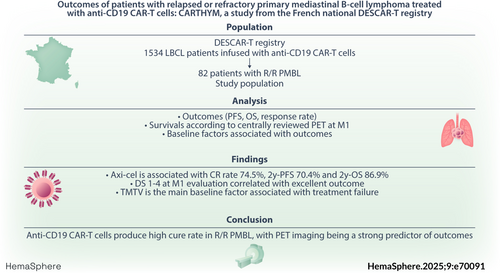
Outcomes of patients with relapsed or refractory primary mediastinal B-cell lymphoma treated with anti-CD19 CAR-T cells: CARTHYM, a study from the French national DESCAR-T registry
Presented at the 29th European Hematology Association (EHA) Annual Meeting & Exposition, June, 2024, in Madrid, Spain.
Graphical Abstract
ABSTRACT
Primary mediastinal B-cell lymphoma (PMBL) is often cured with dose-dense anthracycline-based regimens but the prognosis at relapse or progression remains poor. While anti-CD19 CAR-T cell therapy has dramatically improved outcomes in relapsed or refractory large B-cell lymphoma, far less is known about their efficacy in PMBL. Using the systematic record of all patients treated with CAR-T cells prospectively included in the DESCAR-T registry in France, along with centrally reviewed positon-emission tomography (PET) imaging, we describe the outcomes and key determinants of treatment success in PMBL patients treated over a 6-year period. Among 82 patients infused in the registry we observed a best complete response (CR) rate, 2-year progression-free survival (PFS), and 2-year overall survival (OS) of 68.1%, 57.4%, and 73.8%, respectively. Outcomes were even better for the 62 patients infused with axicabtagene ciloleucel, with best CR rate, 2-year PFS, and 2-year OS reaching 74.5%, 70.4%, and 86.9%, respectively. Achieving a Deauville score of 1–4 or a ΔSUVmax reduction of more than 24% at the 1-month evaluation was associated with excellent outcomes, whereas increased total metabolic tumor volume baseline PET increased the risk of treatment failure. Surprisingly, neither the response to bridging therapy nor the type of bridging therapy (chemotherapy versus immune checkpoint inhibitors) were associated with long-term outcomes. In conclusion, this study confirms that anti-CD19 CAR-T cells as a valid standard-of-care for relapsed and refractory PMBL and highlights key determinants of treatment success.
INTRODUCTION
Primary mediastinal large B-cell lymphoma (PMBL) is a rare subtype of large B cell lymphoma accounting for approximatively 5%–10% of all cases of non-Hodgkin lymphoma and affecting mostly young patients in their 30s.1, 2 This disease shows both clinical and biological features overlapping with classical Hodgkin lymphoma (i.e., primary mediastinal location, young age, favorable outcome, NFkB, JAK-STAT and PDL1/PDL1 pathways dysregulations) which led to its identification as a distinct entity among large B cell lymphoma (LBCL) since the WHO 2008 classification.3 Outcome after dose dense anthracyclines based-regimen with or without radiotherapy is usually excellent since around 80%–90% of patients are cured after first line treatment.4, 5 However outcome at progression is dismal. For these patients, high dose chemotherapy followed by autologous stem cell transplantation (ASCT) remained for long the standard-of-care but treatment failure were very common and therapeutical options got scarce for more advanced diseases. Immune checkpoint inhibitors have shown promising results in this setting but only small sample-sized studies have been published to date.6, 7
On the other hand, anti-CD19 CAR-T cells therapy dramatically changed the therapeutic landscape of relapsed or refractory (R/R) LBCL as it can cure around 40% of patients after early or multiple relapses.8 Three products are currently available worldwide: tisagenlecleucel (tisa-cel), axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel). According to EMA axi-cel is approved for PMBL in third or more advanced line while liso-cel is approved for PMBL in second line in case of early relapse. These approvals derived from ZUMA-1 trial9 and TRANSFORM trial,10 respectively, in whom patients with PMBL histology could be included – though they accounted for fewer than 10% of all patients, which is insufficient to draw definitive conclusions regarding CAR-T cells efficacy in this rare subset of aggressive lymphoma and to set this treatment as a standard-of-care of R/R PMBL. Real world evidence are still needed to further describe actual cure rates, to precise the main determinants of outcome, and to describe the kinetic of response to therapy. Notably, positon-emission tomography (PET) has emerged as a critical tool to predict outcome of R/R LBCL treated with CAR-T cells,11, 12 both at baseline and at early evaluation, but such evidence is still lacking for PMBL.
Using the systematic record of patients treated with CAR-T cells in the French nation-wide prospective DESCART registry, we sought to study the real-life outcome of patients with R/R PMBL treated with anti-CD19 CAR T cells in France, and highlight the clinical and imaging factors associated with treatment failure.
