Inflammatory pathways and anti-inflammatory therapies in sickle cell disease
Abstract
Sickle cell disease (SCD) is a monogenic disease, resulting from a single-point mutation, that presents a complex pathophysiology and high clinical heterogeneity. Inflammation stands as a prominent characteristic of SCD. Over the past few decades, the role of different cells and molecules in the regulation of the inflammatory process has been elucidated. In conjunction with the polymerization of hemoglobin S (HbS), intravascular hemolysis, which releases free heme, HbS, and hemoglobin-related damage-associated molecular patterns, initiates multiple inflammatory pathways that are not yet fully comprehended. These complex phenomena lead to a vicious cycle that perpetuates vaso-occlusion, hemolysis, and inflammation. To date, few inflammatory biomarkers can predict disease complications; conversely, there is a plethora of therapies that reduce inflammation in SCD, although clinical outcomes vary widely. Importantly, whether the clinical heterogeneity and complications are related to the degree of inflammation is not known. This review aims to further our understanding of the roles of main immune cells, and other inflammatory factors, as potential prognostic biomarkers for predicting clinical outcomes or identifying novel treatments for SCD.
INTRODUCTION
Sickle cell disease (SCD) is caused by a single nucleotide polymorphism (SNP) in the first exon of the beta-globin gene.1 This SNP promotes a glutamic acid to valine change in the protein chain, and this variant of beta-globin binds to an alpha-globin to form hemoglobin S (HbS).1, 2 HbS, when deoxygenated, polymerizes into the red blood cell (RBC), giving it the characteristic sickle shape. Polymerization of HbS leads to a cascade of events that include hemolysis, activation of inflammation in several ways, and vaso-occlusion, the latter being the hallmark of the disease.3, 4
Patients with SCD face several acute and chronic clinical complications, some of them life-threatening, leading to high morbidity and earlier mortality compared with the general population1. Interestingly, despite being a monogenic disease, SCD is characterized by a high phenotypic heterogeneity that might be, in part, driven by in vivo inflammatory interactions not fully understood. Patients with SCD exhibit high levels of inflammatory markers, such as C-reactive protein (CRP) and an increased number of leukocytes. These elevated leukocyte counts are associated with a higher risk of adverse outcomes.4
Hemolysis is closely associated with the RBC membrane damage caused by HbS multipolymers,3 reduction of RBC deformation capacity, changes in the influx of calcium and potassium through the Gardos channel, and increased expression of adhesion molecules on their membrane. Approximately 30% of the hemolysis in SCD is intravascular.5 Free heme and free HbS released in the bloodstream lead to degradation and impaired synthesis of nitric oxide (NO),3 contributing to inflammatory and endothelial activation.6, 7 Inflammation activates leukocytes which, in turn, can adhere to the endothelium and trap other cells, forming heterocellular aggregates that cause vaso-occlusion,8 a reversible phenomenon that results in ischemia-reperfusion injury (I/RI) that releases reactive oxygen species (ROS). ROS induces vascular inflammation and endothelial activation via the activation of redox-sensitive transcription factors, such as NF-κB, leading to a vicious cycle that perpetuates vaso-occlusion and inflammation. Therefore, hemolysis, inflammation, endothelial activation, and vaso-occlusion are closely associated.
The growing evidence of the pivotal role of inflammation in SCD has contributed to improving the understanding of the pathophysiology of the disease and bridging vaso-occlusion and hemolysis.5 However, inflammatory mechanisms and pathways still need to be better understood. Currently, there are limited inflammatory biomarkers and targets that help predict SCD complications.9 Although some clinical trials already use drugs that target inflammatory pathways, the efficacy of most drugs is lower than expected.10-12 The analysis of the therapeutic efficiency of these targets poses another challenge since key outcomes, such as pain, are subjective and assessed by self-reported scales due to the lack of a biological measure. Thus, unveiling new biomarkers and biological measures are fundamental to improve the management of SCD.
