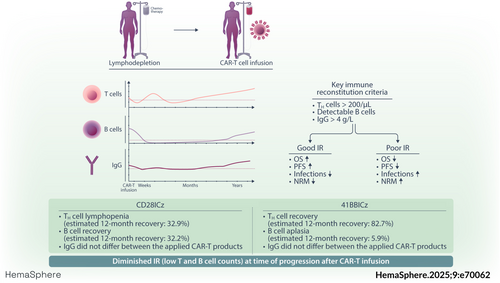
Prognostic significance of immune reconstitution following CD19 CAR T-cell therapy for relapsed/refractory B-cell lymphoma
Graphical Abstract
Abstract
Immune deficits after CD19 chimeric antigen receptor (CAR) T-cell therapy can be long-lasting, predisposing patients to infections and non-relapse mortality. In B-cell non-Hodgkin lymphoma (B-NHL), the prognostic impact of immune reconstitution (IR) remains ill-defined, and detailed cross-product comparisons have not been performed to date. In this retrospective observational study, we longitudinally characterized lymphocyte subsets and immunoglobulin levels in 105 B-NHL patients to assess patterns of immune recovery arising after CD19 CAR-T. Three key IR criteria were defined as CD4+ T helper (TH) cells > 200/µL, any detectable B cells, and serum immunoglobulin G (IgG) levels >4 g/L. After a median follow-up of 24.6 months, 38% of patients displayed TH cells, 11% showed any B cells, and 41% had IgG recovery. Notable product-specific differences emerged, including deeper TH cell aplasia with CD28z- versus longer B-cell aplasia with 41BBz-based products. Patients with any IR recovery experienced extended progression-free survival (PFS) (median 20.8 vs. 1.7 months, p < 0.0001) and overall survival (OS) (34.9 vs. 4.0 months, p < 0.0001). While landmark analysis at 90 days confirmed improved PFS in patients with any recovery (34.9 vs. 8.6 months, p = 0.005), no significant OS difference was noted. Notably, 72% of patients with refractory disease never displayed recovery of any IR criteria. Early progressors showed diminished IR at the time of progression/relapse compared to patients with late progression/recurrence (after Day 90). Our results highlight the profound immune deficits observed after CD19 CAR-T and shed light on the intersection of IR and efficacy in B-NHL. Importantly, IR was impaired considerably postprogression, carrying significant implications for subsequent T-cell-engaging therapies and treatment sequencing.
INTRODUCTION
Genetically modified chimeric antigen receptor (CAR) T cells represent a transformative immunotherapy for a range of refractory B-cell malignancies.1-6 The commercially available CAR-T products target common B-cell antigens such as CD19 or BCMA and endow autologous T cells with HLA-independent specificity against the target antigen.7 While long-term disease remissions are frequently observed, CAR T cells are also associated with distinct immune-related adverse events such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).8, 9 Furthermore, grade 3 or higher cytopenias are common and typically follow either a biphasic or aplastic trajectory.10-12 Such hematologic side effects are multifactorial and were recently termed immune effector cell-associated hematotoxicity (ICAHT).13-17
While the lymphodepleting chemotherapy administered prior to CAR T-cell infusion (typically fludarabine [flu] and cyclophosphamide [cy] or bendamustine) is essential to facilitate effective CAR T-cell expansion and persistence, it also contributes to delayed recovery of endogenous lymphocytes.18 Low CD4+ T-cell counts can last longer than 1 year postinfusion and lead to an increased risk for opportunistic infections.19 Next to the cellular immune deficits conferred by protracted neutropenia and decreased CD4+ T-cell counts, B-cell aplasia (BCA) and hypogammaglobulinemia are well-characterized “on-target/off-tumor” side effects of CAR T-cell therapies targeting B-lineage surface antigens.20, 21 For example, CD19 is expressed on a wide range of B-cell precursors and mature B cells and plays a critical role during B-cell development and maturation.22 Due to the nature of CAR T-cells as a “living drug,” humoral immune deficits can thus be long-lasting and extend beyond 10 years.23 The extent of BCA closely mirrors CAR T-cell expansion and its duration can be viewed as a pharmacokinetic read-out of functional CAR T-cell persistence.24 Concomitantly, loss of BCA has been implicated as a harbinger of relapse, particularly in patients with B-cell acute lymphoblastic leukemia (B-ALL).24
Even though CRS and ICANS represent the prototypical side effects of CAR T-cell therapy, infectious complications represent the main driver of early and late non-relapse mortality (NRM).25, 26 Bacterial infections predominate early after CAR T-cell infusion (Days 0–90), while viral infections are more common in the late phase (after Day 90).27-30 Fungal infections are rare overall,31 but mortality can be high when patients present with severe coincident immunotoxicity.32, 33 Characterizing the detailed patterns of T- and B-cell recovery after CD19 CAR-T can help treating physicians understand when and how long patients are at risk for a specific subtype of infection. Currently, it remains poorly understood which baseline and postinfusional factors predispose to protracted immune deficits. Due to specific differences in their lymphodepletion dosing regimens and co-stimulatory domains (CD28z vs. 41BBz), we expect that there will be distinct and characteristic changes in IR patterns across CAR-T products. For example, it is hypothesized that the critical phase of immune suppression may be shorter with CD28z-costimulated products like axicabtagene ciloleucel due to diminished CAR T-cell persistence.34 A further knowledge gap pertains to the relationship between protracted T- and B-cell reconstitution and survival outcomes, which remains less well-defined for lymphoma patients compared to B-ALL patients. Finally, little is known about immune cell counts at the time of post-CAR-T progression or relapse, which would carry important implications for the expected efficacy of subsequent T-cell-engaging therapies like bispecific antibodies.
In this retrospective observational study, we aimed to address these knowledge gaps by characterizing IR patterns (including at the time of progression or relapse), examining predictors of delayed recovery, and exploring associations between IR and survival outcomes in 105 patients receiving CD19 CAR-T for relapsed/refractory B-cell non-Hodgkin lymphoma (r/r B-NHL). The primary objectives of this study were to (1) descriptively analyze IR patterns following CAR-T therapy according to prespecified subgroups and (2) assess the impact of IR on infectious events, NRM, and survival.
METHODS
Patient cohort
We included all B-NHL patients consecutively treated with CD19 CAR-T at the University Hospital of the LMU Munich between January 2019 and December 2023 (data cutoff). Patients were treated with axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), lisocabtagene maraleucel (liso-cel), or brexucabtagene autoleucel (brexu-cel) in a standard-of-care setting. Treatment for a disease entity other than r/r B-NHL (n = 21), follow-up less than 30 days prior to data cutoff (n = 3), or insufficient data (n = 2) represented the key exclusion criteria, resulting in a final study population of 105 patients, including 90 large B-cell lymphoma (LBCL) and 15 mantle cell lymphoma (MCL) patients (Supporting Information S1: Figure S1). LD chemotherapy (e.g., flu, cy) was administered before CAR-T according to the manufacturers' instructions.35, 36 Clinical metadata were collected with institutional review board approval (project number 19-817).
