Delving deeper into the pathogenesis and genomics of posttransplant diffuse large B-cell lymphoma
Abstract
Posttransplant lymphoproliferative disorders (PTLDs) are a well-known complication of solid organ transplantation and allogeneic hematopoietic stem cell transplantation. The diffuse large B-cell lymphoma subtype (PT-DLBCL) is the most frequent monomorphic PTLD and is associated with poor prognosis. Transplant recipients have an increased risk of abnormal proliferation of lymphoid cells because of diminished immune surveillance. In about 60% of the cases, Epstein–Barr virus infection seems to contribute to the cancer phenotype. Although clinical and research interest in the disorder has increased during the last two decades, the pathology of the disease remains largely elusive. In this review, we summarize current knowledge of PT-DLBCL pathogenesis, and we discuss how a better understanding of PT-DLBCL can lead to improved diagnostics and therapeutic strategies.
INTRODUCTION
Posttransplant lymphoproliferative disorders (PTLDs) are a heterogeneous group of lymphoid neoplasms complicating both solid organ transplantation (SOT) and allogeneic hematopoietic stem cell transplantation (aHSCT). Transplant recipients have an increased risk of abnormal proliferation of lymphoid cells because of (i) diminished immune surveillance due to iatrogenic immunosuppression and (ii) Epstein–Barr virus (EBV) infection.1, 2 Lymphoma represents 21% of all malignancies occurring in SOT recipients, compared to 4% of malignancies in the immunocompetent (IC) population.3
Based on the histopathological classification, WHO 2017 and ICC 2022 classify PTLD as follows: (1) non-destructive lesions, (2) polymorphic PTLD (P-PTLD), (3) monomorphic PTLD (M-PTLD, including diffuse large B-cell [PT-DLBCL]), and (4) classical Hodgkin lymphoma-like PTLD.4-6 While M-PTLDs resemble neoplastic lymphoproliferations in IC patients, P-PTLDs are characterized by an extensive proliferation of stromal immune cells and few transformed cells. Yet, it is sometimes difficult to distinguish between P-PTLD and bona fide PT-DLBCL. In addition, WHO-HAEM5 proposed a three-part nomenclature for the classification of immune deficiency and dysregulation-associated lymphoproliferative disorders and lymphomas, in which viral status, particularly EBV, is specified.7
As a leading life-threatening malignancy in the transplant population, PTLD is receiving increasing attention. However, its disease biology remains poorly understood. An additional confounding factor is the high genomic heterogeneity among the different histopathological PTLD subgroups. Despite this heterogeneity, studies often investigate the pathophysiology of PTLD without distinguishing between different subgroups, complicating any attempt to unravel the genetic signature of PTLD.
In this review, we focus on PT-DLBCL, the most common monomorphic PTLD. We summarize current knowledge of PT-DLBCL pathogenesis, and we discuss how a better understanding of PT-DLBCL can lead to improved disease diagnostics and therapeutic strategies.
PATHOGENESIS
Although PT-DLBCL was first considered to be uniformly EBV-driven, the rate of EBV-negative (EBV−) cases has risen to 40% of PT-DLBCL following SOT.8 Because EBV− PT-DLBCL cases generally manifest later compared to EBV+ cases, the rise in reports of EBV− PT-DLBCL could at least partially be attributed to the extended survival of transplant patients in recent decades.
EBV+
Affecting up to 95% of the population, EBV hijacks the normal B-cell program by expressing different latent antigens during B-cell development, promoting B-cell proliferation and transformation.
After EBV reactivation or primary infection, the virus transforms its genome into an episome, limiting viral gene expression and establishing chronic latency.5, 9 Instead of viral particles, the infected B-cell expresses different latent antigens in accordance with the latency stage. Figure 1 provides an overview of all latent EBV antigens and the different cancer phenotypes to which they contribute. In addition, all latency forms are characterized by high expression levels of Epstein–Barr-encoded small RNAs (EBERs) and microRNAs (miRNAs). In IC hosts, these viral constituents elicit a T-cell response destroying most of the EBV-infected B-cells, keeping the virus under control.10 However, in transplant recipients, the T-cell immune surveillance is decreased by immunosuppressants. Impaired T-cell immunity due to immunosuppressive therapy allows for EBV-infected B-lymphocytes to become prone to somatic hypermutation of proto-oncogenes, alterations of BCL6 and MYC expression, activation of NF-κB, PI3K/AKT/mTOR and BCL2 pathways, and aberrant immunoglobulin class switching.11, 12 Ultimately, this results in B-cell transformation and lymphoma development.13
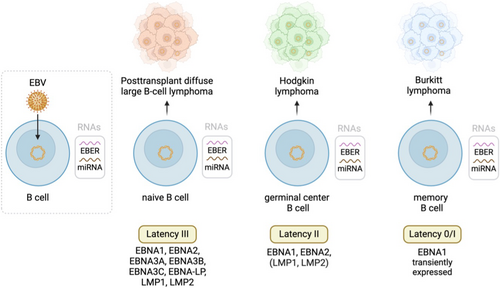
In EBV+ PT-DLBCLs, tumor development is automatically attributed to the oncogenic potential of EBV. However, the EBV status of PT-DLBCLs is determined through EBER in situ hybridization,14 which detects latent EBV infection in all its forms, while true EBV-driven PT-DLBCLs are mostly classified as latency type III.11 The current EBV detection method is thus not selective for true EBV-driven lymphoma.