PATIENTS AND METHODS
Data source
The DESCAR-T registry is the French national registry sponsored by the LYSARC for all patients treated with commercial CAR T cells across all hematologic malignancies. In the CARTHYM study, we analyzed data from all PMBL patients registered in the DESCAR-T registry and who received commercial CD19 CAR T cells between July 2018 and January 2024. Pathological diagnosis was made by expert hematopathologist within the French LYMPHOPATH network.13 A total of 21 French centers participated in this study. All patients signed a consent letter before enrollment. The protocol was approved by the institutional review board, and the study was undertaken in accordance with the Declaration of Helsinki. DESCAR-T is registered under the ClinicalTrials.gov identifier NCT04328298.
Study design
Data on baseline patient and disease characteristics, cellular therapy, and outcome after CAR T-cell therapy were obtained from the DESCAR-T registry. Eligible patients were adults (aged ≥18 years) with relapsed or refractory PMBL treated with commercial anti-CD19 CAR-T cells (axi-cel, tisa-cel or liso-cel). Patients were treated through the French Early Access Program or routine commercial use covered by French health insurance in approved centers. All patients received conditioning regimen consisting in fludarabine and cyclophosphamide for 3 days. The main objective was to report outcomes in the study population, including 2 years progression-free survival (PFS), 2 years overall survival (OS) and response rate: response at 1-month (M1), 3-months (M3) and best overall response rate (bORR). We also planned to describe 2y-PFS, 2y-OS and response rate separately in patients treated with axi-cel, as it might represent the main group of patients following axi-cel early EMA approval in R/R PMBL, and to compare these outcomes with other LBCL treated with axi-cel in the DESCAR-T registry. Another objective of the study was to report baseline clinical, biological and imaging factors (including total metabolic tumor volume [TMTV]) associated with PFS, and the association between early response at M1 PET evaluation and outcomes in patients treated with axi-cel. Finally, we planned to report toxicities—especially immune-mediated toxicity graded according to the American Society for Transplantation and Cellular Therapy (ASTCT).14
Imaging
PET performed at baseline (after any bridging therapy and before the beginning of conditioning regimen) and M1 after CAR-T cells infusion were reviewed by an expert nuclear medicine physician (C.M) when centrally available inside the DESCAR-T study. TMTV (obtained from the sum of the metabolic volumes of all nodal and extranodal lesions) was computed with the 41% maximum standardized uptake value threshold method.15 Deauville 5-point scale evaluation was performed at M1. In order to assess the ΔSUVmax between baseline and M1, the hottest tumor in any region or organ was used for comparison, even if its location differed from the initial hottest tumor in baseline PET-CT.
Statistical analysis
Quantitative variables were displayed using mean, standard deviation, median, range and quartiles. Qualitative variables were expressed as frequencies and percentages. The best overall response (best CR and best PR) was the best response recorded during the evaluation period of patients. If a patient had a subsequent treatment for progression, all responses recorded after the initiation of this new treatment were censored. The follow-up duration was defined as the time between the date of CAR-T infusion and date of last contact for alive patients. Deceased patients were censored at the date of death and follow-up duration was estimated using reverse Kaplan-Meier method. Survival curves were generated using the Kaplan–Meier estimation method. PFS was measured from the date of CART-cell infusion to the date of death from any cause or disease progression. Duration of response (DOR) and CR (DOCR) were measured from the time of first response or CR, respectively, to the date of death of any cause or disease progression. OS was calculated from the date of CAR T-cell infusion until the date of death from any cause. Survival distributions were compared using the log-rank test. A two-sided p value of less than 0.05 was considered significant. Survival analyses of ΔSUVmax and Deauville score at M1 used landmark at M1. Cut-offs for ΔSUVmax and TMTV pre CAR-T are calculated according to Lausen method.16 Cut-offs correspond to values of quantitative variable where the log-rank statistics is maximum. Adjustment is made on the p values to account for type I error inflation due to multiple testing. Prognostic factors for PFS were estimated using proportional hazard Cox model. Covariates with univariate p values < 0.25 by AIC were selected for multivariate analysis. Prognostic factors for Toxicity were estimated using logistic regression on toxicity indicators. Statistical analyses were performed using SAS software version 9.4 and R version 4.2.3. The maxstat package version 0.7 was used for cutoffs analyses.