Data collection and definitions of IR
- (1)
CD4+ T helper (TH) cell count above >200/µL.
- (2)
B-cell recovery defined as any detectable B cells.
- (3)
IgG recovery defined as >4 g/L.29
- (1)
“sustained response” with ongoing complete remission or partial remission status,
- (2)
“early relapse” with relapse or death within the first year,
- (3)
“late relapse” with relapse or death after 1 year, and
- (4)
“refractory” patients with continuous stable disease or progressive disease (PD) status, or death.
CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria,8 with toxicity management following institutional guidelines.27, 38, 39 ICAHT was graded according to the European Hematology Association (EHA) and European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines.13, 40 The CAR-HEMATOTOX score was calculated as previously described.10, 12 Institutional policies regarding anti-infective prophylaxis, G-CSF use, and intravenous immunoglobulin (IVIG) administration are outlined in Supporting Information S1: Table S1. Detailed and comprehensive data regarding predominantly outpatient IVIG replacement therapy could not be retrieved.
Infection categorization and grading of infection severity
Infectious events were collected for the first 100 days and graded as mild, moderate, severe, life-threatening, or fatal as outlined in previous studies.27, 28 Infections were defined as bacterial, viral, or fungal on the basis of microbiologic/histopathologic data or as a clinical syndrome of infection (e.g., pneumonia, cellulitis, cystitis) based on retrospective chart review. All infections occurring before CAR-T infusion were excluded. Infection onset was specified as the first day on which the diagnostic test was performed and/or the onset of symptoms. The clinical source of infection was determined from the combination of symptomology, microbiologic isolates, and radiographic findings. Fever alone, in the absence of clinical signs of infection or microbiologic data, was not counted as an infection.
Statistical considerations
Cumulative incidence curves of recovery, infections, and NRM were calculated using the inverse Kaplan–Meier method, censoring for PD, next-line treatment, or death. In addition, competing risk analysis was performed as indicated using cause-specific hazard models (Kalbfleisch and Prentice method) or the Fine-Gray test. Kaplan–Meier estimates of progression-free (PFS) and overall survival (OS) were calculated from the time of CAR-T infusion. Prespecified landmark analyses were performed at 30 days, 90 days, and 180 days postinfusion; only subjects without progression or relapse at the landmark time were analyzed.
Statistical significance between groups was explored by the Mann–Whitney test for continuous variables and Fisher's exact test for comparison of percentages. For the Kaplan–Meier survival analysis, p values were determined using the log-rank method, and hazard ratios (HRs) were calculated using a univariate Cox proportional hazards model. Statistical analysis and data visualization were performed using GraphPad Prism (v9.0), SPSS (IBM, v28.0), and R Statistical Software (v4.1.2).
RESULTS
Overview of baseline features and toxicity profile of study cohort
The median age of the study cohort was 65 (range 19–85). Axi-cel represented the most commonly used CAR-T product (47.6%), followed by tisa-cel (31.4%), brexu-cel (13.3%), and liso-cel (7.6%). Overall, 22 patients (21%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater (Table 1). The median number of lines of therapy before CAR-T indication was 3 (IQR: 2–4), with 28 patients (27%) having received a prior autologous hematopoietic cell transplantation. A total of 44 patients (41.9%) were exposed to bendamustine, including 11 patients (10.5%) treated with bendamustine in the 9 months preceding CAR-T therapy.41 Holding therapy (i.e., between indication and apheresis) was applied in 45 patients (42.9%), with a median “brain-to-vein” time of 29 days.42 Bridging therapy (i.e., between apheresis and lymphodepletion) was used in 75 patients (71.4%), with a median “vein-to-vein” time of 38 days. At lymphodepletion, patients presented with median serum lactate dehydrogenase (LDH) levels of 260 U/L (IQR: 194.5–402 U/L) and a median CAR-HEMATOTOX score of 2 (IQR: 0–3).
| All patients (n = 105) | No recovery (n = 41) | Any recovery (n = 64) | p Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Ages, years (range) | 65 (19–85) | 60 (19–80) | 66 (25–85) | 0.02 |
| Sex (female), n (% of total) | 40 (38.1%) | 13 (31.7%) | 27 (42.2%) | 0.3 |
| PS | ||||
| Median ECOG at LD (IQR) | 1 (0–1) | 1 (0–2) | 1 (0–1) | 0.002 |
| ECOG 0–1, n (% of total) | 83 (79%) | 28 (68.3%) | 55 (85.9%) | |
| ECOG ≥ 2, n (% of total) | 22 (21%) | 13 (31.7%) | 9 (14.1%) | 0.048 |
| Therapy management | ||||
| Prior SCTa, n (%) | 28 (26.7%) | 11 (26.8%) | 17 (26.6%) | >0.9 |
| Median lines of therapy before bridging (IQR) | 3 (2–4) | 4 (2–4) | 3 (2–4) | 0.06 |
| Any bendamustine before CAR, n (% of total) | 44 (41.9%) | 16 (39%) | 28 (43.8%) | 0.7 |
| Bendamustine last 9 months before CAR, n (% of total) | 11 (10.5% | 6 (14.6%) | 5 (7.8%) | 0.3 |
| Bendamustine for bridging or LD, n (% of total) | 19 (18%) | 6 (14.6%) | 13 (20.3%) | 0.6 |
| Holding therapy, n (% of total) | 45 (42.9%) | 23 (56.1%) | 22 (34.4%) | 0.043 |