Moreover, it is puzzling that all transplant recipients undergo chronic immunosuppression targeting T-cells, and despite the widespread prevalence of EBV infection, only a subset of patients develops PT-DLBCL. This implies that the pathogenesis of PT-DLBCL involves more complex factors beyond general immunosuppression. It even suggests a scenario where nuanced aspects of the immune system such as the tumor microenvironment (TME) play a crucial role, which could explain why certain patients are susceptible to PT-DLBCL development while others are not.15-17 The importance of the TME would not be surprising, as the composition of the TME is affected by numerous factors in PTLD. These include (i) immune stimulation by the graft organ through chronic antigen presentation, (ii) the oncogenic EBV which influences immune responses, (iii) immunosuppressive therapy to prevent organ rejection or GVHD, and (iv) interaction with donor-derived immune cells originating from the graft. Moreover, it seems that an inflamed TME with higher proportions of immune cells correlates with a better outcome compared to a non-inflamed TME, which further underpins a role for the TME in PTLD.18
EBV−
As opposed to EBV+ PT-DLBCL, the pathogenesis of EBV− cases is ambiguous. In the early decades of transplantation, prolonged survival following SOT was limited, hampering the manifestation of EBV− PT-DLBCL and causing almost all PT-DLBCL cases to be EBV+. Consequently, the hypothesis of a hit-and-run EBV infection was proposed. It speculates that EBV infects B-lymphocytes, induces chromosomal aberrations, and then exits the cell. This mechanism potentially explains the small number of EBV− PT-DLBCL cases. However, in vivo PCR assays could not detect any involvement of the EBV genome in EBV− PT-DLBCL pathogenesis.19 Moreover, since we now know that about 40% of PT-DLBCL are EBV−, the hit-and-run hypothesis seems improbable. Other hypotheses for viruses associated with PT-DLBCL include cytomegalovirus (CMV) co-infection, human herpes virus 8 (HHV8) or other unknown viral infections in PT-DLBCL development. However, this theory seems improbable for different reasons. First, HHV8+ PT-DLBCL cases are extremely rare.20 Second, there is no evidence that CMV infects B-cells or that it has a direct oncogenic effect on B-cells.21 Finally, EBV is currently the only virus that was found to be significantly associated with PT-DLBCL. A third hypothesis involves chronic antigen stimulation by the graft. It postulates that B-cells synthesize antigens against the graft, eventually leading to the development of lymphoma. Yet again, this theory has not been sufficiently supported. It is thus not known whether EBV− cases should be considered a true immunosuppression-related disease or coincidental lymphoma in a transplant recipient.
Overall, based on the notable differences in their pathogeneses, EBV+ and EBV− PT-DLBCL could be considered at least two distinct pathologies and might need different approaches and/or treatments.
DIFFERENCES BETWEEN EBV+ AND EBV− PT-DLBCL
Clinical presentation
The clinical presentation of PT-DLBCL varies greatly, from incidental asymptomatic findings to fulminant presentations, such as organ failure and spontaneous tumor lysis. Like many other aggressive non-Hodgkin lymphomas, PT-DLBCL is characterized by a high incidence of extranodal involvement.5
Based on the time between transplantation and the development of lymphoma, PT-DLBCL is categorized as early onset (<1 year) and late onset (>1 year). Both subtypes have distinct characteristics: compared to late-onset cases, early PT-DLBCL is typically characterized by a positive EBV status, frequent graft organ involvement, and less extranodal disease. However, risk factors and response to treatment seem similar for both early and late-onset subtypes.22-25
While EBV association is not required for PT-DLBCL diagnosis, staining by EBER in situ hybridization is generally recommended and obligatory according to WHO-HAEM5.7, 26 A detailed discussion on PTLD and PT-DLBCL diagnosis is beyond the scope of this review and can be found in WHO and ICC guidelines.4, 6, 7
As stated earlier, PT-DLBCL is subtyped as EBV+ and often considered EBV-driven upon EBV detection. However, this does not necessarily mean that the lymphoma is EBV-induced. In some cases, EBV could be an innocent bystander rather than the driving force behind oncogenesis. Since the oncogenic potential of EBV is considered a swift driver of tumor development, it is possible that only early-onset EBV+ cases are truly EBV-driven lymphomas.