Therefore, the aim of this review is to discuss the role of molecules, cells, and inflammatory targets relevant to SCD, as well as to discuss some of the therapies that reduce inflammation in these settings.
Genesis of SCD inflammation
Hemolysis, which is caused by the destabilization of the RBC membrane, is a hallmark of SCD.9 Although intravascular hemolysis corresponds to a fraction of total hemolysis in SCD,5 the release of heme and other RBC products that act as damage-associated molecular patterns (DAMPs)13, 14 activate the Toll-like receptor (TLR)4 inflammatory pathway, leading to the secretion of tumor necrosis factor (TNF)-alpha and to neutrophil and monocyte activation,6 which will be further discussed. Heme and DAMPs also activate endothelial cells through the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome pathway and lead to the production of IL-1Beta.15 When free hemoglobin is released into the vessels, heme interacts with NO and binds to oxygen to convert ferrous iron into ferric iron; as a result, NO loses oxygen and turns into an inactivated molecule, impairing vasodilation.16 In addition, sickle RBC also releases arginase, which hydrolyzes l-arginine, preventing NO production.16 Therefore, intravascular hemolysis causes vasoconstriction, which contributes to vaso-occlusion in SCD settings. Hemolysis has been associated with several SCD complications, namely priapism, leg ulcers, pulmonary hypertension, and ischemic stroke,1, 3 and it is hypothesized that the aforementioned NO reduction plays a role in such complications.
Vaso-occlusion is a very complex phenomenon, mainly triggered by HbS and driven by the interaction between the sickle RBC, other cells, and vessels. Polymerization of HbS causes higher expression of adhesion molecules, such as CD36, CD47, phosphatidylserine, integrin alpha4beta1, SO4 glycolipid, and Lu/B-CAM on the membrane of RBC17; these cells bind to the endothelium directly through vascular-cell adhesion molecule 1 (VCAM-1), P-selectin, or interacting with proteins such as thrombospondin, laminin and endothelial Lutheran blood group, and basal cell adhesion molecule Lu/BCAM.17, 18 To unbind sickle RBC from the endothelium, a 20-fold higher shear stress is required compared with normal RBC.19 Once they bind to the endothelium, sickle RBCs trap other RBCs, leukocytes, and platelets, and this heterocellular aggregate promotes vaso-occlusion in small vessels.8 In murine models, Turhan et al. showed that sickle RBC interacted more with adherent leukocytes than with the endothelium, and the number of rolling and adherent leukocytes is higher in SCD mice than in controls.8 Vaso-occlusion is reversible, leading to I/RI). I/RI alters ATP metabolism and calcium ion efflux in the cell, determining the production of ROS such as superoxide and hydrogen peroxide. The interaction between hemolysis, vaso-occlusion, inflammation, and I/RI is complex and is summarized in Figure 1.
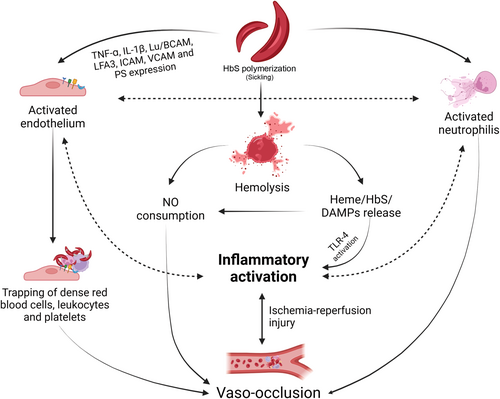
Neutrophils, vaso-occlusion, and inflammation
Leukocytosis, in particular neutrophilia, has been associated with SCD complications, including higher severity scores and a higher risk of death; acute chest syndrome; and hemorrhagic stroke.4, 20-22 Use of GCSF in patients with SCD has led to fatal events because of the acute rise of neutrophil counts,23 so that patients with SCD that undergo stem cell mobilization for gene therapy use plerixafor.24
Leukocyte rolling and adhesion are likely some of the underlying mechanisms that contribute to maintain the inflammatory milieu and promote vaso-occlusion, leading to adverse outcomes. In murine models, leukocyte rolling, adherent leukocytes and interaction between leukocytes and RBC occur more in SS mice than in controls8; adherent leukocytes are also higher after ischemia-reperfusion injury in SS mice.25
Chronic neutrophil activation might be enhanced during sickling episodes.26 Zhang et al. proposed a model of activation of vaso-occlusion in which neutrophils are recruited by activated endothelial cells, adhere to the endothelium and, upon inflammatory signaling, trap circulating RBC, promoting obstruction and increasing transit time, causing deoxygenation and sickling.27 This model is represented in Figure 2.
