in the original 8-color AGI tool study (90%).20
Next, we determined whether the AGI tool could reliably be used with CD19-negative BCP-ALL samples as well. Therefore, 14 samples (with or without parallel molecular MRD data being available) were analyzed manually and by the AGI tool by the same expert (daily applying the AGI tool for routine BCP-ALL MRD analyses, referred to as daily user). All samples were reported MRD-positive by both methods, with an excellent correlation in MRD levels (R2 = 0.99) (Figure 4A). These samples were then re-analyzed by an additional daily user, yielding a 100% concordance and maintaining an excellent correlation (R2 = 0.99) (Figure 4B). These data show that the 12-color tubes in combination with the 8-color AGI tool can also reliably be used for the BCP-ALL follow-up samples containing CD19-negative BCP-ALL cells.

Finally, to validate the inter-expert variation of the 12-color BCP-ALL MRD tubes between experts (both daily and non-daily users), 14 CD19-negative BCP-ALL follow-up samples were stained with the 12-color tubes and processed with the 8-color AGI tool. Each processed file was analyzed by five non-daily users and two daily users from different centers. In total 89 out of 98 analyses (91%) showed concordant results (Figure 4C), with non-daily users showing lower concordance compared to daily users (84% vs. 100%). Overall, it can be concluded that the 8-color BCP-ALL MRD AGI tool can reliably be used to analyze follow-up samples stained with the 12-color BCP-ALL MRD tubes, though intensive training remains necessary. In addition, it shows that when expert-based manual data analysis is used the new 12-color panel improves the level of inter-expert concordance among CD19- MRD samples to 91% compared with the previously reported 81% with the 8-color EuroFlow BCP-ALL MRD panel.19
DISCUSSION
The presence of MRD, assessed by molecular techniques or flow cytometry, remains the most important prognostic factor in BCP-ALL management.6, 7, 30 Protocols have traditionally relied on CD19 as a B-cell gating marker. However, the advent of CD19-targeted immunotherapies has resulted in CD19-negative relapses,16-18 potentially compromising the reliability of CD19-based gating in MRD monitoring by flow cytometry. To address this, we evaluated a two-tube 12-color BCP-ALL MRD antibody panel including CD22, CD24, HLA-DR, and CD3/CD7. These markers not only facilitate (expert-guided) B-cell gating in the absence of CD19 but also help identify MRD-positive patients treated with CD19 (or other) therapies that may benefit from CD22-targeted treatment strategies.31 Our findings are consistent with previous studies32-36 that have utilized CD22 and CD24, although Cherian et al. (2018) found them insufficient alone for B-cell identification. Cherian et al.33 introduced a two-tube 7- and 8-color protocol, incorporating CD66b as an exclusion marker. Others included cyCD79a as an additional B-cell marker in their 11-color panel,35 or proposed a 15-color panel including HLA-DR as an additional B-cell marker.32 These studies suggest that combining CD22 and CD24 with an additional B-cell-associated marker such as HLA-DR and pan-T/NK-cell markers to exclude non-B lymphoid cells might be generally sufficient to identify B-cells.
To standardize the gating strategy, we opted to use the semi-automated 8-color BCP-ALL MRD AGI tool for analyzing the 12-color BCP-ALL MRD panel, which shares the first eight markers with the 8-color tubes. Manual gating strategies are inherently dependent on the analyst's knowledge and experience, making (semi)-automated analysis preferable. In our study, we assessed the applicability of the AGI-tool-supported analysis strategy for CD19-negative BCP-ALL MRD cases. We observed an excellent correlation (concordance = 100%, R2 = 0.99) when samples were blindly analyzed by one expert, both manually and using the AGI tool, with results confirmed by a second daily user, clearly above the previously reported concordance rate when a standardized manual gating approach with the 8-color EuroFlow BCP-ALL panel was used in similar settings.19
When the same files were analyzed by different experts, an overall concordance of 91% was achieved for these CD19-negative cases, with daily users finding highly comparative results and non-daily users showing somewhat greater variation (100% versus 84%). These findings show that interpretation for CD19-negative cases remains expert-dependent, emphasizing the need for training experts in new software tools or the development of new expert-independent analysis strategies. Overall, the concordance was similar to the reproducibility observed for CD19-positive cases in the original AGI tool study (91% vs. 90%, respectively).20 It's important to note that this analysis was performed without knowledge of the patient, treatment, treatment phase, and immunophenotype at diagnosis or relapse. In practice, such information will typically be available, aiding MRD analysis.