Genomic landscape
To gain a better understanding of PT-DLBCL pathogenesis, different approaches have been used to characterize its genomic landscape (Table 1).
| Number of samples | Technique | Most frequent aberrations | Reference |
|---|---|---|---|
| 36 (21 M-PTLD; 18 B-cell, of which 15 PT-DLBCL, 2 EBV− PT-DLBCL, 8 EBV+ PT-DLBCL, the rest not determined; and 3 T-cell) | Karyotyping | M-PTLD: trisomy 9 and/or 11, rearrangements of 8q24.1 (MYC rearrangement in BL-PTLD), 3q27 (BCL6), and 14q32 (IGH). | Djokic et al. (2005)27 |
| 35 (26 M-PTLD: 22 B-cell, of which 19 PT-DLBCL, 5 EBV− PT-DLBCL, 14 EBV+ PT-DLBCL and 4 T-cell) | CGH, FISH | Gains: 8q24, 3q27, 2p24p25, 5p, 9q22q34, 11, 12q22q24, 14q32, 17q, 18q21 (BCL2). Losses: 17p13, 1p36, 4q, 17q23q25, Xp Amplifications: 4p16, 9p22p24, 18q21q23 |
Poirel et al. (2005)28 |
| 20 PTLD (13 PT-DLBCL), 25 IC-DLBCL | aCGH | Gains: 5p, 11p Losses: 12p, 4p, 4q, 12q, 17p, and 18q Chromosome 12p was the most frequent target of deletions among PT-DLBCL. Small deletions and gains involving BCL2, PAX5, and ZDHHC14 were identified. |
Rinaldi et al. (2006)29 |
| 44 PT-DLBCL, 105 IC-DLBCL, 28 HIV-DLBCL | High-resolution SNP-based CGH arrays | PT-DLBCL: lack of del (13q.14.3), lack of copy neutral LOH at 6p, and frequent breakage at fragile sites (deletions at 2p16.1 [FRA2E]). | Rinaldi et al. (2010)30 |
| 28 PT-DLBCL (10 EBV−, 18 EBV+) | Targeted NGS for TP53 mutations, FISH for MYC rearrangements | PT-DLBCL: EBV− cases were more frequently TP53-mutated and p53-positive, CD30L negative and of GC type compared with EBV+ cases. | Courville et al. (2016)31 |
| 36 PT-DLBCL (25 EBV−, 11 EBV+), 79 IC-DLBCL | Targeted NGS | PT-DLBCL: more TP53 mutations and absence of ATM and B2M mutations compared to IC-DLBCL. | Menter et al. (2017)32 |
| 17 M-PTLD after SOT (10 EBV−, 7 EBV+) | lcWGS to detect CNVs, targeted NGS to identify EBV-DNA load and SNVs in cfDNA | Most frequent mutations in EBV+ and EBV− PTLD: TP53 and KMT2D (41%), SPEN, TET2 (35%), ARID1A, IGLL5, and PIM1 (29%). CNVs were more prevalent in EBV− lymphoma, no difference was observed in the number of SNVs. | Veltmaat et al. (2023)33 |
| 29 EBV+ BL (latency I) and 28 EBV+ PT-DLBCL (latency III) | Gene and miRNA expression profiling | EBV-encoded latent proteins and miRNAs affect the signature of genomic aberrations depending on latency type. | Navari et al. (2014)34 |
| 49 PT-DLBCL (26 EBV−, 23 EBV+) 170 IC-DLBCL | CNA | PTLD has higher genomic stability than IC-DLBCL; genomic stability is further enhanced in EBV+ PT-DLBCL. | Armitage et al. (2023)35 |
| 33 PT-DLBCL (11 EBV−, 22 EBV+), 15 IC-DLBCL | GEP | EBV+ and EBV− PT-DLBCL were similar except for decreased T-cell signaling in EBV+ cases. | Morscio et al. (2013)36 |
| 31 Pediatric mPTLDs, 24 PT-DLBCLs (21 EBV+), and 7 PT-BL (7 EBV+) | FISH, CNA analysis, targeted NGS | PT-DLBCL showed a very heterogeneous genomic profile with fewer mutations and CNA than IC-DLBCL. PT-BL carries a higher mutational burden than PT-DLBCL. | Salmerón-Villalobos et al. (2023)37 |
| 50 PTLDs (28 PT-DLBCL), 15 immunosuppressed DLBCL, and 14 IC-DLBCL) | IHC, FISH, targeted sequencing, GEP | Higher number of mutations in general and a particularly high prevalence of TP53 mutations in EBV− PT-DLBCL | Ivanova et al. (2025)38 |
Comparative genomic hybridization (CGH) and fluorescence in situ hybridization (FISH) were performed on 35 M-PTLD cases, showing that M-PTLDs harbor some chromosomal imbalances known to be involved in lymphomagenesis in IC patients, such as 8q24 (c-MYC), 17p13 (TP53), 18q21 (BCL2), and 3q27 (BCL6), while other nonrandom gains (5p) and losses (4q, 17q, Xp) had not been reported previously.28 Nonrandom alterations that involved larger chromosomal regions (such as 2p, 9q, 11, and 12q gains) were also observed, containing large numbers of genes, however, with no obvious candidate genes involved in lymphomagenesis. Notably, EBV− PT-DLBCL was more often associated with complex CGH imbalances than EBV+ PT-DLBCLs.