| Bridging therapy, n (% of total) | 75 (71.4%) | 32 (78.1%) | 43 (67.2%) | 0.3 |
| Pola-based bridging, n (% of total) | 27 (25.7%) | 8 (19.5%) | 19 (29.7%) | 0.3 |
| Immunochemotherapy-based bridging, n (% of total) | 68 (64.8%) | 30 (73.2%) | 38 (59.4%) | 0.2 |
| Brain-to-vein time, days (IQR) | 29 (20–48.5) | 35 (19–52.5) | 28 (20–41) | 0.1 |
| Vein-to-vein time, days (IQR) | 38 (33–46) | 35 (30–42.5) | 40 (34–47.8) | 0.01 |
| Laboratory parameters prior to LD | ||||
| LDH, U/L (IQR) | 260 (194.5–402) | 383 (206.5–545) | 229 (191.8–318.8) | 0.006 |
| GFR, mL/min (IQR) | 83 (67–97) | 88 (72–104.5) | 82 (65.3–121) | 0.07 |
| CRP, mg/dL (IQR) | 0.9 (0.2–3.6) | 3.1 (0.6–5.3) | 0.4 (0.13–1.4) | <0.0001 |
| Ferritin, ng/mL (IQR) | 455 (160–1294) | 778 (213–2164) | 316 (118.8–839.8) | 0.004 |
| ANC, cells/µL (IQR) | 2463 (1350–3600) | 2330 (1120–3460) | 2565 (1740–3820) | 0.09 |
| PLT, G/L (IQR) | 169 (108–220.5) | 146 (64.5–210.5) | 176 (133.3–225.3) | 0.046 |
| Hemoglobin, g/dL (IQR) | 9.9 (8.6–11.5) | 9.0 (7.8–10.9) | 10.3 (8.9–11.9) | 0.02 |
| CAR-HEMATOTOX score | ||||
| CAR-HEMATOTOX score absolute (IQR) | 2 (0–3) | 3 (1–4.5) | 1 (0–2) | 0.0003 |
| CAR-HEMATOTOX score low (0–1), n (% of total) | 49 (46.7%) | 12 (29.3%) | 37 (57.8%) | 0.005 |
| CAR-HEMATOTOX score high (>2), n (% of total) | 56 (53.3%) | 29 (70.7%) | 27 (42.2%) | 0.005 |
| Disease entity, n (% of total) | ||||
| Nontransformed lymphoma (DLBCL, PMBCL, THRLBCL) | 58 (55.2%) | 26 (63.4%) | 32 (50%) | 0.2 |
| Transformed lymphoma (trFL, trHL, trMZL, trCLL, trMALT) | 32 (30.5%) | 8 (19.5%) | 24 (27.5%) | 0.06 |
| MCL | 15 (14.3%) | 7 (17.1%) | 8 (12.5%) | 0.6 |
| Infused CAR T cell product | ||||
| CAR product, n (% of total) | ||||
| Axi-cel | 50 (47.6%) | 24 (58.5%) | 26 (40.6%) | 0.1 |
| Tisa-cel | 33 (31.4%) | 9 (22%) | 24 (37.5%) | 0.1 |
| Brexu-cel | 14 (13.5%) | 6 (14.6%) | 8 (12.5%) | 0.8 |
| Liso-cel | 8 (7.6%) | 2 (4.9%) | 6 (9.4%) | 0.5 |
| Co-stimulatory domain (ICD) of CAR-T product, n (% of total) | ||||
| CD28-based ICD | 64 (61%) | 30 (72.2%) | 34 (53.1%) | 0.044 |
| 41BB-based ICD | 41 (39%) | 11 (26.8%) | 30 (46.9%) | |
| Immunotoxicity | ||||
| CRS, n (% of total) | ||||
| No CRS | 15 (14.3%) | 6 (14.6%) | 9 (14.1%) | >0.9 |
| CRS grade 1–2 | 80 (76.2%) | 26 (63.4%) | 50 (78.1%) | 0.1 |
| CRS grade ≥3 | 10 (9.5%) | 5 (12.2%) | 5 (78%) | 0.5 |
| ICANS, n (% of total) | ||||
| No ICANS | 55 (52.4%) | 18 (43.9%) | 37 (57.8%) | 0.23 |
| ICANS grade 1–2 | 33 (31.4%) | 13 (31.7%) | 20 (31.3%) | >0.9 |
| ICANS grade ≥3 | 17 (16.2%) | 10 (24.4%) | 7 (10.9%) | 0.1 |
| Toxicity management, n (% of total) | ||||
| Received tocilizumab | 88 (83.8%) | 35 (85.4%) | 53 (82.8%) | 0.8 |
| Received dexamethasone | 47 (44.8%) | 21 (51.2%) | 26 (40.6%) | 0.3 |
| ICU admission necessary | 14 (13.3%) | 10 (24.4%) | 4 (6.3%) | 0.02 |
- Note: Patient baseline characteristics prior to CAR-T infusion of all patients (n = 105) and for patients with “no recovery” (n = 41) or “any recovery” (n = 64) after 3 months. All laboratory values were determined prior to lymphodepleting chemotherapy with a leniency time period of 3 days. If a measurement was not available for all patients, the denominator is indicated in the table. p Values < 0.05 are highlighted in bold.
- Abbreviations: ANC, absolute neutrophil count; CAR-T, chimeric antigen receptor T-cell; CI, confidence interval; CLL, chronic lymphocytic leukemia; CRP, C-reactive protein; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; GFR, glomerular filtration rate; HL, Hodgkin lymphoma; ICANS, immune effector cell-associated neurotoxicity syndrome; ICD, intracellular co-stimulatory domain; ICU, intensive care unit; IQR, interquartile range; LD, lymphodepletion; LDH, lactate dehydrogenase; MALT, mucosa-associated lymphoma tissue lymphoma; MCL, mantle cell lymphoma; PLT, platelet; PMBCL, primary mediastinal large B-cell lymphoma; PS, performance status; SCT, stem cell transplant; THRLBCL, T-cell/histiocyte-rich large B-cell lymphoma; tr, transformed.
In terms of the coincident toxicity profile, severe CRS and ICANS (ASTCT grade 3 or higher) were noted in 9.5% and 16.2% of patients, respectively (Table 1). In this study cohort, 88 patients (84%) received tocilizumab, 47 patients (45%) received high-dose glucocorticoids, and 14 patients (13%) required a transfer to the intensive care unit. The median duration of severe neutropenia (absolute neutrophil count [ANC] < 500/µL) within the first 60 days was 9 days (Supporting Information S1: Figure S2A). The most common neutrophil recovery phenotype10 was “intermittent” (57.3%), followed by “quick” (24.3%) and “aplastic” recovery (18.4%) (Supporting Information S1: Figure S2B). For two patients, neutrophil recovery was not assessed due to early death. According to the recently developed EHA/EBMT criteria13 for early ICAHT (Day 0–30), 68% and 25% of patients displayed mild-to-moderate (grade 1–2°) and severe-to-life-threatening (grade 3–4°) hematotoxicity, respectively (Supporting Information S1: Figure S2C). Regarding late ICAHT (after Day +30), 43% of patients displayed grade 1 or 2 hematotoxicity, while grade 3 or 4 late ICAHT was noted in 37% of patients. Five patients could not be graded for early ICAHT and 14 patients for late ICAHT due to early death or progression.
