Additional parameters to improve the prognostic value of the 8th edition of the UICC classification for human papillomavirus-related oropharyngeal tumors
Abstract
Background
The prognostic reliability of the UICC's TNM classification (8th edition) for human papillomavirus (HPV)-positive tonsillar squamous cell carcinomas (TSCCs) compared to the 7th edition was explored, and its improvement by using additional anatomical and nonanatomical parameters.
Methods
One hundred and ten HPV-positive and 225 HPV-negative TSCCs were retrospectively analyzed. Survival was correlated with patient and tumor characteristics (7th and 8th edition UICC TNM classification).
Results
In HPV-positive TSCCs, the 8th edition UICC's TNM classification correlated better with prognosis than the 7th edition. Also, smoking status was a stronger prognosticator of survival than UICC staging. Non- or former smokers had a 5-year overall survival of 95.1% regardless of tumor stage. Furthermore, age (>65 years), cN3, and M1 classification were significant prognostic factors.
Conclusion
The prognostic value of the 8th edition UICC's TNM classification improved significantly when compared to the 7th edition. Nonetheless, further improvement is possible by adding nonanatomical factors (smoking, age >65 year) and separating N0-N2 from N3.
1 INTRODUCTION
In head and neck squamous cell carcinomas (HNSCCs), the UICC tumor staging system, based on T, N, and M classification, is crucial in predicting patient prognosis and guiding therapeutic decision-making.1 Within this staging system, N classification is considered to be the strongest prognosticator.2-4 However, for oropharyngeal tumors the prognostic value of N classification has become questionable in light of the epidemic rise of human papillomavirus (HPV)-associated carcinomas in recent decades.5-12
At diagnosis, HPV-positive tumors have smaller primary tumor sizes, but equally affected lymph nodes (positive N classification) compared to HPV-negative tumors. Moreover, their prognosis is more favorable than that of similarly staged HPV-negative tumors. The different clinical presentation and biological behavior of HPV-positive tumors, in combination with an increasing incidence of HPV, has shifted the prognostic value of “traditional” tumor classification models for HPV-dominant head and neck tumor sites. This became clear from our review of articles reporting the prognostic value of T and N classification in tonsillar squamous cell carcinomas (TSCCs). We found 10 studies published prior to 1990 all reporting N classification to be of prognostic importance (although only 2 studies provided results based on statistical analysis), while only 4 out of 12 studies published from 1990 onwards showed N classification to be of prognostic relevance (see Table 1).13-34 In a previous study we tested the prognostic value of N classification in a group of 81 HPV-positive and HPV-negative TSCCs and found a decrease in prognostic value of N classification in the whole group.35 N classification was of prognostic relevance only when tonsillar tumors were not HPV-associated. Similar findings have been reported by Klozar et al.36 in HPV-positive oropharyngeal SCCs and by Fritsch et al.37 in tumors located in HPV-dominant head and neck sites compared to non-HPV-dominant tumor sites.