Conventional cytogenetics of 36 PTLD cases showed that the most frequent clonal abnormalities in B-cell M-PTLD were trisomies 9 and/or 11, associated with EBV positivity, followed by rearrangements of 8q24 (MYC), 3q27 (BCL6), and 14q32 (IGH), which are known aberrations in lymphomagenesis.27 Moreover, 72% of all B-cell M-PTLD showed chromosomal abnormalities, as opposed to only 15% in P-PTLD. Two other studies reported different frequencies of cytogenetic abnormalities: 46%–75% in M-PTLD and 33%–57% in P-PTLD.28, 39, 40
In 2006, Rinaldi et al. used whole genomic DNA profiling (array CGH) to compare the DNA profiles of 20 PT-DLBCL to those of IC-DLBCL patients.29 Frequent gains in PT-DLBCL included 5p and 11p, common losses were 12p, 4p, 4q, 12q, 17p, and 18q. Chromosome 12p was the most prevalent target for deletions among PT-DLBCL. In IC-DLBCL, DNA loss did not always result in loss of heterozygosity (LOH), a common form of allelic imbalance in cancer, where a heterozygous somatic cell becomes homozygous because one of the two alleles is lost. However, in PT-DLBCL, LOH with no loss of DNA was more prevalent. The different patterns of DNA loss and/or LOH indicate that the genomic lesions are brought about by different mechanisms. LOH in the absence of DNA loss suggests that the mutant allele was doubled, leading to uniparental disomy (UD). Such mechanism was observed in chromosome 10 of PT-DLBCL, pointing toward UD as a transforming mechanism in PT-DLBCL and not in IC-DLBCL. This genomic aberration is also known as copy-neutral LOH.
In a follow-up study, the authors used a high-density genome-wide single-nucleotide polymorphism (SNP)-based array CGH (aCGH) to compare PT-DLBCL to IC-DLBCL and HIV-related DLBCL (HIV-DLBCL).30 They found that the genomic profile of PT-DLBCL resembled that of IC-DLBCL. Nevertheless, PT-DLBCL showed distinctive features. For example, PT-DLBCL lacked a deletion in the long arm of chromosome 13, at position 14 (13q14). This deletion leads to the loss of important miRNAs that maintain the apoptotic balance in B-lymphocytes. Moreover, PT-DLBCL did not show a copy-neutral LOH at 6p as opposed to IC-DLBCL. Frequent breakage at fragile sites (FRA1B, FRA2E, and FRA3B) was found in PT-DLBCL but not IC-DLBCL, with deletions at 2p16.1 (FRA2E) being the most common lesions. FRA2E resembled an EBV insertion site discovered in a Burkitt lymphoma (BL) cell line. Like DLBCL, BL is known to be associated with EBV,41, 42 suggesting that the genomic instability of PT-DLBCL might be due to the integration of viral DNA upon infection. The FRA2E site contains the genes FANCL (a ubiquitin ligase important in DNA repair) and VRK2 (a negative regulator of the MAPK pathway), which may play a role in oncogenesis.30, 40, 43 Recurrent gains of 1q, 11q, and chromosome 7, as well as losses at 17p (TP53) were observed at a similar frequency in PT-DLBCL and IC-DLBCL.
Accordingly, Salmerón-Villalobos et al. showed that PT-DLBCL had less CNA and less driver mutations than IC-DLBCL; however, EBV− PT-DLBCLs carried more driver mutations than their EBV+ counterparts.37 Frequent mutations in PT-DLBCL were epigenetic modifiers and genes of the Notch pathway.