RESULTS
Patients
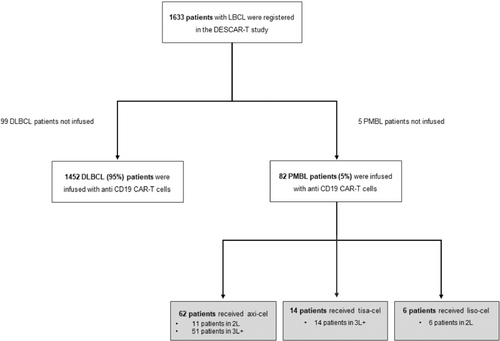
From July 2018 to January 2024, 87 PMBL patients underwent leukapheresis and were recorded in the DESCART registry, and 82 were included in this study as 5 patients did not receive CAR-T cells infusion. Axi-cel CAR-T cells were used in 62 patients, tisa-cel in 14 patients, and liso-cel in 6 patients. Flowchart is shown in Figure 1 and the characteristics of the patients are shown in Table 1. Briefly, the median age at infusion was 33 years, with a sex ratio of approximately 1:1 (male/female). The median number of prior lines of therapy was 2 (ranges 1–7) with 20% of patients being treated at relapse or progression after first line: 17% (n = 11) for axi-cel, 0% for tisa-cel, and 100% (n = 6) for liso-cel. At CAR-T cells decision, most patients had stage I–II disease and approximately two-thirds of patients had a low (0–1) age-adjusted IPI score. Median vein-to-vein time was 41 days (ranges 29–105).
| CAR T | ||||
|---|---|---|---|---|
| Axi-cel | Tisa-cel | Liso-cel | ALL | |
| N = 62 | N = 14 | N = 6 | N = 82 | |
| Age at first infusion (years) | ||||
| Median (ranges) | 33.5 | 37 | 33 | 33.5 |
| Sex, n (%) | ||||
| Male | 32 (51.6) | 7 (50) | 4 (66.7) | 43 (52.4) |
| Female | 30 (48.4) | 7 (50) | 2 (33.3) | 39 (47.6) |
| Prior lines of therapy, n (%) | ||||
| Median | 2.0 | 3.0 | 1.0 | 2.0 |
| Min; Max | 1; 7 | 2; 8 | 1; 1 | 1; 8 |
| 1 | 11 (17.7) | 0 (0) | 6 (100.0) | 17 (20.7) |
| 2 | 39 (62.9) | 5 (35.7) | 0 (0.0) | 44 (53.7) |
| 3 | 9 (14.5) | 4 (28.6) | 0 (0.0) | 13 (15.9) |
| >4 | 3 (4.8) | 5 (35.7) | 0 (0.0) | 8 (9.8) |
| Prior autograft, n (%) | ||||
| No | 60 (96.8) | 10 (71.4) | 6 (100) | 76 (92.7) |
| Yes | 2 (3.2) | 4 (28.6) | 0 (0) | 6 (7.3) |
| Ann Arbor at decision, n (%) | ||||
| Missing | 1 | 1 | 0 | 2 |
| I-II | 38 (62.3) | 3 (23.1) | 2 (33.3) | 43 (53.8) |
| III-IV | 23 (37.7) | 10 (76.9) | 4 (66.7) | 37 (46.3) |
| aaIPI at RCP, n (%) | ||||
| 0 | 12 (20) | 2 (15.4) | 0 (0) | 14 (17.7) |
| 1 | 29 (48.3) | 4 (30.8) | 5 (83.3) | 38 (48.1) |
| 2 | 19 (31.7) | 6 (46.2) | 0 (0) | 25 (31.6) |
| 3 | 0 (0) | 1 (7.7) | 1 (16.7) | 2 (2.5) |
| Missing | 2 | 1 | 0 | 3 |
| ECOG at lymphodepletion, n (%) | ||||
| 1 | 57 (95) | 14 (100) | 5 (83.3) | 76 (95) |
| 2 | 2 (3.3) | 0 (0) | 1 (16.7) | 3 (3.8) |
| 3 | 1 (1.7) | 0 (0) | 0 (0) | 1 (1.3) |
| Missing | 2 | 0 | 0 | 2 |
| LDH at infusion > ULN, n (%) | ||||
| Normal | 38 (64.4) | 7 (53.8) | 3 (50) | 48 (61.5) |
| >Upper limit | 21 (35.6) | 6 (46.2) | 3 (50) | 30 (38.5) |
| Missing | 3 | 1 | 0 | 4 |
| CRP at infusion (mg/L) | ||||
| Missing | 1 | 2 | 1 | 4 |
| Median | 6 | 16.5 | 12 | 8 |
| Min; Max | 0; 188 | 0; 119 | 1; 49 | 0; 188 |
| Time from first apheresis to CAR-T injection (days) | ||||
| Median | 39.5 | 43 | 54 | 41 |
| Min; Max | 29; 105 | 33; 64 | 48; 77 | 29; 105 |
- Abbreviations: aaIPI, age adjusted international prognostic index; Axi-cel, axicabtagene ciloleucel; Liso-cel, lisocabtagene maraleucel; SUV, standard uptake value; Tisa-cel, tisagenlecleucel.