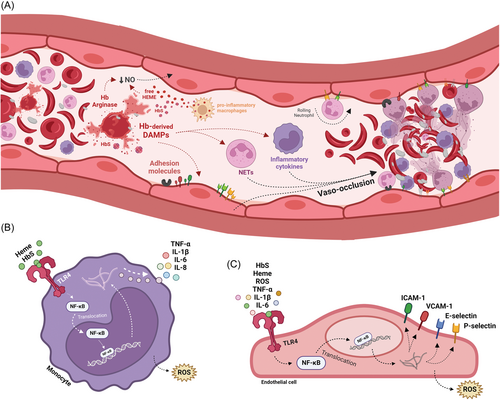
E-selectin and P-selectin are endothelial selectins that mediate leukocyte rolling and adhesion. Mice with a knockout in P-selectin and E-selectin genes had less interaction of SS RBC with leukocytes, resulting in the absence of vaso-occlusion.8 Both selectins are potential targets for therapies in SCD, which will be further discussed in this article. Leukocyte adhesion in SCD might also be regulated by overexpression of cluster differentiation (CD)18 integrins, which participate in the regulation of intercellular adhesion molecule (ICAM)−1,28 as SCD neutrophils express more CD18 than controls.29
In addition to the role of neutrophils in adhesion, emerging evidence suggests that neutrophil extracellular traps (NETs) modulate inflammation in SCD. NETs are formed in response to the production of reactive oxygen species (ROS) and are a mechanism of defense against pathogens and sterile inflammation triggers.30 During VOC, higher expression of genes that drive NET formation has been demonstrated.30, 31 In SCD animal models, NET formation in the lungs, induced by heme, occurred upon stimulation with TNF-alpha.13 Vessel injury and vaso-occlusion in the lungs during acute chest syndrome might be driven by the formation of NETs in the liver and their migration to the lung.32
Monocytes and macrophages, vaso-occlusion, and inflammation
Monocytes are innate immune cells that can phagocyte and produce cytokines, helping to regulate other immune cell populations. There are three main monocyte populations: classical (CD14++CD16−), intermediary (CD14+CD16+), and nonclassical (CD14dimCD16+).33 Despite the variability in the immunophenotypic and transcriptomic profiles between populations, the differences in their functional roles remain a matter of debate.34 It is hypothesized that classical monocytes have a pivotal role in triggering inflammation, whereas nonclassical monocytes have a patrolling function and might be involved in anti-inflammatory response.33 High monocyte counts are common in patients with SCD and might be linked to hemolysis, as they have been associated with high reticulocyte counts.35 Also, monocytes express more heme-oxygenase 1 (HO-1), an enzyme that degrades heme and protects the endothelium from its adverse effects, than other leukocyte lineages.36 Patients with SCD have a higher expression of HO-1 in nonclassical monocytes compared with healthy controls.36
Patients with SCD express higher levels of some cytokines, such as IL-1Beta, IL-6, IL-8, and TNF-alpha, when compared with healthy controls.37-39 These cytokines are secreted in response to nuclear factor kappa B (NFκB) pathway activation when Toll-like receptors (TLRs) are stimulated.40 TLRs are transmembrane and intracellular proteins that recognize pathogen-associated molecular patterns (PAMPs) from several types of fungi and bacteria, and DAMPs, which are normally endogenous. Monocytes express several types of TLR on their membrane, namely TLR2 and TLR4.41