| Study | Prognostic value of N classification | Site | Patients (number) | Inclusion (years) | UICC/AJCC | Therapy | Statistics | Survival tested based on N classification | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N0 > N+ | N0–N1 > N2–N3 | N0 = N+ | N0–N1 = N2–N3 | ||||||||||
| Author | Year | ||||||||||||
| Edström | 1978 | X* | Tonsil | 37 | 50–′70 | AJCC ′67 | RT | None | 5-year OS | Unknown | |||
| Mantravadi | 1978 | X* | Tonsil | 117 | 55–′73 | AJCC ′67 | Combined | None | 2–5-year DFS | Unknown | |||
| Petrovich | 1980 | X* | Tonsil | 205 | 46–′76 | AJCC ′78 | Combined | None | 5-year OS | Unknown | |||
| Tong | 1982 | X* | Tonsil | 104 | 65–′75 | AJCC ′78 | RT | None | 3-year OS, DFS, LRC | Unknown | |||
| Dubois | 1983 | X* | Tonsil | 215 | 70–′76 | UICC/WHO ′62–′79 | RT | None | 3–5-year OS | Unknown | |||
| Orregia | 1983 | X | Tonsil | 79 | 60–′79 | UICC ′78 | Combined | Life-table method, log rank | 5-year OS | 0.001 | |||
| Amornmarn | 1984 | X* | X* | Tonsil | 185 | 56–′77 | AJCC ′83 | RT | None | 5-year DFS, LC, RC | Unknown | ||
| Mizono | 1986 | X* | Tonsil | 203 | 57–′79 | AJCC ′78 | Combined | None | 5-year DFS | Unknown | |||
| Vallis | 1986 | X | X | Tonsil | 87 | 62–′81 | UICC ′78 | RT | Life-table method, log rank | 10-year OS, DFS | 0.001 | ||
| Lusinchi | 1989 | X | Tonsil | 193 | 70–′82 | UICC ′79 | RT | Kaplan–Meier | 3–5-year OS | Unknown | |||
| Di Marco | 1990 | X (no N2) | X | Tonsil | 183 | 70–′84 | UICC ′78 | RT | Log rank | 5-year OS | <0.005 | ||
| Mak-Kregar | 1990 | X | X | Tonsil | 92 | 66–′85 | UICC ′82 versus UICC ′87/AJCC ′88 | Combined | Kaplan–Meier, Cox regression | 3-year DFS | NS | ||
| Al-Abdulwahed | 1997 | X | Tonsil | 102 | 75–′95 | UICC ′87 (restaged) | Combined | Kalpan–Meier | 5-year OS, 4-year DSS | 0.02 | |||
| Perez | 1998 | X | Tonsil | 384 | 59–′91 | Macomb and Fletcher ′67 | Combined | Not described, (life-table method?) | 4–10-year DFS | Multivariate DFS: 0.001 | |||
| Friesland | 1999 | X | Tonsil | 167 | 80–′95 | AJCC ′97 | Combined | Kaplan–Meier, Cox regression | 5–15-year OS, DSS | 0.0057 | |||
| Mellin | 2000 | X | Tonsil (HPV) | 60 | 84–′96 | UICC ′97 | Combined | Kaplan–Meier, Cox regression | 2–10-year DSS | NS | |||
| Charbonneau | 2006 | X | Tonsil | 164 | 90–′99 | AJCC ′97 | Combined | Kaplan–Meier, Cox regression | 2–5-year OS | Univariate OS: 0.0028, multivariate OS: 0.043 | |||
| Pitkin | 2007 | X | Tonsil | 84 | 90–′03 | AJCC ′97 | Combined | Kaplan–Meier, Cox regression | 2–13-year OS, DSS, DFS | Univariate DFS: 0.248, univariate DSS: 0.005, multivariate DSS: N1 0.521, N2 0.022, N3 0.832 | |||
| Chien | 2008 | X | Tonsil (HPV) | 111 | 92–′05 | AJCC ′97 | Combined | Kaplan–Meier, Cox regression | 5-year DSS | Univariate DSS: 0.557 | |||
| Hafkamp | 2008 | X | Tonsil | 81 | 92–′01 | AJCC ′97 | Combined | Kaplan–Meier, Cox regression | 5-year OS, DSS | NS, unadjusted HR: 1.0 (95%CI 0.5–2.9) | |||
| Aziz | 2010 | X | X | Tonsil | 69 | 98–′00 | AICC ′97 | RT en brachy | Kaplan–Meier, Cox regression | 3-year OS, DSS | Univariate DSS: 0.304 | ||
| Bachar | 2010 | X | Tonsil | 640 | 70–′90 | Not described | Combined | Kaplan–Meier, Cox regression | 20-year OS, DSS | Univariate OS: 0.0003, multivariate DSS: N1 0.0654, N2 0.0483, N3 <0.0001 | |||
- Note: Results of the meta-analysis performed in Pubmed: inclusion criteria: studies 1960–July 2013; search terms: “cancer,” “neoplasm*,” “carcinoma*,” “oropharyn*,” “tonsil*,” “lymph*,” “node*,” “nodal,” “neck,” “survival*,” “prognos*,” “mortality,” “morbidity,” “outcome.” 634 abstracts were evaluated for inclusion, and in case of doubt the full text article was read. References of all read articles were checked. In total, 22 articles were selected for inclusion (publication years: 1978–2010; range in number of patients per study: 37–640; range in year of inclusion: 1950–2005).