Temporal course of T- and B-cell recovery as well as immunoglobulin levels
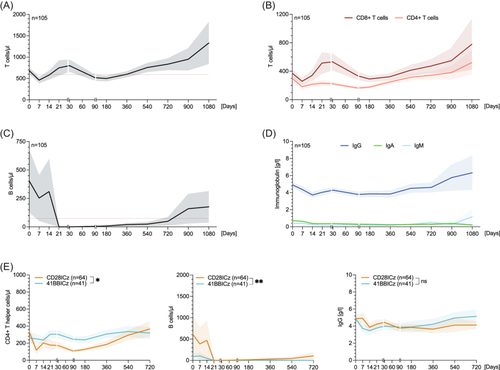
To identify characteristic CAR-T-associated IR patterns, we first analyzed the evolution of leukocytes (Supporting Information S1: Figure S3A), platelets (Supporting Information S1: Figure S3B), lymphocytes (Supporting Information S1: Figure S3C), T cells (Figure 1A), B cells (Figure 1B), natural killer (NK) cells (Supporting Information S1: Figure S3D), and immunoglobulin levels (Figure 1D, Supporting Information S1: S3E) over time. The respective numbers at risk for each time point are depicted in Supporting Information S1: Table S2. We confirmed the previously observed biphasic pattern of leukocyte recovery (Supporting Information S1: Figure S3A), with intermittent recovery followed by a second dip.43, 44 A similar biphasic reconstitution was noted for CD3+ T-lymphocytes, particularly the CD3+ CD8+ T-lymphocyte population (Figure 1A,B)—consistent with likely CAR T-cell expansion and contraction during the first month.45 We observed T-cell lymphopenia until approximately Day +180, which was followed by steady count recovery. The NK cell population initially decreased following lymphodepletion, but displayed early recovery to above baseline levels by Day +30 (Supporting Information S1: Figure S3D). A rapid decrease of B cells to undetectable levels was observed by Day 21, which was followed by a sustained period of BCA (Figure 1C). Above-normal B-cell counts (>70/µL) were detected on average by the third year following CD19 CAR-T (in the noncensored patients). Similarly, serum IgG and IgM levels increased after the second year following CAR-T, although serum IgA levels remained persistently low during the study period (Figure 1D, Supporting Information S1: S3E).
Influence of patient- and treatment-related factors on post-CAR-T IR
Next, we examined how disease histology, therapy lines (e.g., greater than 4 vs. 0–3 lines), tumor burden (LDH), co-stimulatory domain (i.e., CD28z vs. 41BBz), baseline CAR-HEMATOTOX score (e.g., high- vs. low-risk group), and CRS/ICANS severity (i.e., grade ≥2 vs. grade 0–1) impact the temporal courses of the different immune cell subsets and immunoglobulin level in prespecified subgroup analyses outlined in Supporting Information S1: Figures S4–S11. While TH cell counts and IgG levels were not significantly influenced by the underlying lymphoma entity, MCL patients showed higher initial CD19+ B-cell counts and only patients with transformed lymphoma displayed sustained B-cell recovery after 1 year (Supporting Information S1: Figure S4). Above median LDH levels at the time of lymphodepletion, a surrogate for tumor burden, did not significantly impact recovery kinetics (Supporting Information S1: Figure S5). Compared to CD28z co-stimulated CAR-T products (e.g., axi-cel or brexu-cel), patients receiving products harboring a 41BBz endodomain (e.g., tisa-cel or liso-cel) displayed less pronounced TH cell lymphopenia but also lower B-cell counts over time (Figure 1E, Supporting Information S1: Figure S8). Patients with grade 2 or higher CRS exhibited increased TH cell counts compared to patients with absent or grade 1 CRS (Supporting Information S1: Figure S9).
CAR-T products with a CD28z co-stimulatory domain exhibit delayed T-cell reconstitution but more rapid B-cell detection
To characterize IR according to clinically relevant thresholds, we next calculated the cumulative incidence of TH cell, B cell, and IgG recovery, censoring for next-line treatment, PD, and death (Figure 2). Across the entire patient cohort, the probability for recovery of at least one IR criteria (TH cells, B cells, or IgG) was 89.3% after 12 months in surviving patients without relapse (Figure 2A). For TH cell, B cell, and IgG recovery, the cumulative incidence of recovery at one year was 55.4%, 17.5%, and 52.7%, respectively (Figure 2B–D). Of interest, we observed delayed TH cell recovery in the patients treated with CD28z-based compared to 41BBz-based CAR-T products (estimated 12-month recovery for CD28z vs. 41BBz: 32.9% vs. 82.7%, p < 0.0001; Figure 2E). On the other hand, CD28z-based CAR-T products were associated with more rapid B-cell detection (estimated 12-month recovery for CD28z vs. 41BBz: 32.2% vs. 5.9%, p = 0.0014; Figure 2F). IgG recovery did not differ by the applied CAR-T product (Figure 2G). Similar differences in IR recovery by co-stimulatory domain were obtained when accounting for relapse and NRM within a competing risk framework (Supporting Information S1: Figure S12; p values from Fine-Gray and Kalbfleisch tests in Figure 2E–G).
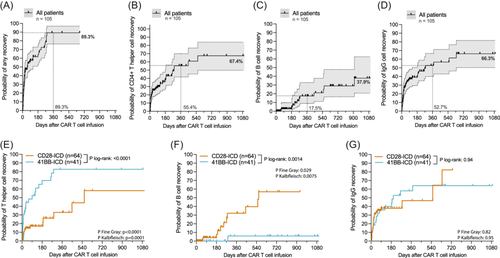
Only a minority of CAR T-cell patients show reconstitution of all three lineages
When evaluating the total study denominator (n = 105), 40 patients (38.1%) displayed TH cell recovery, 12 patients (11.4%) showed any B-cell recovery, and 43 patients (41%) had IgG recovery at any timepoint (Figure 3A,B). For patients fulfilling the respective recovery criteria, the median time to TH cell and B-cell recovery was 0.7 and 8.5 months, respectively (Figure 3C). While 39.1% of patients never displayed reconstitution of any lineage, 37.1% recovered at least one IR criterium, 18.1% recovered more than two criteria, and only 5.7% recovered all three criteria (Figure 3D). Patients refractory to CD19 CAR-T were more likely to never achieve recovery compared to patients with any objective response (% without recovery: 72.4% vs. 26.3%, p = 0.0002, Figure 3E). Similar observations were noted at the 3-month PET-based imaging assessment (% without recovery for non-responders vs. responders: 66.7% vs. 15.8%, p < 0.0001; Figure 3E). When analyzing IR by the duration of response, we found that patients with refractory disease had the highest proportion of absent recovery (dark red, 72.4%), followed by patients with early relapse (50.0%) and late relapse (23.1%), while the sustained responders displayed the lowest proportion of absent recovery (8.6%). The opposite trend was noted for recovery of all three IR criteria, which was more commonly observed in sustained responders (0% vs. 3.6% vs. 9.1% vs. 10.8%, p = 0.03; dark green Figure 3D). Of note, the absence of IR was significantly associated with aplastic neutrophil recovery (Figure 3F) and coincident severe hematological toxicity (Figure 3G,H).