In SCD, Belcher et al. described vaso-occlusion following TLR4 activation by heme and NFκB response.6 However, in another study, heme did not directly activate TLR4, leading to the hypothesis that heme-bound iron might activate monocytes by amplifying TLR4 signaling in the presence of TLR4 stimulators.42 More recently, Allali et al. demonstrated that HbS, and not heme, is responsible for triggering TLR4 and inducing an inflammatory response mediated by monocytes that results in NFkB activation and higher production of interleukins.7 Altogether, these data point out that monocyte activation through TLR4 due to HbS or heme plays a major role in maintaining the inflammatory status in SCD. Polymorphisms in TLR2, which has a pivotal role in defeating some bacteria and yeast, might modulate response to infections in SCD and be associated with less clinical complications, although reports remain conflicting.43, 44 Other activating or inhibitory monocyte receptors, such as NKG2D, might also modulate some disease complications, such as sickle cell retinopathy.45
In addition, TLR stimulation serves as a primer for inflammasome activation through NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) transcription; activation of inflammasome complexes leads to the cleavage of pro-interleukins IL-1Beta and IL-18 mediated by caspase-1.5, 46 IL-1Beta stimulates E-selectin, P-selectin, VCAM-1, and ICAM-4 expression in endothelial cells, enhancing adherence of leukocytes and platelets to the endothelium, thus contributing to vaso-occlusion.46
Macrophages are fundamental for maintaining heme and iron homeostasis, tissue repair, and clearance of apoptotic cells, which is highly relevant in SCD because of chronic hemolysis and constant tissue damage.47, 48 Capture and internalization of heme or Hb by macrophages can be done directly or mediated by haptoglobin (Hp) and hemopexin (Hx).49 However, the overload of free Hb and free Heme in SCD causes increasingly well-known phenotypic and functional changes in macrophages.50
Defective efferocytosis is associated with suppression of the transcription factor proliferator-activated receptor γ (PPARγ) and peroxisome proliferator-activated receptor coactivator 1α (PGC1α). In addition to the reduction of efferocytic receptors, macrophages undergo metabolic reprogramming that limits the absorption capacity of apoptotic cells. Finally, reduced IL-4 and IL-10 expression by macrophages reinforces defective efferocytosis.50
As reported in monocytes, free heme is an activator of the inflammasome via TLR4 in macrophages and stimulates a pro-inflammatory profile. When activated by heme, macrophages express and release IL-1β, IL-6, IL-12, IL-23, TNFα, and ROS, assuming a profile simplistically known as M1 that contributes to inflammation and tissue damage in SCD.51, 52
Studies in sickle cell mice have demonstrated that macrophages may contribute to liver disease, increase glomerular permeability and cell motility in glomerular capillaries, and lead to higher expression of IL-6 through sphingosine-1-phosphate (S1P).52-54 Finally, Redinus et al. suggested that intracellular iron accumulation macrophages have a critical role in pulmonary vascular remodeling and exacerbation of pulmonary hypertension in mice and patients with SCD.55 Monocytes and macrophages are being increasingly recognized as inflammatory cells with a central role in SCD complex pathophysiology; however, further studies are warranted to better characterize all inflammatory pathways and cytokine secretion.