Menter et al. performed targeted next-generation sequencing (NGS) on a customized panel of 68 genes in 9 P-PTLD, 37 PT-DLBCL, and 76 IC-DLBCL samples.32 The median number of mutated genes per case was zero, two, and four for P-PTLD, PT-DLBCL, and IC-DLBCL, respectively. The frequency of mutations in P-PTLDs was significantly lower as compared to PT-DLBCLs. Frequently mutated genes in the PT-DLBCL group were the lysine methyltransferase KMT2D and TP53. Other differences in the mutational landscape of PT-DLBCL versus IC-DLBCL included (i) fewer mutations in genes related to NF-κB signaling (CARD11, IRF4, MYD88, PIM1, PRDM1, and TNFAIP3) and (ii) a lack of Beta2-microglobulin (B2M) downregulation, a mechanism of immune evasion in lymphomas in immune-competent setting, in PT-DLBCL. The latter observation may be attributed to the small number of tumor-infiltrating T-cells in PT-DLBCL.32, 44 In another study, EBV− PT-DLBCL cases were more frequently TP53-mutated and p53-positive, CD30L negative and of GC type compared to EBV+ cases.31
Veltmaat et al. performed low-coverage WGS (lcWGS) to detect copy number variations (CNVs), and targeted NGS to identify EBV-DNA load and single nucleotide variants (SNVs) in cell-free DNA of 17 M-PTLD patients.33 They found that the most frequently mutated genes were TP53 and KMT2D (41%), SPEN and TET2 (35%), ARID1A, IGLL5, and PIM1 (29%). Moreover, CNVs were more prevalent in EBV− lymphoma, but no difference was observed in the number of SNVs.
Compared to IC-DLBCLs, EBV+ PT-DLBCL had fewer mutated genes and particularly fewer mutations in NF-κB pathway-related genes. The overall mutational frequency of EBV− PT-DLBCL resembled IC-DLBCLs, yet TP53 mutations were more frequent in EBV− PT-DLBCL. When comparing PTLD by cell of origin (COO, i.e., germinal center or non-germinal center B-cell) subsets, no significant differences were found. Similarly, Ivanova et al. detected a higher number of mutations in general and a particularly high prevalence of TP53 mutations in EBV− PT-DLBCL.38
To date, there seems to be a consensus that EBV+ PT-DLBCL bears fewer mutations compared to EBV− PT-DLBCL,35 whose mutational landscape resembles that of IC-DLBCL. The substantial genomic differences between EBV+ and EBV− PT-DLBCL strengthen the rationale to subtype PT-DLBCL based on EBV association. Similarly, several genomic studies have postulated that EBV+ DLBCLs in IC hosts represent a distinct entity.45 It was even found that less than 20% of EBV+ IC-DLBCLs could be attributed to an established molecular DLBCL subtype.45-47
The reason why genetic abnormalities are less frequent in EBV+ PT-DLBCL remains elusive. Possibly, the virus' oncogenic activity contributes substantially to lymphoma development, overcoming the need for activating mutations of oncogenes or silencing mutations of tumor suppressors in EBV+ PT-DLBCL.32, 38 Since the accumulation of such genomic aberrations is a gradual process, this could explain why EBV+ PT-DLBCL occur sooner than their EBV− counterparts. Accordingly, Navari et al. found that the genomic landscape of EBV+ BL (latency I) in IC patients was mostly affected by EBV-miRNAs, while EBV+ PT-DLBCL (mostly latency III) presented a significant enrichment in genes regulated by the viral latent proteins.34 In addition, Salmerón-Villalobos et al. found that the mutational burden of PT-BL (latency I) was higher than PT-DLBCL (latency II and III), indicating that the high expression levels of viral oncoproteins might overcome the need for genomic abberrations.37
The observation that the genomic signature in EBV-driven cancers is largely dictated by the latency type of the tumor further supports the hypothesis that EBV+ and EBV− could be considered distinct pathologies and might require a different clinical approach.
Transcriptional profiling
Gene expression profiling (GEP) was used to further investigate the pathogenesis of EBV+ and EBV− PT-DLBCL (Table 2). Craig et al. found that EBV+ cases demonstrated a subset of gene expression changes associated with EBV infection of B-cells,48 but this could not be confirmed in a later study.39 Micro-array-based GEP showed that EBV+ cases have upregulated genes involved in (i) immunogenic tolerance, and (ii) innate immune responses, probably directed against EBV.36 The presence of viral particles in EBV+ PT-DLBCL elicits an immune response, recruiting immunogenic cells to the TME. As a result, the distinct gene expression profiles of EBV+ and EBV− PT-DLBCLs result in different TMEs.