Most patients (86.6%) received bridging therapy after leukapheresis and before CAR-T cell infusion. This bridging therapy included various regimen of polychemotherapy (i.e., platinum-containing regimens such as ifosfamide, carboplatin, etoposide, or dexamethasone, high-dose cytarabine, carbo or cisplatin) in 60.6%, immune checkpoint inhibitors (ICI, pembrolizumab or nivolumab) in 29.6%, and/or radiotherapy in 19.6% of patients (Table 2). Nine out of 21 patients received ICI in combination with Brentuximab-Vedotin (Bv). Among the 68 (95%) patients evaluated after bridging therapy and prior to CAR-T cell infusion, 31.5% were responder (complete response, CR, or partial response, PR), while the remainder were in stable disease (SD) or progressive disease (PD).
| CAR T | ||||
|---|---|---|---|---|
| Axi-cel | Tisa-cel | Liso-cel | ALL | |
| N = 62 | N = 14 | N = 6 | N = 82 | |
| Bridging therapy | ||||
| No | 10 (16.1%) | 1 (7.1%) | 0 (0.0%) | 11 (13.4%) |
| Yes | 52 (83.9%) | 13 (92.9%) | 6 (100.0%) | 71 (86.6%) |
| If bridging, | ||||
| Including ICI | ||||
| No | 32 (61.5%) | 12 (92.3%) | 6 (100.0%) | 50 (70.4%) |
| Yes | 20 (38.5%) | 1 (7.7%) | 0 (0.0%) | 21 (29.6%) |
| Including chemotherapy | ||||
| No | 25 (48.1%) | 3 (23.1%) | 0 (0.0%) | 28 (39.4%) |
| Yes | 27 (51.9%) | 10 (76.9%) | 6 (100.0%) | 43 (60.6%) |
| Including radiotherapy | ||||
| No | 41 (78.8%) | 11 (84.6%) | 5 (83.3%) | 57 (80.3%) |
| Yes | 11 (21.2%) | 2 (15.4%) | 1 (16.7%) | 14 (19.7%) |
| Response (Lugano 2014) | ||||
| CR | 5 (10.0%) | 0 (0.0%) | 0 (0.0%) | 5 (6.5%) |
| PR | 12 (24.0%) | 3 (25.0%) | 2 (33.3%) | 17 (25.0%) |
| SD | 12 (24.0%) | 3 (25.0%) | 1 (16.7%) | 16 (23.5%) |
| PD | 21 (42.0%) | 6 (50.0%) | 3 (50.0%) | 30 (44.1%) |
| Missing | 2 | 1 | 0 | 3 |
- Abbreviations: Axi-cel, axicabtagene ciloleucel; ICI, immune checkpoint inhibitor; Liso-cel, lisocabtagene maraleucel; Tisa-cel, tisagenlecleucel.
Regarding PET findings at baseline (see Table S1), >5 cm bulk was present in 37.8% of patients. The median SUVmax was 14, ranging from 0 to 39. The median TMTV was centrally assessed in 58 patients and evaluated at 35.7 mL, with a wide range values (from 0 to 2611 mL).
Outcome of the whole cohort
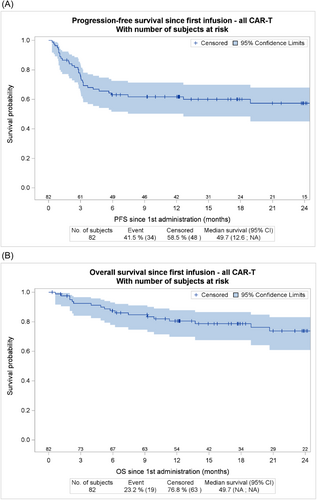
The bORR for the 82 infused patients was 84.7% (68.1% CR and 16.8% PR). Of note CR and PR rates were 40% and 38.5% at M1 and 51.5% and 15.6% at M3, respectively. The proportion of PR patients at M1 who converted to CR at M3 was 48.1%. After a median follow-up of 21 months, 2-year PFS of all infused patients was 57.4% (95% confidence interval [CI], 45%–67.9%) (Figures 2A) and 2-year OS was 73.8% (95%, 60.9% to 83%) (Figure 2B).
Outcome of patients receiving axi-cel
For the 62 patients infused with axi-cel, bORR was 89.1%, with CR rate of 74.5% and PR of 14.5%. Of note, CR and PR rates were 41.5% each at M1 and 63.8% and 17% at M3, respectively. The proportion of PR patients at M1 who converted to CR at M3 was 59.1%.
After a median follow-up of 21 months, 2-year PFS of patients infused with axi-cel was 70.4% (95% CI, 57.1% to 80.3%) (Figure 3A) and 2-year OS was 86.9% (95% CI, 76.6% to 93.8%) (Figure 3B). Two-year DOR for responder patients was 71.9% (95% CI, 55.4% to 84.1%) and it was 79.9% (95% CI, 62.2% to 90%) for patients reaching CR (Figure S1).

PET evaluation at M1 according to 5-PS Deauville score (DS) strongly correlated with outcome: 2-year PFS was 90.5% (95% CI, 67 to 97.5) for patients with a DS 1 to 3, 84.4% (95% CI, 49.3 to 96%) for patients with a DS 4, and 33% (95% CI, 7.8% to 62.3%) for patients with a DS 5 (Figure 3C). DS 5 at M1 was associated with a significantly shorter PFS in comparison with DS 1 to 4 (p < 0.001) and a reduced 2-year OS (70% vs. 100% for Deauville score 1–4, p = 0.013) (Figure S2).