Lymphocytes and inflammation
The distribution of lymphocyte subpopulations might be altered in SCD. Patients with SCD have less circulating TCD4, TCD8, and T regulatory lymphocytes (Treg) than healthy controls56, 57 and altered Th17/Treg imbalance, which is driven by higher levels of IL-6.58 T-lymphocyte associated antigen 4 (CTLA4), a protein expressed on activated T lymphocytes and Tregs, is more expressed in Tregs from SCD patients.59 Although polymorphisms in the CTLA4 gene were associated with higher susceptibility to alloimmunization in SCD,60 expression of CTLA4 is not higher in alloimmunized patients.59 Patients with osteonecrosis had more IFN-gamma and IL-17 producing TCD4+ lymphocytes56; susceptibility to osteonecrosis, stroke, and infections has been associated with polymorphisms in the HLA loci.61-64
Platelets and inflammation
Platelet activation is a well-established phenomenon in SCD, driven by several factors that include upregulation of VCAM, ICAM-1, P-selectin, and E-selectin caused by sterile inflammation, ischemia-reperfusion injury, and heme release in the blood stream.65, 66 Activated platelets contribute to shape inflammatory response by interacting with immune cells, releasing DAMPs, chemokines, and recognizing inflammatory signaling (reviewed in Scherlinger et al.67). They also interact with neutrophils and regulatory T lymphocytes (Tregs) through P-selectin56, 57 and form aggregates with leukocytes.68 In patients with SCD, higher levels of platelet–neutrophil and platelet–monocyte aggregates compared with controls have been described.66, 67 These platelet–neutrophil aggregates were shown to mediate pulmonary vaso-occlusion in murine SCD models.69 In patients, thrombocytopenia predicts rapidly progressive acute chest syndrome70 and this might be partially explained by platelet sequestration in vaso-occlusive sites.65
Furthermore, because platelets express several TLRs, including functional TLR4,71 some DAMPs might contribute to platelet activation and aggregation. High mobility group box 1 (HMGB1), a chromatin binding protein that maintains DNA structure, is a DAMP that enhances platelet aggregation via TLR4 activation in mice.72 In patients with SCD, circulating HMGB1 levels are higher in steady state compared with controls, and even higher during VOC episodes, activating TLR4.73 This might be an additional mechanism for platelet activation in these settings; nevertheless, further studies are warranted to elucidate how TLR4 triggering contributes to platelet activation in SCD.
Other inflammatory mechanisms
In addition to the significant data supporting the role of neutrophils, monocytes, macrophages, lymphocytes, and platelets in the inflammatory processes of SCD, there are also newer data supporting other mechanisms of inflammation, such as pro-resolving mediators (SPMs) and alternative complement pathways. SPM are molecules derived from omega-3 fatty acids involved in the resolution of acute inflammation by stimulating key cellular events, for instance, limiting neutrophil infiltration and macrophage clearance of cells.74, 75 In murine SCD models, administration of 17R-resolvin D1 resulted in a failure of inflammation-resolution programs,76, 77 and a reduction of inflammation in target organs by mechanisms that involve lowering NF-kB activation, some cytokines, and E-selectin expression.
Activation of the complement pathway has also been reported in SCD. The alternative complement pathway is activated in patients in steady-state,78 during VOC79 and due to the exposition of phosphatidylethanolamine (PE) or PS on the RBC external membrane.80 In vitro and in vivo models revealed higher levels of C5b-9 complex in steady-state SCD.81, 82 Ischemia-reperfusion injury and irreversibly sickled RBC also caused complement activation.82, 83 Infusion of C5a in murine models caused VOC, NFkB activation, and increased expression of TLR4, VCAM-1, ICAM-1, and E-selectin.82
Anti-inflammatory therapies in SCD
Advances in the understanding of inflammatory pathways in SCD have stimulated several clinical trials and allowed the discovery of auxiliary drugs for the management of SCD complications. Several drugs act on inflammatory pathways, even if their primary action is not strictly anti-inflammatory.
Hydroxyurea and inflammation in SCD
Hydroxyurea, primarily a chemotherapy agent, induces reactivation of gamma-globin expression by poorly understood mechanisms.84 After a clinical trial that showed a reduction of complications in patients with SCD,85 in 1998, HU was the first drug approved by the FDA for treating SCD.86 The current recommendation is to offer HU treatment to all infants 9 months or older, children, and adolescents with SS and SBeta0 genotypes. Despite the recent plethora of pharmacological therapies for SCD, HU remains the gold standard and, to date, cannot be replaced by any other drug.