| Number of samples | Technique | Transcriptomic features | Reference |
|---|---|---|---|
| 8 M-PTLD | GEP | EBV+ cases demonstrated a subset of gene expression changes associated with EBV infection of B-cells | Craig et al. (2005)48 |
| 12, of which 8 EBV+ (1 IM-like lesion, 5 P-PTLD, 6 M-PTLD) | GEP | Distinct clustering of PTLD compared to other B-NHL. No segregation of EBV+ PTLD from EBV− PTLD | Vakiani et al. (2008)39 |
| 33 PT-DLBCL (11 EBV−, 22 EBV+), 15 IC-DLBCL | GEP | EBV+ and EBV− PT-DLBCL were similar except for decreased T-cell signaling in EBV+ cases | Morscio et al. (2013)36 |
| 21 EBV+ PT-DLBCL, 6 EBV− PT-DLBCL, 11 IC-DLBCL | aCGH, GEP | EBV− PT-DLBCL: at least 10 aberrations also found in IC-DLBCL (e.g., gain 3/3q and 18q, loss of 6q23/TNFAIP3 and 9p21/CDKN2A) EBV+ PT-DLBCL: amplification of 9p24.1 |
Finalet Ferreiro et al. (2016)49 |
| 49 PT-DLBCL (26 EBV−, 23 EBV+) 170 IC-DLBCL | GEP | EBV−: upregulated DNA repair genes RAD51, CCNA2, POLR2D, PCNA, WHSC1, GTF2H3 EBV+: downregulated DNA repair genes CHEK1 |
Armitage et al. (2023)35 |
| 60 PTLDs (42 EBV+ and 18 EBV−) | GEP | EBV− PTLD: upregulated BCL6, BCL11A, CXCR5, UBE2A, CUL3, CUL4B, BZW2, and LMO2 EBV+ PTLD: upregulated CCL3, CCL4, CCL8, IL15, IFNG, CD38, CD163, CD300A, SIGLEC1, OAS1, OAS2, OASL, ISG15, IFI44L, IFIT3, IFIT5, APOBEC3G, CTC-338M12.4, and PIK3R5 |
Toh et al. (2024)50 |
Compared to EBV+ PT-DLBCL, the transcriptomic profile of EBV− PT-DLBCL is similar to that of IC-DLBCL, except for decreased T-cell signaling, which is likely related to the chronic immunosuppression in posttransplant patients.36 These observations were confirmed using aCGH combined with transcriptomics on 27 PT-DLBCLs and 11 IC-DLBCL.49 Recently, the gene expression datasets of Craig et al.,48 Vakiani et al.,39 and Morscio et al.49 were included in a multi-cohort analysis by Toh et al.50 They found that upregulated genes in EBV+ PTLD included cytokines and chemokines, immune cell surface markers, interferon-stimulated genes, cytotoxic mediators, and the antiviral apolipoprotein B mRNA editing catalytic polypeptide-like (APOBEC) protein family member APOBEC3G. In addition, PIK3R5 was found to be upregulated, which encodes the p101 regulatory subunit of PI3Kγ, a kinase in the PI3K/Akt/mTOR pathway that was found to be constitutively active in EBV+ PTLD.51, 52 Upregulated genes in EBV− PTLD included anti-apoptotic transcription factor genes, chemokine receptor genes, genes encoding proteins involved in ubiquitin-mediated protein degradation, and oncogenes.
Ferreiro et al. showed that EBV+ and EBV− PT-DLBCL displayed distinct aCGH profiles, while EBV− PT-DLBCL and IC-DLBCL had features in common. These data support the hypothesis that EBV− PT-DLBCLs are de novo lymphomas in transplant recipients and EBV+ PT-DLBCLs represent true PTLDs.
Only one aberration recurred in both EBV− and EBV+ PT-DLBCL (i.e., gain of 12q21q21), while EBV− PT-DLBCL and IC-DLBCL shared 11 recurrent CNVs (among others gain of 3/3q and 18q, loss of 6q23.3 [TNFAIP3], 9p21 [CDKN2A/-2B]). It is remarkable that the latter aberrations are characteristic of non-GCB DLBCL, while EBV− are generally considered to be GCB subtypes.53
EBV+ PT-DLBCL showed the least cases with genomic imbalances. The most frequent genomic alteration in this group was gain/amplification of the 9p24.1 region, which harbors PD-L1, PD-L2, and JAK2 and possibly contributes to PD-L1 overexpression. Copy number gain of 9p24.1 is a known aberration in lymphoma and facilitates immune surveillance evasion. The clinical potential of checkpoint inhibitors blocking the PD-L1/PD1 axis in PT-DLBCL could be interesting to test in future clinical trials. Of note, boosting the immune response has shown promising results in other lymphomas, but this approach might be associated with an increased risk of graft rejection in the posttransplant setting.40, 49, 54
While the gain of chromosome 3/3q was absent in EBV+ PT-DLBCL, in EBV− cases it represented the CNV with the strongest impact on the expression level of the encoded genes.49 The aberration correlated with a differential expression of thirteen genes, including FOXP1 (3p13), encoding a transcriptional regulator suggested to be an oncogene that is particularly important in B-cell lymphomas.55 In contrast to FOXP1 overexpression in EBV− PT-DLBCL, low FOXP1 expression in EBV+ PT-DLBCLs further support the idea that EBV− PT-DLBCLs can be considered DLBCL in a posttransplant setting.