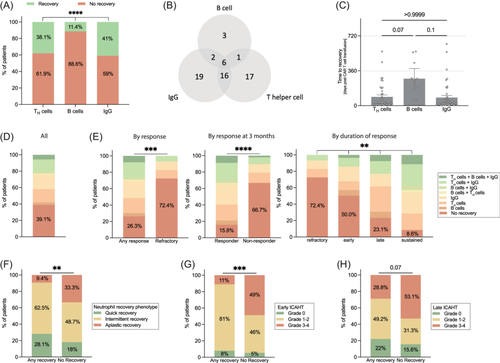
Impaired IR associates with infectious complications and infection-driven NRM
Across the entire study cohort, 57 patients (54%) displayed an infection event while 47 patients (45%) developed a bacterial infection within the first 100 days (Figure 4A). Most of these infections were of bacterial origin followed by viral and fungal infections (Supporting Information S1: Figure S13). Severe infections and severe bacterial infections were noted in 39% and 29% of patients, respectively. When censoring for progression and death, the cumulative 100-day incidence of any-grade and severe infections was 55.9% and 40.1%, respectively (Figure 4B,C). We noted significantly higher rates of infections, including bacterial infections, in the patients with absent recovery of any IR criteria (Figure 4D). Accordingly, these patients had a significantly higher cumulative incidence of any infections (74.4% vs. 45.8%, p = 0.04, Figure 4E, left) and severe infections (59.2% vs. 28.7%, p = 0.004, Figure 4E, right). Cause-of-death analysis revealed that infections were the main driver of NRM (Figure 4F). The overall 1-year NRM rate was 7.5% and was significantly elevated in the patients who never displayed immune recovery (23.5% vs. 0%, log-rank p = 0.004, Figure 4G).
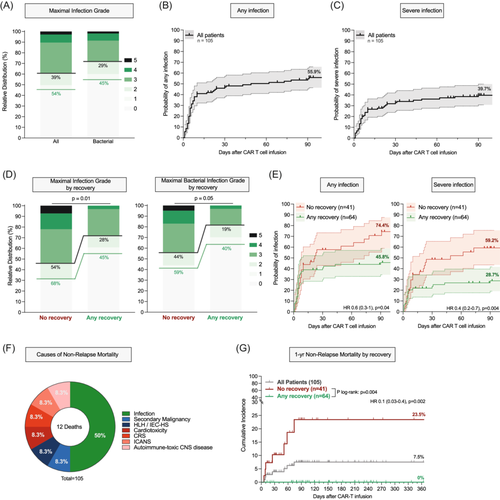
Impaired IR associates with poor treatment outcomes
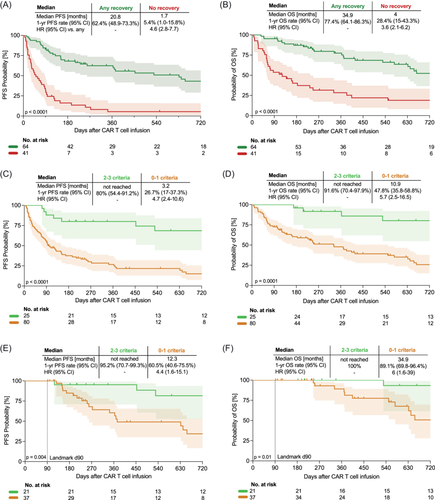
After a median follow-up of 24.6 months, the median PFS was 5.0 months and the median OS was 17.4 months across all patients (Supporting Information S1: Figure S14A,B). We found that CAR T-cell patients who did not recover any IR criteria experienced markedly inferior PFS compared to patients with any recovery (mPFS: 1.7 vs. 20.8 months, estimated 1-year PFS 5.4% vs. 62.4%, log-rank p < 0.0001; Figure 5A). Furthermore, patients with absent recovery also exhibited worse OS (mOS 4.0 vs. 34.9 months, estimated 1-year OS 28.4% vs. 77.4%, log-rank p < 0.0001; Figure 5B). Concomitantly, “no recovery” markedly increased the risk of inferior survival on univariate Cox regression analysis (HRPFS = 4.6, 95% confidence interval [CI]: 2.8–7.7; HROS = 3.6, 95% CI: 2.1–6.2). Increasing fulfillment of IR criteria (e.g., recovery of 3 vs. 2 vs. 1 vs. none) was associated with improved PFS (1-year PFS: 100% vs. 73.7% vs. 50.5% vs. 5.4%; Supporting Information S1: Figure S15A) and improved OS (1-year OS: 100% vs. 88.8% vs. 68% vs. 28.4%; Supporting Information S1: Figure S15B). Consequently, patients with (poor) recovery of 0–1 IR criteria showed markedly worse clinical outcomes compared to patients with (good) recovery of 2–3 IR criteria (mPFS: 3.2 months vs. not-reached, mOS 10.9 months vs. not-reached; Figure 5C,D).
Patients with impaired IR display poor-risk baseline features
To assess if IR-related survival differences reflect variations in key baseline prognostic factors, we next studied baseline characteristics according to the “no recovery” versus “any recovery” groups (Table 1, right). Overall, the CAR-T recipients with absent recovery had poorer ECOG performance status (≥2: 32% vs. 14%, p < 0.05) and more frequently received holding therapy (56% vs. 34%, p < 0.05). In terms of baseline laboratory parameters, we found higher serum LDH levels (383 vs. 229 U/L, p = 0.006) and marked elevations of serum inflammatory markers such as CRP (3.1 vs. 0.4 mg/dL, p < 0.0001) and ferritin (778 vs. 316 ng/mL, p = 0.004) in the “no recovery” group (Table 1). Furthermore, absent recovery was associated with baseline cytopenias, including lower platelet counts (146 vs. 176 G/L, p < 0.05) and lower hemoglobin (9.0 vs. 10.3 g/dL, p = 0.02). Accordingly, we noted both higher absolute CAR-HEMATOTOX scores (median 3 vs. 1, p < 0.0001) and a higher proportion of high-risk patients (score ≥2: 73% vs. 27%, p = 0.004) in the “no recovery” group.10 After CAR T-cell infusion, the “no recovery” group also demonstrated numerically higher rates of severe ICANS (24.4% vs. 10.0%, p = 0.1) and significantly increased intensive care unit admissions (24.4% vs. 6.3%, p = 0.02). The observed differences in baseline prognostic features were similar to the changes stratified by the response assessment at 3 and 6 months (Tables S3 and S4).