Unlike our semi-automated approach, other recent next-generation flow cytometry protocols do not implement automated analysis strategies. Instead, standardized manual gating strategies are provided. Cherian et al. used a “rough B-cell gate” for B-cell identification based on CD22 and CD24 expression in absence of CD66b, followed by BCP-ALL cell identification using aberrant expression of maturation markers (CD10, CD20, CD34, and CD38).33 A similar gating strategy was used by Gao et al.34 Mikhailova et al.35 used a strategy based on the expression of CD22, cyCD79a, CD24, and CD10, while Singh et al. proposed a gating strategy using CD22, CD10, and CD34.36 Chatterjee et al. employed a gating strategy based on CD22, CD24, CD81, and CD33.37 Lebecque et al.38 developed a 14-marker 12-color panel using CD43 and CD81 to distinguish BCP-ALL from normal BCPs, adding CD72 as alternative B-cell marker for patients treated with CD19-targeted therapy. While these gating approaches facilitate B-cell gating without CD19, reliance on CD22 can be problematic after CD22-targeted therapy due to the potential loss of CD22 expression.39 CyCD79a's requirement has the disadvantage that it requires intracellular staining. In summary, each gating approach presents distinct advantages and disadvantages.
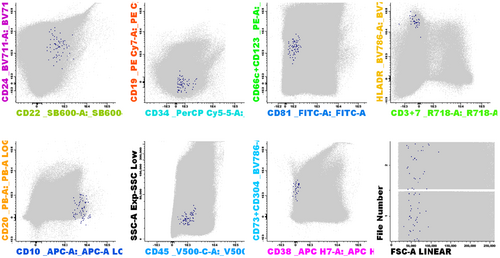
We utilized the 12-color BCP-ALL MRD panel and aimed to achieve a sensitivity of 10−5. Consequently, we implemented a minimum of 4 × 106 acquired cells, with the limit of detection (LOD) set at a cluster of 10 cells and a lower limit of quantitation requiring at least 40 cells. Similar sensitivity was reached by the panels proposed by Chatterjee et al. and Gao et al.34, 37 The protocols of Mikhailova et al. and Singh et al. use less cells (3 × 105 and 1 × 106, respectively) and consequently, the sensitivity of 10−5 is not reached by these protocols.35, 36 Figure 5 shows an example of a 12-color analysis, where a very low percentage of CD19-negative BCP-ALL cells is detected; this population would have been very hard to recognize using the 8-color panel. Unfortunately, despite multiple years of sample collection in multiple medical centers, such CD19-negative cases with low MRD levels were only limited available. In our cohort, the 12-color panel did not outperform the 8-color panel in most cases, although it facilitated the (expert-based) analysis. Certainly for cases that are both CD19-negative and CD22-negative (which may become more frequent in the future) the additional markers may improve the appropriate identification of BCP-ALL cells (see example in Figure S6).
We conclude that the 12-color BCP-ALL MRD panel performs well and allows reliable detection of MRD. The tubes can be used in combination with the 8-color AGI tool, allowing (semi)-automated analysis. Our 12-color panel did not perform better than the 8-color BCP-ALL MRD panel using the evaluated data set but has the advantages that B-cells can easier be defined and that information about the targetable marker CD22 is provided.
ACKNOWLEDGMENTS
We gratefully acknowledge all EuroFlow participants for fruitful discussions. We thank all laboratory staff of the EuroFlow laboratories for collecting data, and Marieke Bitter, Bart Lubbers, Laura Bargeman, Evelien Rijkers, and Yvonne Buis-Franken for organizational and management support.