GEP data comparing EBV+ to EBV− PT-DLBCL showed that pathways involved in the innate immune system, immune response, and inflammation were often dysregulated in EBV+ PT-DLBCL but not in EBV− cases.49 These findings imply that the host's response to a viral infection in B-cells has a significant impact on the transcriptomic signature of EBV+ PT-DLBCL. Furthermore, EBV+ PT-DLBCL shares clinical, genomic, and transcriptomic characteristics with EBV+ DLBCL, NOS (previously referred to as EBV+ DLBCL of the elderly), pointing toward overlapping EBV-driven oncogenic pathways in both conditions.56 Finally, Armitage et al. show that genes involved in DNA repair mechanisms are upregulated in EBV− PT-DLBCL and downregulated in EBV+ cases.35
Proteomics
Alsayed et al. used a protein micro-array approach in six PTLD patients, five of which were EBV+.57 Upregulated proteins in the tumor samples included members of the PI3K/Akt/mTOR, MAPK and PKC pathways, cell cycle regulators, endoplasmic reticulum homeostasis (HSP90), NF-κB pathway, and apoptosis-related proteins (caspase 7-8 and MAP2K4) (Table 3).
| Number of samples | Technique | Proteomic features | Reference |
|---|---|---|---|
| 6 (5 EBV+) | Protein micro-array | Upregulated proteins: PI3K/Akt/mTOR, MAPK and PKC pathways, cell cycle regulators, endoplasmic reticulum homeostasis (HSP90), NF-κB pathway, and apoptosis-related proteins (caspase 7-8 and MAP2K4) | Alsayed et al. (2008)57 |
| 15 E-PTLDs | IHC | E-PTLD: Significantly higher number of pS6-positive (downstream mTOR effector) cells | Nelson et al. (2012)58 |
| 60 primary PTLD | IHC | Upregulated proteins in EBV+: BCL-2, downregulated proteins: BIM and cleaved PARP | Ghigna et al. (2013)59 |
Early-onset PTLD (E-PTLD) cases, which are almost exclusively EBV+, showed increased pS6, a downstream effector of mTOR.58 Moreover, a significant downregulation of pro-apoptotic proteins BIM and PARP has been observed in EBV+ compared to EBV− PTLD cases.59 The same study reported a tendency toward increased BCL-2-protein expression in EBV+ PTLD. Indeed, the viral protein LMP1 is known to induce overexpression of anti-apoptotic proteins such as BCL-2 and MCL-1.60-62 Such findings prompted Robert et al. to assess the efficacy of ABT-737, a dual BCL-2 and BCL-XL inhibitor, in EBV+ PTLD.63 Interestingly, combining ABT-737 and rituximab, the current first-line treatment of PTLD, was highly efficient and induced approximately 70% remission in PTLD xenograft mice.63 These results suggest that BCL-2 inhibitors, either alone or in combination, might show promise in treating EBV+ PTLD.
Epigenetics
Epigenetic alternations such as DNA hypermethylation of tumor suppressor genes and subsequent downregulation of gene expression may contribute to PT-DLBCL development.64 One study showed significant hypermethylation of O6 -methylguanine-DNA methyltransferase (MGMT) in four of eight PT-DLBCL cases and hypermethylation of death-associated protein-kinase (DAP-K) in 14 of 15 PT-DLBCL cases.64 Promotor hypermethylation in at least one of four analyzed genes (MGMT, DAP-K, TP53, and CASP8) was present in 93% of PT-DLBCL cases, suggesting that aberrant promotor hypermethylation occurs frequently in PTLD.