- Abbreviations: AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control Tumor staging system; brachy, brachytherapy; DFS, disease-free survival; DSS, disease-specific survival; LRC, locoregional recurrence rate; NS, not significant; OS, overall survival; RT, radiotherapy.
The issue of restaging HPV-associated head and neck carcinomas using N classification alone or in combination with other clinical parameters has been addressed from various perspectives in the literature. Ang et al.38 presented a predictive model on prognosis for stage III and IV oropharyngeal SCCs (OPSCCs) treated with concomitant chemoradiotherapy, whereby smoking status was combined with N stages “N0-2a” and “N2b-3.” Spector et al.39 subdivided N classification on the basis of diameter and number of nodes in three risk groups. Huang et al.40 discriminated four prognostic groups in HPV-associated oropharyngeal carcinomas without hematogenous metastases, based on N classification (N0-2c vs. N3), T classification (T1-3 vs. T4), smoking behavior (fewer vs. more than 20 pack-years history), and age (younger vs. older than 70 years). Dahlstrom et al.41 were not able to validate Huang's results; consequently, they proposed an HPV-associated system in which N classification was staged corresponding to nasopharyngeal carcinomas. Finally, the classification system of O'Sullivan et al.42 (ICON-S) was adopted for the 8th edition of the clinical TNM staging for HPV-related carcinomas (based on the sidedness and maximum diameter of the nodes rather than on the number of nodes). Regarding pathological staging, the system proposed by Haughey et al.43 was accepted for the 8th edition. In contrast to clinical staging, the number of nodes (with a cut-off point of 4) determines N classification (ranging from N0 to N2 without differentiation between N2a, N2b, and N2c) for pathological staging.
Both the clinical and the pathological staging systems were recently validated by Cramer et al. in a population of more than 15 000 HPV-positive oropharyngeal carcinomas.44 They have shown that the new 8th edition of the UICC's TNM classification for HPV-related oropharyngeal squamous cell carcinomas overcomes some of the main shortcomings of the 7th edition.
However, given the findings cited from the literature, this study questions whether the 8th edition UICC classification system already reaches an optimum level to predict prognosis for HPV-positive OPSCCs. As mentioned above, the study of Ang et al. emphasized the role of both smoking and age.38 Therefore, it is of interest to study the influence of tobacco use and senescence on tumor biology and thus on the validity of staging systems. Second, from 2009 onwards, as it became clear that the N classification is not a valid predictor in HPV-positive TSCCs, there were indications that the prognosis for N0 tumors was even worse than for N+ tumors.35 It has been hypothesized that the presentation of a well treatable neck metastasis would lead to earlier detection of the primary tumor.44 Thus, it is important to investigate the extent to which this is demonstrable in the currently adopted staging system (UICC 8th edition). A third factor that influences the prognostic reliability is the method for detection of HPV-association used in the 8th edition. The new system uses p16-IHC, which is widely available and frequently applied due to the low cost. However, it yields false positives in HPV-negative cases.
The primary aim of this study was to improve the classification system for HPV-associated tumors. We focused on a large series of 368 tonsillar SCCs which were subjected to HPV analysis using p16 overexpression, HPV-specific PCR and/or FISH. Tonsils were chosen for two reasons: they form the predominant oropharyngeal subsite where HPV-positive tumors develop; and different patterns of nodal dissemination have been reported between tonsillar SCCs and base of tongue carcinomas.45 All cases were evaluated according to the 7th and the 8th edition UICC TNM classification. We examined the prognostic value of T, N, and M classification and investigate to what extent patient-associated clinical variables of age, smoking behavior, alcohol consumption, tumor differentiation grade, and treatment influence prognosis.