ΔSUVmax between baseline PET and M1 evaluation was also strongly associated with worse PFS. In an exploratory analysis, the optimal cut-of value to predict progression was ΔSUVmax ≥ 24%, with 2-year PFS of 84% (95% CI, 65.3% to 93.1%) for patient with ΔSUVmax ≥ 24% versus 34.1% (95% CI, 9.1% to 61.6%, p = 0.028) for patients with ΔSUVmax < 24% (Figure 3D).
Comparison of outcome of PMBL and DLBCL treated with axi-cel in the DESCART registry
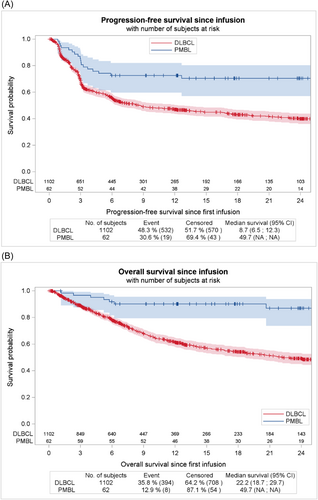
For 1102 diffuse large B-cell lymphoma (DLBCL) patients (PMBL excluded) infused with axi-cel and for whom response was available, bORR was 83.5%, with CR rate of 65.9% and PR of 17.6%. These rates seem comparable with those reported in our cohort (89.1%, 74.5% and 14.5% for ORR, CR and PR respectively). However 2-year PFS and OS were evaluated at 39.9% (95% CI, 36.1% to 43.7%) and 49.2% (95% CI 45.1% to 53.2%) respectively for DLBCL, compared to 70.4% and 86.9% respectively for PMBL patients. Survival curves are shown in Figure 4. It is noteworthy that, considering the distinct histology, clinical course and likely imbalance in baseline factors between the two populations, we did not perform statistical test to compare the two populations.
Baseline factors associated with outcome for patients treated with axi-cel
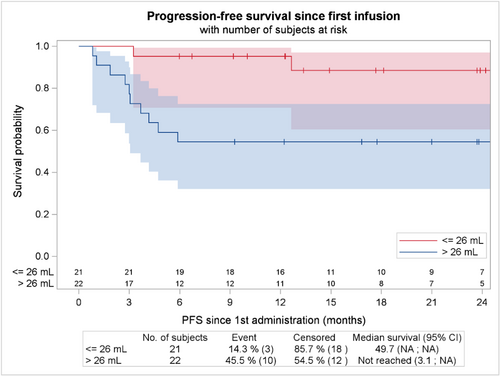
Baseline TMTV as a continuous variable was associated with a shorter PFS (p = 0.001). Of note, the optimal cut off for discriminating survival in our population was 26 ml (Figure 5). Baseline CRP was also associated with a shorter PFS (p = 0.005). Sex and receipt of two or more prior line of therapy was not risk factor for progression or death, but a trend was observed between elevated LDH at infusion and PFS (p = 0.073). Interestingly, neither the type of bridging therapy (chemotherapy vs. immunotherapy), nor response to the bridge before axi-cel infusion (CR + PR vs. SD + PD) were associated with outcome (p = 0.576 and p = 0.379, respectively) (Figure S3). Poor performance status or extranodal involvement, two factors commonly associated with worse outcome in the setting of CAR-T cells treatment, were too rare in this population to be evaluated. In multivariate analysis after selection of covariate with univariate value < 0.25, only TMTV remains as a predictor of PFS events (p = 0.004) (see Table 3).
| Univariate analysis | Multivariate analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||
| Variable | Modality | Number of patients | Hazard ratio | Lower | Higher | p Value (global likelihood ratio test) | Hazard ratio | Lower | Higher | p Value (global likelihood ratio test) |
| CRP at infusion | CRP (unit = 1) | 61 | 1.05 | 1.01 | 1.09 | 0.005 | 1.183 | 0.953 | 1.468 | 0.124 |
| LDH at infusion | Upper limit vs. Normal | 59 | 2.38 | 0.92 | 6.19 | 0.07 | 2.385 | 0.378 | 15.033 | 0.355 |
| Number of prior lines | 2 L vs. 3 L+ | 62 | 0.77 | 0.25 | 2.34 | 0.65 | - | - | - | - |
| Response to bridging | No Bridging vs. SD/PD | 56 | 1.53 | 0.47 | 4.9 | 0.37 | - | - | - | - |
| RC/PR vs. SD/PD | 0.48 | 0.10 | 2.22 | |||||||
| Sex | Female vs. Male | 62 | 0.77 | 0.30 | 1.97 | 0.59 | - | - | - | - |
| TMTV pre CAR-T | TMTV (unit = 1) | 43 | 1.08 | 1.02 | 1.14 | 0.001 | 1.181 | 1.054 | 1.322 | 0.004 |
| Type of bridging | Chimio vs. Other | 62 | 1.25 | 0.43 | 3.61 | 0.57 | - | - | - | - |
| No bridging vs. Other | 2.01 | 0.56 | 7.16 | |||||||
- Abbreviations: 2 L, second line; 3 L+, third line or more; CI, confidence interval; CR, complete response; LDH, lactate dehydrogenase; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; TMTV, total metabolic tumor volume.