Patients progressing after CD19 CAR-T exhibit profound immune deficits
Because of the observed interdependence between the “no recovery” group and 3-month non-responders, we applied landmark analyses to ascertain the prognostic role of IR in lymphoma patients without immediate relapse or primary progression.46 The median PFS was 14.9 months, 26.8 months, and not reached at the Day 30, Day 90, and Day 180 landmarks, respectively (Supporting Information S1: Figure S14C,E,G). The median OS was 34.9 months at the Day 30 landmark and was not reached at the Day 90 and Day 180 landmarks (Figure S14D,F,H). Considering the rate of subsequent progression was still high at the Day 30 landmark, we examined the impact of IR on clinical outcomes in more detail for Day 90 (Figure 4E,F, Supporting Information S1: S15C–F) and Day 180 landmarks (Supporting Information S1: Figure S16). We confirmed inferior PFS in the patients with “no recovery” in comparison to patients with “any recovery” at the Day 90 landmark (mPFS: 8.6 vs. 34.9 months, estimated 1-year PFS 25% vs. 83.7%, log-rank p = 0.005; Supporting Information S1: Figure S15C) and Day 180 landmark (mPFS: 11.3 vs. not-reached, estimated 1-year PFS 40% vs. 94.1%, log-rank p = 0.06; Supporting Information S1: Figure S16A). However, we did not detect a significant difference in OS between the groups at both landmark timepoints (Supporting Information S1: Figures S15 and S16). Survival differences were more pronounced when comparing CAR-T recipients with (poor) recovery of 0–1 IR criteria to patients with (good) recovery of 2–3 IR criteria (mPFS: 12.3 months vs. not-reached, mOS 34.9 months vs. not-reached, Figure 5E,F). Overall, we observed minimal differences in baseline characteristics between CAR-T recipents with and without recovery at the later landmark times (Tables S5 and S6).
Prognostic role of BCA and NK cell recovery in lymphoma patients
In the B-NHL patients responding at 3 months, loss of BCA (i.e., B-cell recovery) was more prevalent with CD28z-based CAR-T products (Supporting Information S1: Table S7), but was not associated with inferior survival outcomes (Supporting Information S1: Figure S17). Of interest, patients who went on to have any detectable B-cell counts exhibited a decreased probability of early infections compared to the patients with sustained BCA (Supporting Information S1: Figure S18).
When studying NK cell recovery patterns, we observed the nadir at 7 days post-CAR-T infusion with the lowest NK cell counts being 20 NK cells per µL (Supporting Information S1: Figure S19). We noted a trend toward both an earlier and deeper NK cell nadir in the CAR-T recipients responding at the 3-month follow-up (Supporting Information S1: Figure S19D,E).
IR is impaired at the time of progression or relapse after CAR-T therapy
Analysis of laboratory parameters and immune cell counts revealed significant disruptions of cellular and humoral immunity in the 72 patients who progressed following CD19 CAR-T (Table 2). For example, cytopenias at the time of progression or relapse were common, with a median ANC of 1.7 G/L, platelet count of 73 G/L, and hemoglobin of 9.4 g/dL. The overwhelming majority of patients had no detectable B cells and showed marked T-cell lymphopenia (median: 332 CD3+ T cells/µL), particularly TH cell aplasia (median: 105 CD3+ CD4+ T cells/µL). We found that patients with early progression (within 90 days) exhibited diminished immune cell counts at the time of progression or relapse when compared to patients with late progression (Table 2). For example, early progressors had a significantly decreased platelet count (56 vs. 150 G/L, p = 0.003), decreased T cells (236 vs. 500 cells/µL, p = 0.03), and reduced NK cells (76.5 vs. 158 cells/µL, p = 0.002) compared to the patients with later relapse. While the co-stimulatory domain had no significant impact on immune cell counts at the time of progression (Supporting Information S1: Table S8), MCL patients had higher postprogression lymphocyte, T-cell, B-cell, and NK cell counts compared to LBCL patients (Supporting Information S1: Table S9).
| All patients (n = 72) | Early progression <90 days (n = 48) | Late progression >90 days (n = 24) | p Value | |
|---|---|---|---|---|
| Laboratory parameters at progression | ||||
| White blood cell count, G/L (IQR) | 3.6 (1.5–6.2) | 2.8 (1.3–5.8) | 4.5 (3.1–6.4) | 0.075 |
| Platelet count, G/L (IQR) | 73 (29–157) | 56 (15–115) | 150 (57-194) | 0.003 |
| Absolute neutrophil count, G/L (IQR) | 1.7 (0.8–3.7) | 1.1 (0.4–3.5) | 2.2 (0.9–4.2) | 0.12 |
| Hemoglobin, g/dL (IQR) | 9.4 (7.8–11.5) | 8.8 (8–10) | 11.5 (9.3–14.3) | 0.0003 |
| LDH, U/L (IQR) | 293 (217–486) | 349.5 (248–708) | 224 (185–333) | 0.002 |
| Lymphocyte subpopulations at progression | ||||
| Lymphocytes, cells/µL (IQR) | 480 (260–968) | 435 (179–829) | 712 (393–1201) | 0.05 |
| T cells, cells/μL (IQR) | 332 (124–613) | 236 (96–528) | 500 (235–825) | 0.03 |
| T helper cells, cells/µL (IQR) | 105 (50–220) | 83 (33–213) | 150 (88–239) | 0.07 |
| Cytotoxic T cells, cells/µL (IQR) | 135 (58–422) | 102 (49–280) | 278 (62–578) | 0.06 |
| CD4/CD8 ratio (IQR) | 0.8 (0.3–1.6) | 0.9 (0.3–1.6) | 0.6 (0.4–1.3) | 0.6 |
| B cells, cells/μL (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–9) | 0.5 |
| NK cells, cells/µL (IQR) | 116 (52–194) | 76.5 (17–149) | 158 (126–201) | 0.002 |
- Note: Laboratory parameters and immune cell counts at the time point of progression or relapse for all patients and comparing the patients with early progression (within 90 days) and late progression (after Day 90). p values determined by the Mann–Whitney test. p values < 0.05 are highlighted in bold.
- Abbrtevaitions: IQR, interquartile range; LDH, lactate dehydrogenase A; NK, natural killer.
DISCUSSION
In this retrospective observational study, we demonstrate that CD19 CAR-T confers profound and long-lasting immune deficits. We show that TH cell aplasia is more common with CD28z CAR-T products, while sustained BCA represents a hallmark of 41BBz CAR-T products. Notably, IR was associated with lower rates of infection, decreased NRM, and improved long-term survival in this lymphoma patient cohort. Finally, we highlight that patients progressing after CD19 CAR-T display significant disruptions of T-cell and B-cell immunity.
In our cohort, only 11.4% of all patients exhibited B-cell recovery while TH cell and IgG recovery was noted in around 40% of patients with a follow-up of more than 2 years. The observed rate of B-cell recovery was lower than in previous studies from Logue (58% at 1 year)20 and Baird et al. (40% at 1 year),19 which most likely reflects their exclusive reporting of axi-cel-treated patients. In terms of CD4+ TH cell recovery, our findings were similar to previous reports that ranged between 40 to 67% at 1 year.19, 20, 47, 48 We also provide a comprehensive overview of NK cell reconstitution over time, demonstrating that the expected nadir is mainly restricted to the first month after CAR T-cell infusion.