2 MATERIALS AND METHODS
2.1 Tumor material and patient data
The study population consisted of 368 patients with TSCC diagnosed between 1987 and 2011 at the Maastricht University Medical Centre. That population was an expansion of our study group on which results were published in 2009 (n = 81).35 Their formaldehyde-fixed, paraffin-embedded archival biopsy and resection materials were classified by histopathology at the Department of Pathology, University Hospital Maastricht, the Netherlands. The materials were analyzed for the presence of oncogenic HPV16 DNA by means of PCR and/or FISH as well as p164INKA immunostaining in 335 available specimens.2 Data on age, sex, TNM classification, tumor differentiation grade, tobacco and alcohol consumption, treatment modality, and follow-up (5 years after treatment) were collected from the head and neck tumor database of our institute and from reviewing clinical, pathological, radiological, and surgical reports. All tumors were reclassified according to the 7th and 8th edition of the UICC's TNM classification.
Classification of smoking and alcohol consumption conformed to Hafkamp et al.: patients were classified as daily smokers (≥1 cigarette, pipe and/or cigar per day), nonsmokers (never smokers), or former smokers (those who had stopped smoking more than 10 years before the diagnosis of TSCC).6 During multidisciplinary counseling, treatment plans were based on tumor size, neck staging, presence of distant metastases, tumor histology and cytology of metastases, feasibility of surgery, clinical condition, comorbidities, and histopathology of resection specimens in case of surgery. Elective treatment of the neck was performed routinely—also in the N0 neck—because of the high incidence of occult metastases in TSCC.15, 19-21
The investigation was conducted in accordance with the declaration of the 18th meeting of the world medical association in Helsinki 1964 and subsequent revisions. Approval for the study protocol was granted by the institutional ethical committee. Written informed consent was obtained from all included patients.
2.2 Statistical analysis
Variables considered were age at time of diagnosis, sex, TNM classification, tumor differentiation grade, smoking and alcohol consumption, T, N, and M classification, and therapy.
The Youden Index was calculated to determine the optimal cut-off point in the ROC displaying the relation between sensitivity and specificity for the range of age value.46
Survival analysis was performed; disease-free survival (DFS) and overall survival (OS) were calculated using the Kaplan–Meier method for each of the considered independent variables. Five-year survival (OS) was calculated from date of diagnosis until death or until discharge from follow-up. DFS was calculated from date of diagnosis until date of recurrence (local, regional or distant). Patients without recurrence were censored at date of last follow-up or death. Level of significance was determined at p ≤ 0.05. Four patients who initially presented with distant metastases were excluded from the survival analysis. Multivariate analyses were performed using the Cox proportional hazards model. Variables remained in the model if p-values were below 0.10.2 IBM SPSS Statistics version 20 was used for the statistical analysis.
3 RESULTS
3.1 Demographic data and outcome related to HPV association
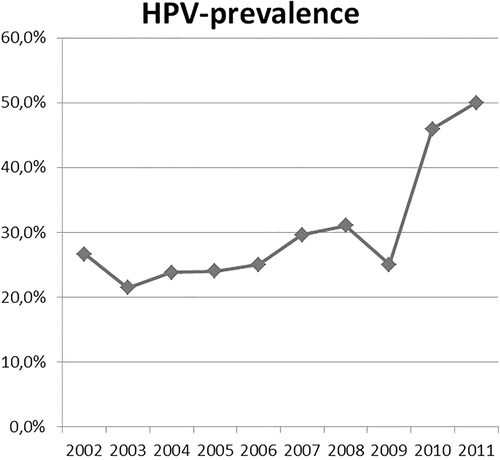
In total, 368 patients with TSCC were included with an average age of 60.3 years (range 39–87). HPV association could be tested in 335 patient samples, of which 32.8% were HPV-positive (n = 110/335). An increasing prevalence of HPV-associated TSCCs during the last decade (since 2002) was observed (Figure 1).
HPV-positive TSCCs were significantly related with a non- or former smoking status, with alcohol use of less than 2 units/day and a poorly differentiated tumor status. There was an equal distribution among age and sex groups regardless of HPV association.