AUTHOR CONTRIBUTIONS
Study concept and design: Martijn W. C. Verbeek and Vincent H. J. van der Velden. Development of methodology: Alberto Orfao, Jacques J. M. van Dongen, and Vincent H. J. van der Velden. Acquisition, analysis, and interpretation of the data: Martijn W. C. Verbeek, Michaela Reiterová, Anna Laqua, Beatriz Soriano Rodríguez, Lukasz Sedek, Chiara Buracchi, Malicorne Buysse, Elen Oliveira, Robby Engelmann, Joana Desterro, Anja X. De Jong, Sebastian Boettcher, Romana Jugooa, Susana Barrena, Saskia Kohlscheen, Stefan Nierkens, Joana G. Rodriques, Mattias Hofmans, Giuseppe Gaipa, Elaine Sobral de Costa, Ester Mejstrikova, Tomasz Szczepanski, Monika Brüggemann, and Vincent H. J. van der Velden. Statistical analysis: Martijn W. C. Verbeek. Writing and revision of the paper: Martijn W. C. Verbeek and Vincent H. J. van der Velden. Review and approval of the final paper: All authors.
CONFLICT OF INTEREST STATEMENT
J. J. M. v. D., A. O., and V. H. J. v. d. V. each report being one of the inventors on the EuroFlow-owned patent PCT/NL2010/050332 (methods, reagents and kits for flow cytometric immunophenotyping of normal, reactive, and malignant leukocytes). The Infinicyt software is based on the intellectual property (IP) of some EuroFlow laboratories (University of Salamanca in Spain) and the scientific input of other EuroFlow members. All aforementioned intellectual property and related patents are licensed to Cytognos (Salamanca, ES) and BD Biosciences (San José, CA), companies that pay royalties to the EuroFlow Consortium. These royalties are exclusively used for the continuation of the EuroFlow collaboration and sustainability of the EuroFlow Consortium. V. H. J. v. d. V. reports a Laboratory Services Agreement with BD Biosciences, Cytognos, and Agilent; all related fees are for the Erasmus MC. J. J. M. v. D. and A. O. report an Educational Services Agreement from BD Biosciences (San José, CA) and a Scientific Advisor Agreement with Cytognos; all related fees and honoraria are for the involved university departments at Leiden University Medical Center and University of Salamanca. M. B. reports a Laboratory Services Agreement with BD Biosciences and Cytognos; all related fees are for the UKSH. M. B. received personal fees from Incyte (advisory board); financial support for reference diagnostics from Amgen and Celgene; grants and personal fees from Amgen (advisory board, speakers bureau, travel and support); and personal fees from Janssen and BD (speakers bureau), all outside the submitted work. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FUNDING
The EuroFlow Consortium received support from the FP6-2004-LIFESCIHEALTH-5 program of the European Commission (grant LSHB-CT-2006-018708) as Specific Targeted Research Project (STREP). The EuroFlow Consortium is part of the European Scientific Foundation for Hemato-Oncology (ESLHO), a Scientific Working Group (SWG) of the European Hematology Association (EHA). This study has been funded by the CB16/12/00400 (CIBERONC) and PI19/01183 grants by Instituto de Salud Carlos III (ISCIII) and co-funded by the Fondo Europeo de Desarrollo Regional (FEDER). M. R. and E. M. were supported by Charles University Research Centre (Program No. UNCE/24/MED/003) and the Ministry of Health of the Czech Republic (grant no. NU23-05-00353). E. M. was further supported by the National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102), funded by the European Union–Next Generation EU. M. B. was supported by the Deutsche José Carreras Leukämie-Stiftung (grants DJCLS R 15/11 and DJCLS 06R/2019). L. S. and T. S. were supported by internal grants from the Medical University of Silesia: PCN-1-215/K/2/I and PCN-1-153/N/2/K, respectively. C. B. and G. G. were partially supported by Fondazione M. Tettamanti M. De Marchi ONLUS for this project.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.