Outcome of patients receiving tisa-cel or liso-cel
For the 14 patients who received off-label Tisa-cel infusion, mainly between 2018 and 2019, best ORR and CR rates were 61.5% and 30.8% respectively. Outcome was very poor with 2-year PFS of 7.1% (95% CI 0.5 to 27.5%) and 2-year OS of 25.7% (95% CI, 6.7% to 50.6%) (see Figure S4). Of note, among the 13 PFS events, 11 were lymphoma progression and 2 were late death in remission.
Six patients received liso-cel treatment after early failure of first line chemotherapy, starting from 2023 and following end-of-2022 EMA approval. Among those six patients, four reached CR and remained in remission after a median follow-up of 6 months. The two other patients experienced very rapid progressive disease leading to early death.
Safety
For the 62 patients infused with axi-cel, the cytokine release syndrome (CRS) rate was 90.6%, mostly of low grade (grade 1, 62.9%; grade 2, 25.8%; grade 3, 1.6%). The immune effector cell associated neurologic symptoms (ICANS) rate was 45.2% and consisted in grade 1 in 9.7%, grade 2 in 9.7%, grade 3 in 21% and grade 4 in 4.8%. Rate of ICU admission was 32%. Type of bridge (especially: previous exposition to ICI) was not associated with grade 3–4 immune-mediated toxicity (p = 0.546). Non-relapse mortality events consisted in one toxic death before D30 from infection in the setting of grade 2 ICANS treated with corticosteroids, and one late death in remission from infection >1 year after infusion.
Among the 14 patients infused with tisa-cel, 12 experienced CRS and 2 experienced ICANS, with 4 patients being admitted in ICU and only one patient experiencing grade 3 immune mediated toxicity (grade 3 CRS + grade 3 ICANS). Two late toxic deaths (cardiogenic shock and cerebral hemorrhage) unrelated to tisa-cel occurred 7 and 18 months after infusion.
Among the four patients infused with liso-cel, four experienced CRS (three grade 1 and one grade 2) and none experienced ICANS.
DISCUSSION
Here, we show that CAR-T cells therapy is associated with excellent cure rates in a real-life cohort of 82 R/R PMBL and underline the crucial importance of PET imaging to predict outcomes. These findings are clinically extremely relevant since PMBL experiencing failure to anthracycline-based regimens are characterized by chemoresistance and poor outcomes. Prospective and real-world data are scarce for this rare subtype of R/R LBCL, with most of cohorts comprising between 30 and 60 patients,17-19 but 2-year PFS after failure of R-CHOP-like regimens may approach 25% in modern series.4 Although salvage chemotherapy and high-dose chemotherapy followed by autologous stem cell transplantation can be curative, some data suggest that outcomes may be worse than for other R/R DLBCL, primarily due to the challenge of achieving a second complete response.20 Innovative strategies that extend beyond the unsatisfactory efficacy of conventional chemotherapy are therefore necessary. Anti-PD1 monoclonal antibodies pembrolizumab and nivolumab, with or without the anti-CD30 conjugate monoclonal antibody brentuximab-vedotin, have shown very promising results in terms of response (ORR 40%–70%) and 2-year PFS (40%–50%) in several phase II studies and limited real-world cohorts,6, 7, 21, 22 but these data remains preliminary and further evidence is still needed to establish an indisputable standard of care—especially in the CAR-T cells era.
Anti-CD19 CAR-T cells are now the cornerstone of treatment of LBCL that relapse within 1 year after completion of first-line therapy or following subsequent lines of chemotherapy. These approvals stem from practice-changing pilot studies where cure rates approached 40–50%, surpassing those of conventional chemotherapy.8-10, 23, 24 Risk factors for treatment failure include elevated baseline TMTV, CRP, and LDH, as well as extranodal involvement and refractoriness to bridging therapy.8, 12, 25-27 The kinetics of response to CAR-T cell therapy have also been described,11 offering more precise insights into key management aspects, such as: how to control tumor burden, which patients to infuse, and how to assess responses to treatment. These essential data are still lacking for the rare subset of R/R PMBL patients. While pilot studies have only included a very small number of PMBL patients (e.g., eight in the ZUMA-1 trial and eight in the TRANSFORM trial), registry studies have recently provided more consistent real world evidence regarding the outcomes of PMBL patients treated with anti-CD19 CAR-T cells. In a small set of 13 patients treated with axi-cel from the German registry, the CR rate was 54%, and 2-year PFS was 54%.28 Another multicenter cohort of 33 patients treated with axi-cel showed a CR rate of 67% and 2-year PFS of 64%.29 Finally, CR rates and 12-month PFS were 65% and 62%, respectively, in an Italian cohort of 70 patients treated with axi-cel, significantly better than the outcomes of 190 other DLBCL patients from the same registry.30 However, baseline features and key response dynamics, including PET-based evaluations, remain largely unexplored.