No statistical differences were noted in the development of second primary tumors regardless of HPV association. Also, no differences were seen in prior history of head and neck cancer or cancer at other sites in both groups. For HPV-positive and HPV-negative tumors, an equal number of patients presented with distant metastases. HPV status relative to patient characteristics is summarized in Table 2. The presented small numbers on failure of control of disease in HPV-positive patients did not allow an adequate uni- or multivariate analysis, in particularly on smoking status and age in this group.
| Patient characteristics | Total (n = 368) | % | HPV-positivea (n = 110) | HPV-negative (n = 225) | p-value | |
|---|---|---|---|---|---|---|
| Sex | Male | 264 | 71.7 | 81 | 161 | NS |
| Female | 104 | 28.3 | 29 | 64 | ||
| Age | Mean | 60, 32 | 60.1 | 60.55 | NS | |
| Range | 39–87 | 39–84 | 41–87 | |||
| Age (years) | <55 | 111 | 30.2 | 36 | 66 | NS |
| 55–65 | 159 | 43.2 | 38 | 103 | ||
| 65 | 98 | 26.6 | 36 | 56 | ||
| Smoking | Nonsmoker | 34 | 9.2 | 21 | 9 | <0.001 |
| Former-smoker (#>10 years) | 34 | 9.2 | 18 | 13 | ||
| Smoker | 265 | 72 | 63 | 181 | ||
| Unknown | 35 | 9.5 | 8 | 22 | ||
| Alcohol | None or <1 unit/day | 74 | 20.1 | 34 | 36 | 0.003 |
| 1–2 units/day | 70 | 19 | 23 | 36 | ||
| >2 units/day | 187 | 50.8 | 45 | 129 | ||
| Unknown | 37 | 10.1 | 8 | 24 | ||
| Tumor differentiation (n = 191) | G1 | 24 | 6.5 | 7 | 16 | 0.041 |
| G2 | 108 | 29.3 | 24 | 79 | ||
| G3 | 57 | 15.5 | 24 | 29 | ||
| Undifferentiated | 2 | 0.5 | 1 | 1 | ||
| p16 | Positive | 124 | 37 | 109 | 15 | <0.001 |
| Negative | 211 | 62.9 | 1 | 210 | ||
| Previous cancer in medical history | Head and neck carcinoma | 3 | 16 | NS | ||
| Other | 2 | 4 | ||||
| Distant metastases | Present | 3 | 3 | NS | ||
| Second primary tumor | 2 | 14 | NS | |||
| Therapy | Surgery | 18 | 4.9 | 3 | 11 | NS |
| (Surgery) + radiotherapy | (93) 237 | (25.3) 64.4 | (42) 78 | (44) 139 | ||
| (Surgery) + concomittant chemoradiotherapy | (10) 79 | (2.7) 21.5 | (3) 21 | (6) 49 | ||
| None/palliative | 23 | 6.1 | 7 | 16 | ||
| Unknown | 11 | 3 | 1 | 10 | ||
- a HPV-association could be tested in the histologic specimens of 335 patients.
- Abbreviations: NS, not significant; SCC, squamous cell carcinoma.
3.2 Analysis of the 7th edition UICC tumor classification system for staging TSCCs
Compared to HPV-negative TSCCs, HPV-positive tumors were associated with significantly smaller primary tumors (Table 3). The extension of neck disease did not differ between both groups. As a consequence, HPV-positive and HPV-negative TSCCs were equally distributed over UICC tumor stages I–IVc in the 7th edition. Within each tumor stage of the 7th edition, HPV-positive TSCCs were associated with a smaller T classification. For TSCCs staged Iva, for example, 38 out of the 61 HPV-positive tumors were classified T1–T2, compared to 31 out of 108 HPV-negative TSCCs (χ2, p < 0.001).