HU promotes cytoreduction, which is one of the potential anti-inflammatory mechanisms associated with this drug. In addition, some studies have shown that patients under the use of HU might show an anti-inflammatory profile, as they secrete less TNF-alpha and more IL-10 than patients not taking HU87 and have higher counts of non-classical monocytes.88, 89 HU is also associated with lower levels of IL-1Beta, IL-6, IL-8, and tissue factor IL-1.88
Nevertheless, although HU induces lower leukocyte and neutrophil count, neutrophils from patients with SCD under HU treatment show more activity than healthy controls, as they form more NETs, degranulate more, and release more myeloperoxidase.90
The use of HU also led to less complement activation in these patients; interestingly, those who had a higher expression of CD46 on neutrophils had lower levels of C5b-9, suggesting that neutrophils might play a role in regulating complement activation.91 Microparticles, which have a role in complement activation in other settings,92 induce endothelial cells to produce ICAM-1 and enhance neutrophil adhesion in SCD, and such effects are diminished using HU.93
Other drugs
l-glutamine is necessary for the synthesis of nicotinamide adenine dinucleotides (NAD), whose reduced form, NADH, lowers RBC oxidative stress.94 A phase 3 trial using l-glutamine per os twice a day showed a small but significant reduction in the occurrence of VOC and acute chest syndrome94 and in 2017 this drug was approved by the Food and Drugs Administration (FDA) in SCD. The use of N-acetylcysteine has been assessed to prevent oxidative stress in SCD95 and showed a decrease in RBC membrane PS exposure, glycation-end products, and free hemoglobin in an early phase trial; however, a phase 3 study failed to show benefits over placebo.96 To increase NO production, N-arginine has been tested in different clinical trials and led to a reduction in opioid use during VOC97 and a rise in nitrite+nitrate levels.98 Intravenous l-citrulline has also been tested in a phase I trial and was shown to be safe in steady-state and patients with VOC, however, the sample size was small.99 Another clinical trial assessed the use of propranolol to reduce RBC adhesion to endothelium since the activity of BCAM/Lu and ICAM4 in sickle RBC is mediated by adrenergic receptors, and propranolol inhibits epinephrine-mediated adhesion.100 The results of this phase 1 trial showed a good safety profile and adherence inhibition in vitro. In another attempt to inhibit adhesion, a phase 2 trial study failed to prove the efficacy of intravenous sevuparin, a heparinoid with a low anticoagulant effect with antiadhesive, anti-inflammatory, and anti-aggregate properties, in treating VOC events in hospitalized patients.101 Poloxamer 188, a drug that blocks cell-to-cell interaction, reduces blood viscosity and adhesion of sickle RBC to the endothelium; however, despite positive results from a previous phase 3 trial, the most recent publication has not shown any positive effects on shortening time to last opioid dose during a VOC.10 Finally, although they do not act specifically in inflammatory pathways, drugs that increase HbS oxygen affinity, such as voxelotor89 and the pyruvate kinase activator mitapivat,90 contribute to lower hemolysis and probably have an indirect effect in lowering inflammatory response.
Targeted anti-inflammatory therapies in SCD
The first therapy targeting a specific inflammatory biomarker that was commercially approved for SCD is crizanlizumab, a humanized P-selectin IgG2 kappa monoclonal antibody that aims to prevent vaso-occlusive events by monthly intravenous infusions. The phase II study demonstrated good efficacy in reducing vaso-occlusive events102 and the drug obtained FDA approval in 2019; however, phase III results did not lead to significantly different outcomes between crizanlizumab and placebo.11 Another fully human P-selectin antibody, inclacumab, is currently being evaluated to prevent VOC and has the advantage of being administered every 12 weeks (NCT04935879). A comparison between both molecules revealed that inclacumab directly binds the P-selectin glycoprotein ligand 1 (PSGL1) binding region on P-selectin, whereas crizanlizumab binds to a more distant epitope from PSGL1 binding region on P-selectin.103 The results of a phase III trial assessing the efficacy of rivipansel, a pan-selectin antagonist administered intravenously during VOC in patients who needed to be hospitalized, showed no major differences in time to hospital discharge, to VOC resolution and in the rate of use of opioids.12 Complement inhibition to prevent VOC is also being assessed using the C5 inhibitor crovalimab (NCT05075824), which has been tested in paroxysmal nocturnal hemoglobinuria104; there are also some reports of the use of eculizumab to treat severe complications, such as thrombotic microangiopathy and bone marrow necrosis.105-107 Canakinumab, a IL-1Beta antagonist, has been assessed in young patients with SCD; although the phase II trial did not reach the primary endpoint of reducing VOC episodes, the drug had an effect on fatigue scores.108 Table 1 summarizes the clinical trials testing potential therapies for SCD that act in inflammatory pathways. Figure 3 shows how these drugs are correlated with such pathways.