Using the systematic and exhaustive records of the French nationwide prospective registry DESCAR-T, we were able to fully describe outcomes and main determinants of treatment success in R/R PMBL patients treated with anti-CD19 CAR-T cells. We confirm here the outstanding efficacy of CAR-T cells in this setting, particularly axi-cel that is associated with CR rates of 74.5% and 2-year PFS of 70.4%. Even though direct comparison has to be interpreted with caution, these results compare very favorably with the outcomes of more than 1000 DLBCL patients from the registry and strongly suggests that axi-cel may be particularly effective in this distinct histology. Moreover, central review of pre- and post-infusion PET allowed us to study the impact of well-recognized imaging tools used in untreated DLBCL, such as pre-treatment TMTV and early response evaluation according to Deauville criteria or reduction in SUVmax. We found that baseline TMTV is associated with an increased risk of treatment failure, though the optimal cut-off in our cohort (26 mL) appears much smaller compared to those observed in DLBCL at diagnosis (around 250–350 mL)31, 32 or before CAR-T cells treatment (81–148 mL),12, 26 likely reflecting the frequent use of bridging therapy and the common early-stage of PMBL. We also demonstrated that early response one month after infusion is a key determinant and a good surrogate marker of long-term outcomes, and that stringent complete response is not required since Deauville 1–4 share excellent outcomes as do patients with moderate reductions in ΔSUVmax. Conversion to CR per Lugano criteria may be slow in this disease and we observed a high rate (56%) of conversion from PR at M1 to CR at M3. These imaging aspects are of significant clinical importance and may help guide high-risk disease definitions and response assessments in future clinical trials involving CAR-T cells in this infrequent lymphoma.
Interestingly, and contrary to findings in other LBCL types,27 we did not observe a significant impact of response to bridging therapy on long-term survival after CAR-T cell treatment. More surprisingly, we did not observe any benefit of receiving ICI before CAR-T cell infusion despite expectations that both enhanced responses and modulation of the tumor microenvironment could improve treatment efficacy.33 Based on these findings, we believe that CAR-T cell infusion should remain the primary therapeutic goal once leukapheresis is performed with bridging therapy only serving as transient tumor burden control.
Several factors could potentially explain the excellent outcome of PMBL patients treated with axi-cel, such as good performance status, a low rate of multiple extranodal involvements, and low TMTV at infusion—these three features being correlated with response and outcome after anti-CD19 CAR-T cells.8, 26 The low rate of non-relapse mortality, compared to what has been reported for LBCL patients in another study from the DESCAR-T registry, is likely due to the younger age of PMBL patients and could explain the lack of late PFS events.34 However, beside clinical factors, biology of the disease is also an key determinant of outcomes after CAR-T cells treatment and recent studies have highlighted the importance of the lymphoma microenvironment—an immunosuppressive milieu being associated with reduced response rate and survival.35 Notably, some aggressive types of LBCL with a “hot” lymphoma microenvironment, such as T-cell and histiocyte-rich LBCL, exhibit extremely poor outcomes following CAR-T cell therapy.36 Given this, the favorable outcomes seen in PMBL patients after anti-CD19 CAR-T cell treatment are somewhat surprising, considering its Hodgkin-like dense immunosuppressive microenvironment. More evidence is needed to fully understand the biological determinants of response to CAR-T cell therapy in this population.
The small number of patients treated with liso-cel (exclusively in the second-line setting following its 2022 EMA approval) provides only preliminary data. However, durable responses in four out of six patients appear to be in line with outcomes observed in axi-cel-treated patients, both in our cohort and in others. In contrast, off-label use of tisa-cel (mostly during the early period of CAR-T cell approval in France, between 2018 and 2020) was associated with poor outcomes—although imbalances in baseline risk factors such as stage at relapse and the number of previous lines of therapy may partially explain these findings.