While IR patterns have been closely linked to short- and long-term infectious complications of CAR-T,28-30 their prognostic impact in lymphoma patients remains enigmatic. In a cohort of 41 r/r LBCL patients, Baird and colleagues found that durable responses to axi-cel were associated with extended BCA, and this duration correlated strongly with poor recovery of CD4+ T-cell counts.19 Yet other studies indicated inverse correlations regarding NK cell49 or T-cell recovery.50 The regular assessment of peripheral blood B cells has been suggested to be an effective proxy for ongoing CAR-T activity.48 In r/r B-ALL, it has been suggested that loss of BCA is associated with relapse, especially for patients with low tumor burden compared to patients with high tumor burden.51 Despite the same target antigen (CD19), our results question this general principle in B-NHL since long-term treatment responses were noted even in the absence of persistent immune deficits. Indeed, this could indicate a “sweet spot” wherein a subset of lymphoma patients receiving CD19 CAR-T are able to reconstitute endogenous immunity in a disease-free state. This may be especially prevalent with CD28z co-stimulated products characterized by faster and higher CAR T-cell proliferation but shorter persistence.34, 52
The underlying causes of protracted immune deficits in lymphoma patients receiving CD19 CAR-T can broadly be separated into disease-, product-, and host-related features. High disease burden prior to therapy initiation often results in the application of holding and bridging therapies.42 For example, patients receiving ≥2 cycles of bridging therapy have a higher risk of infections and diminished OS compared to patients receiving only 1 cycle.53 The type and response to bridging therapy have also been linked to treatment responses,54, 55 and may shape the extent of immune suppression following CAR-T. Next to the use of bridging therapies, prior exposure to T-cell-depleting chemotherapeutics like bendamustine has been shown to be prognostic in the context of CAR-T,41 although we did not find bendamustine-exposed patients to have impaired IR in this study.
While the intensity of bridging therapy can vary, all patients receive LD chemotherapy prior to CAR T-cell therapy, with the commercially available products typically relying on flu and cy.18 Our study represents one of the first to explore IR patterns in a product- and disease-specific manner. We noted distinct IR features for CD28z (deeper TH cell aplasia) versus 41BBz (deeper BCA) CAR-T products. The increased dose of flu/cy lymphodepletion provides a likely explanation for the more extensive T-cell lymphopenia in patients receiving CD28z co-stimulated products like axi-cel. Furthermore, the depth of lymphodepletion shapes the immune cell state at the time of post-CAR-T progression. As a result, patients with early progression or relapse had significantly altered endogenous immune cells compared to patients with late relapse (e.g., lower blood counts, extensive T-cell lymphopenia). One may speculate that this could impact subsequent treatment lines and drive differential efficacy of T-cell-engaging therapies like Glofitamab or Epcoritamab depending on the timing of relapse (e.g., early vs. late).
Host factors such as body composition and renal function may additionally impact lymphodepletion depth and subsequent immune recovery.39, 56 In our study, we found several host-related factors, such as inflammatory markers, blood counts, and certain risk prognostication scores, to be altered in patients who never showed any recovery of IR criteria (Table 1, right). However, many of these same features were noted to be increased in CAR-T non-responders as well, suggesting that there is a subgroup of CD19 CAR-T recipients who are not only at risk for poor treatment outcomes but also carry an inherent risk of severe immunotoxicity and poor IR.43 It should also be noted that we observed strong associations between altered IR and severe hematologic toxicity, including increased severity of early ICAHT and aplastic neutrophil recovery.13
Such a combination of cellular (e.g., low neutrophil counts, T-cell lymphopenia) and humoral immune deficits (e.g., BCA, hypogammaglobulinema) provides a critical context for the high rate of infectious complications observed in CAR-T recipients.29, 30 Indeed, fatal infections are by far the main driver of NRM following CAR-T26 and we could confirm both the higher risk of early infections and NRM in patients with absent IR. Early infections implicate both longer and broader antibiotic exposure, which may shape subsequent lymphocyte recovery—as has been suggested in the context of murine bone marrow transplantation models.57-59 Remarkably, NRM only occurred in patients with absent IR recovery (23.5%) whereas patients with any IR recovery had no NRM events (0%). Lack of IR could therefore represent a key stratification tool for additional monitoring and interventions that aim to prevent or mitigate infection-related NRM. Concomitantly, robust infection prevention guidelines are an important component of CAR T-cell delivery. Furthermore, the long-term nature of post-CAR-T immune deficits underscores that infection prevention and management does not end when the patient is discharged from the hospital. Rather, providers must remain vigilant and aware of potential opportunistic infections even months to years after the infusion of CAR T-cells. The close interaction with infectious disease specialists, well-designed vaccination protocols, and the liberal indication of Immunoglobulin replacement therapy may represent effective strategies to mitigate the serious risk of infections in CAR T-cell patients, and are being actively explored in prospective clinical trials (NCT: 05952804).29, 60 At the same time, it should be noted that the low rates of B-cell recovery reported in this study may portend poor serologic responses to vaccines following B-cell targeting CAR T-cell therapy.61-63
A further clinical implication of protracted immune deficits following CAR-T therapies lies in the potential of dysregulated immune surveillance, which may propagate the emergence of second primary malignancies (SPMs). This subject garnered significant attention after the United States Food and Drug Administration issued a class-wide black box warning for the development of SPM after CAR T-cell therapy.64, 65 While SPM risk is likely multifactorial and many CAR-T patients will have received multiple prior protumorigenic therapies,66 the contributing role of prolonged immunosuppression for SPM development should be explored further. Of interest, pharmacovigilance studies have pointed toward a disproportional increase in the reporting of SPM cases (especially myeloid neoplasms) with CAR-T products that harbor the 41BBz co-stimulatory domain such as tisa-cel and cilta-cel.67, 68
Key limitations of this study include its retrospective nature and monocentric design. While this represents one of the largest real-world studies of post-CAR-T IR, our cohort is still of moderate size and the results need to be confirmed in larger (and ideally prospective) data sets. Such studies may further elucidate the prognostic role of IR by accounting for key confounders using propensity score matching or other statistical methods.69, 70 Unfortunately, detailed and comprehensive data regarding outpatient IVIG supplementation were unavailable for this cohort, which could partly explain increased IgG recovery relative to B-cell recovery. The definition of B-cell recovery as any detectable B cells may have overemphasized the differences in BCA between the costimulatory targets. Moreover, while we examined the kinetics of different endogenous T-cell populations, we were not able to further delineate CAR T-cell expansion and persistence (reported in a subset of patients previously).45 Additionally, only a small proportion of patients displayed long-term follow-up extending beyond 3 years. While we accounted for progression events by censoring, many of the early progressors would simply not have had time to recover their counts. We attempted to address this by performing several landmark analyses, studying patients in sustained remission at 3 and 6 months, and finding largely stable results.