| Drug | Mechanism | Phase of investigation | FDA approval | NCT number |
|---|---|---|---|---|
| Crizanlizumab | P-selectin inhibitor | Phase 3 completed | Yes | NCT03814746 |
| Canakinumab | IL-1Beta inhibitor | Phase 2 completed | No | NCT02961218 |
| Crovalimab | C5 inhibitor | Phase 2 ongoing | No | NCT05075824 |
| Eculizumab | C5 inhibitor | Case reports | No | |
| Inclacumab | P-selectin inhibitor | Phase 3 ongoing | No | NCT04935879 |
| l-arginine | Increase in NO production | Phase 3 ongoing | No | NCT04839354 |
| l-citrulline | Increase in NO production | Phase 1 completed | No | NCT02697240 |
| l-glutamine | Reduction of oxidative stress | Phase 3 completed | Yes | NCT01179217 |
| Mitapivat | Increases pyruvate kinase | Phase 1 completed | No | NCT04610866 |
| N-acetylcysteine | Reduction of oxidative stress | Phase 3 completed | No | NCT01849016 |
| Poloxamer 188 | blocks cell-to-cell interaction, reduces blood viscosity and adhesion of sickle RBC to the endothelium | Phase 2 | No | NCT01737814 |
| Propranolol | Reduction of adhesion dependent on adrenergic receptors | Phase 1 completed | No | NCT01077921 |
| Rivipansel | pan-selectin antagonist | Phase 3 completed | No | NCT02187003 |
| Sevuparin | Anti-adhesion, aggregation, and inflammation | Phase 2 completed, phase 3 ongoing | No | NCT02515838 |
| Tovinontrine | Phosphodiesterase inhibitor | Phase 2 completed | No | NCT04053803 |
| Voxelotor | Allosteric effect on increasing HbS affinity for oxygen | Phase 3 completed | Yes | NCT03036813 |
| Zileuton | Lipoxygenase inhibitor | Phase 1 completed | No | NCT01136941 |
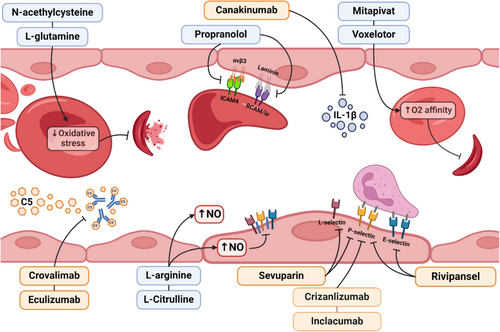
Future perspectives in SCD anti-inflammatory treatment
The growing understanding of the influence of inflammatory pathways on the pathophysiology of SCD opens the possibility for various markers of these pathways to be therapeutic targets. In murine models, an anti-TLR4 molecule, TAK-242, has prevented some SCD complications,109 but to date there are no clinical studies targeting TLR4 in SCD. Drugs that act on multiple inflammatory pathways, such as colchicine, have also been tested in animal models.110 Drugs that promote gamma-globin reactivation, such as LSD-1 inhibitors, can potentially ameliorate inflammation and might promote cytoreduction.111 Finally, definitive curative therapies, such as hematopoietic stem cell transplantation (HSCT) and gene therapy, have the potential for permanent reduction of inflammatory burden in these patients, although mixed chimerism and graft versus host disease might impair the anti-inflammatory effect of HSCT.112, 113
DISCUSSION
The understanding of SCD as an inflammatory disease is relatively recent. Erythrocyte adhesion to the endothelium in SCD has been proposed by Hebbel et al.,114 but the mechanisms of adhesion have been further elucidated during the last two decades.17, 46, 115 Likewise, although adverse SCD outcomes have been associated with high leukocytes long ago, the understanding of the role of leukocytes in adhesion and in driving inflammation in SCD is more recent. The clinical presentation of SCD is highly heterogeneous and disease genetic modifiers, such as HbS haplotypes, hereditary persistence of fetal hemoglobin (HPFH), and alpha-thalassemia trait, are well known.2, 116 Nevertheless, the extension of the inflammation role in such heterogeneity remains a matter of debate. Although the genetic and functional role of inflammation in SCD complications has been reported, studies using other designs are warranted to better establish the causal effect of some inflammatory pathways in these settings.46, 116