Our study has several limitations. First, the relatively small number of patients did not allow us, given the low number of progression events, to fully analyze the clinical factors associated with outcomes. However, to our knowledge, this study remains one of the largest to date to focus on R/R PMBL and may serve as a guide for future backbone of treatment of R/R PMBL. Second, even if all lymphoma diagnosis in France are confirmed by expert hematopathologists within the LYMPHOPATH network, with common use of molecular tools such as CIITA rearrangement and/or gene expression profiling of tumor cells,13, 37 we were unable to perform a central review of the biopsy samples and therefore to rule out the inclusion of misdiagnosed PMBL cases. We also could not study the impact of histological and molecular features, which has been associated with outcome after first line chemotherapy,38, 39 on CAR-T cells response. On the other hand, besides the prospective inclusion of all patients treated with CAR-T cells in France within the DESCAR-T registry, the main strength of this study relies on the central review of PET scans allowing for accurate definitions of response, response dynamics, and imaging features associated with treatment failure.
In conclusion, this study demonstrates the excellent outcomes of R/R PMBL patients treated with anti-CD19 CAR-T cells, particularly axi-cel. These findings further support anti-CD19 CAR-T cells as a standard of care in this indication and highlight the importance of baseline and dynamic PET evaluations as tools to predict outcomes.
ACKNOWLEDGMENTS
The authors thank the patients who accepted to be included in the DESCAR-T study, and all the investigators and their staff involved in the data collection and analyses. The authors thank everyone from the Lymphoma Academic Research Organization (LYSARC) DESCAR-T study group who actively participated in the study The DESCAR-T registry was partly funded by Gilead, Bristol Myers Squibb and Novartis; however, they did not participate in the study design, data collection, statistical analysis, or interpretation, and did not provide assistance for manuscript writing or editorial support.
AUTHOR CONTRIBUTIONS
Conception and design: Jean Galtier, Krimo Bouabdallah, and Roch Houot. Provision of study material or patients: all authors. Collection and assembly of data: Jean Galtier and Charles Mesguich. Data analysis and interpretation: Jean Galtier, Krimo Bouabdallah, Charles Mesguich, and Vivien Dupont. Manuscript writing: Jean Galtier, Krimo Bouabdallah, Vivien Dupont. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
CONFLICT OF INTEREST
Jean Galtier, Roberta Di Blasi, François-Xavier Gros, Sylvain Carras, Krimo Bouabdallah: Honoraria from Kite/Gilead. Vincent Camus: Honoraria from Incyte, AbbVie, AstraZeneca, Bristol Myers Squibb, Ideogen, Janssen, Kyowa Kirin, Kite/Gilead, Lilly, Novartis, Octapharma, Pfizer, Pierre Fabre, Sanofi, and Takeda, all of which are unrelated to the present study. Pierre Sesques: Honoraria, Advisory/Consultancy from Janssen, Roche, BMS, Chugai; Novartis, Abbvie and Kite/Gilead. Catherine Thieblemont: speaker: AbbVie, Amgen, Bayer, Bristol Myers Squibb/Celgene, Gilead Sciences Inc, Incyte Corporation, Janssen, Kite, Novartis, Roche, Takeda; Travel and accommodation: AbbVie, Amgen, Bayer, Bristol Myers Squibb/Celgene, Gilead Sciences Inc, Janssen, Kite, Novartis, Roche, Takeda; Research grant: Janssen, Roche; Consultancy/Honoraria: AbbVie, Amgen, Bayer, Bristol Myers Squibb/Celgene, Gilead Sciences Inc, Novartis, Roche, Incyte Corporation, Janssen, Kite, Takeda. Emmanuel Bachy: AbbVie, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees, Consultancy, Honoraria ADC Therapeutics: Honoraria Amgen: Research Funding BeiGene: Membership on an entity's Board of Directors or advisory committees, Honoraria Bristol Myers Squibb: Honoraria, Other, Research Funding Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees Janssen: Honoraria, Consultancy Kite, a Gilead Company: Consultancy, Honoraria, Other Novartis: Honoraria, Other Pfizer: Honoraria, Other. Franck Morschhauser: consultancy: Roche/Genentech; consultancy and membership on an entity's Board of Directors or advisory committees: Kite/Gilead Sciences, Bristol Myers Squibb, AbbVie, Epizyme, AstraZeneca, Novartis, Genmab; Honoraria: Roche/Genentech, Chugai, Takeda. Guillaume Cartron: Consultancies. Roche, Celgene-BMS, Abbvie, Milteny Scientific Advisory Boards. Onwards Therapeutics, MedxCell, EmerCell, MabQi Honoraria. Sanofi, Abbvie, Takeda, Janssen, Roche, Celgene, Novartis, Incyte. Roch Houot: Honoraria from Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, Incyte, Janssen, MSD, Takeda, Amgen, Abbvie, and Roche; and is a member on an entity's Board of Directors or advisory committees of Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, Tessa Therapeutics, Abbvie and Roche. The remaining authors declare no conflicts of interest.
FUNDING
This research received no funding.
Open Research
DATA AVAILABILITY STATEMENT
Data are subject to controlled access by DESCAR-T owing to privacy and legal requirements and proprietary reasons. Anonymized IPD requests will be promptly reviewed by the corresponding author (JG) and the scientific committee of the DESCAR-T registry.