Despite these limitations, we see several salient implications for clinical practice. First and foremost, these descriptive data provide a benchmark regarding the expected immune recovery of B-NHL patients receiving CD19 CAR T-cells. Clinicians treating CAR-T patients should be aware that both humoral and cellular immune deficits can be long-lasting in nature and monitor patients accordingly. Interestingly, we find that deficits in IR are associated with inferior treatment outcomes. It remains to be studied if moving CAR-T therapies to earlier treatment lines or non-malignant conditions facilitates less profound immune deficits. In autoimmune diseases like systemic lupus erythematosus, the B-cell load or target antigen density is expected to be lower (as these patients have no malignant B cells and are commonly Rituximab-exposed), and as a result, the immune deficits may not be as long-lasting.71 Finally, the observation of diminished immune recovery and T-cell lymphopenia at the time of progression or early relapse following CAR-T raises significant questions regarding the sequencing and efficacy of bispecific antibody therapies if they are applied within the first 2 months after CAR T-cell infusion. These concerns are supported by a report from Iacoboni and colleagues showing differential efficacy of CD20-targeting bispecifics depending on relapse within 2 months (low response rates) versus ≥2 months (higher response rates).72
In conclusion, we demonstrate that IR patterns are associated with early infections, infection-driven NRM, and survival in lymphoma patients. The finding that IR is impaired at the time of progression after CAR T-cell therapy, particularly in earlier compared to late relapses, carries important implications for the sequencing of immunotherapies and the ultimate success of subsequent T-cell-engaging therapies following relapse.
ACKNOWLEDGMENTS
Sophia Stock, Veit L. Bücklein, Viktoria Blumenberg, and Kai Rejeski were supported by the Else Kröner-Fresenius Clinician Scientist Program Cancer Immunotherapy and IOLIN, Munich Clinician Scientist Program (MCSP), and DKTK School of Oncology. Sophia Stock was further supported by the Förderprogramm für Forschung und Lehre (FöFoLe) and the Momente Mentoring Program of the Medical Faculty of the LMU Munich. Viktoria Blumenberg and David M. Cordas dos Santos received the Walter Benjamin Fellowship from DFG. Kai Rejeski was supported by the EHA-ASH Translational Research Training in Hematology (TRTH) program. Open Access funding enabled and organized by Projekt DEAL.
AUTHOR CONTRIBUTIONS
Sophia Stock, Kai Rejeski, Marion Subklewe involved in conceptualization. Sophia Stock, Veit L. Bücklein, Viktoria Blumenberg, Giulia Magno, Alica-Joana Emhardt, Alessandra M. E. Holzem, Stefanie Grießhammer, Lisa Frölich, Kai Rejeski, Marion Subklewe involved in investigation. Sophia Stock involved in formal analysis and visualization. Sophia Stock, Kai Rejeski, Marion Subklewe involved in methodology. Sophia Stock, Kai Rejeski, Marion Subklewe involved in writing original draft. Sophia Stock, Veit L. Bücklein, Viktoria Blumenberg, Giulia Magno, Sebastian Kobold, Michael von Bergwelt-Baildon, Kai Rejeski, Marion Subklewe involved in writing review and editing. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
Veit L. Bücklein—AMGEN: Honoraria; Celgene: research funding; Pfizer: Honoraria; Kite/Gilead: research funding, Honoraria; Novartis: Honoraria. consultancy/advisory, BMS: Consultancy/advisory, Takeda: consultancy/advisory. Viktoria Blumenberg—BMS/Celgene: research funding; Kite/Gilead: consultancy, Honoraria, research funding; Janssen: research funding, Honoraria; Novartis: research funding, Honoraria; Roche: consultancy, research funding; Takeda: research funding. Sebastian Kobold—received honoraria from TCR2 Inc., Cymab, Miltenyi, Galapagos, Novartis, BMS, and GSK, is an inventor of several patents in the field of immuno-oncology, received license fees from TCR2 Inc. and Carina Biotech, received research support from TCR2 Inc., Tabby Therapeutics, Catalym GmBH, Plectonic GmBH, and Arcus Bioscience for work unrelated to the manuscript. Michael von Bergwelt-Baildon—consultancy, research funding, and Honoraria: MSD Sharp & Dohme, Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Astellas, Mologen, and Miltenyi. Kai Rejeski—Kite/Gilead: research funding, advisory, honoraria, and travel support; Novartis: Honoraria; BMS/Celgene: consultancy, Honoraria; Pierre-Fabre: Travel Support. Marion Subklewe—receives industry research support from Amgen, BMS/Celgene, Gilead, Janssen, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, and Takeda and serves as a consultant/advisor to AvenCell, CDR-Life, Ichnos Sciences, Incyte Biosciences, Janssen, Miltenyi Biotec, Molecular Partners, Novartis, Pfizer, and Takeda. She serves on the speakers' bureau at Amgen, AstraZeneca, BMS/Celgene, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, and Takeda. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Clinical metadata were collected with institutional review board approval (LMU Munich: Project Nr. 19-817) and the study was performed in accordance with the Declaration of Helsinki.
FUNDING
Sophia Stock declares research funding from the Else Kröner-Fresenius-Stiftung, DKTK School of Oncology, Novartis InCa Förderpreis 2022 for young researchers, Förderprogramm für Forschung und Lehre (FöFoLe) of the Medical Faculty of the LMU Munich (grant number 1168), and SFB-TRR 338 (start-up funding). This work was further supported by a grant within the Gilead Research Scholar Program (to Kai Rejeski and Marion Subklewe) and Bruno and Helene Jöster Foundation (to Kai Rejeski, Sebastian Kobold, and Marion Subklewe). This work was also supported by a Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) research grant provided within the Sonderforschungbereich SFB-TRR 338/1 2021—452881907 (to Sebastian Kobold and Marion Subklewe), and DFG research grant 451580403 (to Marion Subklewe). The work was further supported by the Bavarian Elite Graduate Training Network (to Sebastian Kobold and Marion Subklewe), the Wilhelm-Sander Stiftung (to Marion Subklewe, project no. 2018.087.1), the Else-Kröner-Fresenius Stiftung (to Marion Subklewe and Sebastian Kobold), and the “CAR-T Control” translational group within the Bavarian Center for Cancer Research (BZKF) (to Kai Rejeski and Marion Subklewe, BZKF-#TLG-22).
Open Research
DATA AVAILABILITY STATEMENT
All data needed to evaluate the conclusions in the paper are present in the manuscript and/or the Supporting Information Materials. Original data are available upon reasonable request to the corresponding authors.