Despite the recent advances, blocking inflammatory pathways to treat SCD presented fewer positive results than expected, which might have multiple causes. Although several in vitro and in vivo studies revealed the key role of some inflammatory cells and molecules in SCD complications, the interactions between inflammatory pathways that drive these complications in the human body are very complex and not always reproducible. In addition, to date, there are scarce inflammatory biomarkers that help predict the occurrence of complications,9 making it more difficult to design drugs to prevent some complications by targeting specific pathways. Unveiling inflammatory biomarkers might not only pave the way for new drugs but also help improve the design of clinical trials, as they could be used as measurable endpoints. Furthermore, some target drugs may offer life-threatening risks; for instance, patients under the use of complement inhibitors have a higher risk of infections caused by encapsulated bacteria, which might hinder a broader use of this class of drugs in SCD due to the risk of functional asplenia and impaired opsonization.117
SCD represents a global health burden, and patients who would benefit more from new drugs are mostly in less developed countries. Efforts towards implementing and running clinical trials in SCD in Africa, India, and South America are necessary in this context, not only to include more patients but mainly to augment representativeness and validate results across ethnically and genetically heterogeneous SCD populations. Furthermore, to date, no specific pharmacological treatment was proven better than HU, and the high costs of treatment associations in SCD might prevent its widespread use. Therefore, the accessibility of novel therapies in SCD might be an upcoming issue.
In conclusion, SCD remains a highly complex disease in which a point mutation results in a myriad of disease manifestations that can be highly heterogeneous across patients. Although it is still difficult to predict some inflammatory interactions using experimental models, the understanding on how inflammation drives SCD pathophysiology and complications has increased exponentially during the last few decades. To conclude, the discovery of novel biomarkers could enhance our knowledge on inflammatory pathways which, in turn, might allow for the development of novel drugs and therapies targeting specific elements of these pathways.
ACKNOWLEDGMENTS
The authors thank the support grant PROADI TIAF from the Ministry of Health, the Center for Education and Research from Hospital Israelita Albert Einstein, the support grant #2022/07503-7, #2013/08135-2 São Paulo Research Foundation (FAPESP) and Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo.
AUTHOR CONTRIBUTIONS
Karina Tozatto-Maio and Felipe A. Rós reviewed the literature. Karina Tozatto-Maio wrote the manuscript. Felipe A. Rós made the figures. Vanderson Rocha and Ricardo Weinlich revised the manuscript. All authors edited and approved the manuscript.
CONFLICT OF INTEREST STATEMENT
Dr. Tozatto-Maio is a subinvestigator of the clinical trial GBT 440042 “A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Efficacy of Voxelotor for the Treatment of Leg Ulcers in Patients with Sickle Cell Disease”. The other authors have no disclosures.
FUNDING
PROADI NUP25000.003071/2021-28; #2022/07503-7, #2013/08135-2 São Paulo Research Foundation (FAPESP).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.