Treatment
As soon as the diagnosis of PTLD—independent of the EBV status—is confirmed, reduction of immune suppression (RIS) is mandatory in all cases, unless there is an absolute contraindication.65 In SOT patients, RIS mostly is performed by reducing calcineurin inhibition and stopping antimetabolites. The level of reduction depends on the clinical situation considering previous episodes of rejection and the possibility of rescuing graft function (i.e., dialysis in kidney transplantation) in case of graft failure. In aHSCT patients, the need for RIS is less clear, since almost all cases are caused by profound T-cell depletion due to the conditioning regimen.5
Although several series have been reported showing complete and durable remissions with RIS alone,66, 67 most physicians agree to add rituximab, a monoclonal anti-CD20 antibody, to all cases of PT-DLBCL. This approach is based on several retrospective analyses and smaller prospective phase II trials.5 In addition, the multicenter international prospective PTLD-1 trial, which included only PTLD patients after SOT, introduced the concept of risk-stratified sequential treatment. Patients reaching complete remission after RIS and rituximab had an excellent prognosis, whereas patients with no complete remission proceeded to chemotherapy. In this trial, the outcome also was independent of tumoral EBV status.68
More recently, the introduction of adoptive immunotherapy with third-party EBV-specific cytotoxic T-lymphocytes (EBV-CTLs) changed the therapeutic landscape of SOT- and aHSCT-related PTLD. In the international, multicentric phase III ALLELE trial, 14 aHSCT-patients and 29 SOT patients with proven EBV+-PTLD refractory to RIS and rituximab with or without chemotherapy were treated with tabelecleucel, an off-the-shelf, allogeneic, EBV-specific T-cell immunotherapy. Overall responses were, although no direct comparison was possible, given the open-label design without randomization, similar to those obtained with chemotherapy, but with a more favorable safety profile.69
Like the impressive progress in the treatment of DLBCL using new immunotherapeutic strategies and targeted therapies, a limited number of these strategies have been explored in the treatment of PT-DLBCL. As most PTLD cases express CD30, the antibody-drug conjugate brentuximab vedotin has been combined with rituximab in phase I/II trial including patients with CD30+ immune-suppression-related lymphomas. Although responses were promising, treatment-related toxicity was high.70 In an American retrospective multicenter analysis, 22 relapsed/refractory SOT-PTLD cases (of which one was EBV+) received anti-CD19 chimeric antigen receptor T-cells (CARTs), showing similar efficacy and toxicities compared to results in immune-competent setting.71 Finally, in the phase II TIDaL trial including 38 patients with SOT-PTLD, adding ibrutinib to rituximab failed to show clinical benefit.72 The increasing interest in new specific EBV inhibitors targeting EBV in both lytic and latent states will hopefully lead to a new class of therapeutic options for patients with relapsed and refractory EBV+ PTLD.73
CONCLUDING REMARKS
PTLD consists of many different lymphoma subtypes, with PT-DLBCL being the most common. In this review, we discussed current knowledge of PT-DLBCL pathophysiology. Although many questions remain unanswered, various studies have shed light on the complex etiology of this disease.
While our understanding of PT-DLBCL pathogenesis remains incomplete, certain findings are consistent across studies. Compared to IC-DLBCL, PT-DLBCLs typically exhibit a lower mutational burden, with notable rearrangements in BCL6 and MYC. Furthermore, EBV+ PT-DLBCLs tend to have fewer mutations and show overactive mTOR signaling, whereas EBV− PT-DLBCLs are characterized by a greater number of mutations and a higher frequency of TP53 mutations.
Overall, genetic profiling and underlying gene expression patterns of PT-DLBCL revealed clear differences between EBV+ and EBV− PT-DLBCL, as the latter shares both genomic and transcriptomic features with DLBCL in IC patients. This supports the hypothesis that EBV− PT-DLBCL represents de novo lymphomas in transplant patients. Moreover, it is not clear whether late-onset EBV+ PT-DLBCLs are truly EBV-driven or if these are cases of coincidental EBV infection. A major drawback in the literature is the lack of subtyping based on (i) lymphoma type (ii) EBV status and (iii) disease onset. Since PTLDs are highly heterogeneous, the disease mechanisms among the subgroups can differ considerably. Not taking these differences into account could cloud the conclusions drawn from studying their genome, transcriptome, and proteome.
Limited knowledge of the genetic landscape in the different disease subtypes complicates our understanding of malignant mechanisms involved in PT-DLBCL. Without a profound insight into oncogenic initiation and development of the different PT-DLBCL subtypes, improving disease management and outcome remains challenging.
AUTHOR CONTRIBUTIONS
All authors were involved in the design and writing of the manuscript and have contributed to the discussions around the manuscript content. All authors critically reviewed the manuscript and approved the final version for submission.
CONFLICT OF INTEREST STATEMENT
Daan Dierickx holds a Fundamental Clinical Mandate (FKM) from the Research Foundation – Flanders (FWO – 18B5824N). Daan Dierickx has received honoraria from Takeda, Incyte, Sanofi, Novartis, Amgen, Atara Biotherapeutics, Kite/Gilead, and Pierre Fabre, all paid to his institution. Vibeke K. J. Vergote has received honoraria from Beigene, Gilead, Roche, Lilly Oncology, Abbvie, and Johnsson & Johnsson, all paid to her institution.
FUNDING
This research received no funding.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