| Total population | HPV-status, n = 335 | p-value | ||||
|---|---|---|---|---|---|---|
| n = 368 | % | Positive (n = 110) | Negative (n = 225) | |||
| 7th edition tumor staging | I | 26 | 7.1 | 5 | 16 | NS |
| II | 38 | 10.3 | 11 | 24 | ||
| III | 78 | 21.2 | 20 | 49 | ||
| IVa | 182 | 49.5 | 61 | 108 | ||
| IVb | 32 | 8.7 | 9 | 21 | ||
| IVc | 7 | 1.9 | 2 | 5 | ||
| ? | 5 | 1.4 | 2 | 2 | ||
| cT classification | 1 | 74 | 20.1 | 22 | 42 | 0.037 |
| 2 | 112 | 30.4 | 44 | 57 | ||
| 3 | 82 | 22.3 | 23 | 54 | ||
| 4a | 83 | 22.6 | 17 | 61 | ||
| 4b | 14 | 3.8 | 3 | 9 | ||
| ? | 3 | 0.8 | 1 | 2 | ||
| T1,T2 vs. T3,T4 | 67 vs. 42 | 99 vs. 124 | 0.014 | |||
| cN classification | 0 | 113 | 30.7 | 27 | 73 | NS |
| 1 | 60 | 16.3 | 16 | 38 | ||
| 2a | 14 | 3.8 | 5 | 9 | ||
| 2b | 112 | 30.4 | 43 | 62 | ||
| 2c | 39 | 10.6 | 11 | 24 | ||
| 3 | 23 | 6.2 | 6 | 15 | ||
| ? | 7 | 1.8 | 2 | 4 | ||
| N0 vs. N+ | 28 vs. 81 | 73 vs. 150 | NS | |||
| N0–N1 vs. N2–N3 | 43 vs. 66 | 112 vs. 111 | NS | |||
| cM classification | 1 | 6 | 1.7 | 3 | 3 | NS |
- Abbreviations: NS, not significant; SCC, squamous cell carcinoma.
In HPV-positive TSCCs, only N3 (affected lymph node >6 cm) was associated with a worse survival (Cox regression, p < 0.001; OR 8.233; 95%CI 2.68–25.29). No significant differences in survival were noted between N stages N0 to N2c, nor between the different stages when we classified tumors according to the 7th edition. Correspondingly, only TSCCs staged IVb or higher showed a worse survival (Cox regression, p < 0.001), and no prognostic differences were noted between stages I and IVa, which altogether cover 90% of the HPV-associated TSCCs.
Among the largest subgroups, stage IVa, for example (n = 181), an overall survival of 85% was found in HPV-positive TSCCs, a significantly higher rate than the 42.1% found in HPV-negative TSCCs (log rank, p < 0.001). This difference may possibly reflect the association of HPV and smaller primary tumor size, given that in stage IVa the 5-year OS for T1-2 and T3-4 were, respectively, 62.3% versus 28.7%, independent of HPV status (log rank, p < 0.001).
In contrast, in the HPV-negative population, a worse survival was associated with increasing T classification, N classification, and UICC tumor stages (Cox regression, p < 0.001).
Thus, in the 7th edition, HPV-positive tumors were mainly staged higher as a consequence of their nodal classification. However, this higher stage seemed to have no prognostic implications.
3.3 Analysis of the 8th edition UICC tumor classification system for staging TSCCs
All HPV-positive tumors were restaged in our study. In the 8th edition, a distinction is made between clinical and pathological staging. Because 38 out of our 110 HPV-positive TSCCs were treated surgically, pathological staging was possible in these 38 tumors (Table 4).
| Five-year overall survival | ||||
|---|---|---|---|---|
| HPV-negative (n = 225) | HPV-positive (n = 110) | |||
| 7th edition | 7th edition | 8th edition: clinical | 8th edition: pathological | |
| I | 75.0% | 80.0% | 83.3% | 81.8% |
| II | 79.2% | 81.8% | 76.2% | 83.3% |
| III | 59.2% | 75.0% | 72% | 100% |
| IVa | 42.1% | 85.0% | 0% | 0% |
| IVb | 33.3% | 55.6% | ||
| IVc | 20.0% | 0.0% | ||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
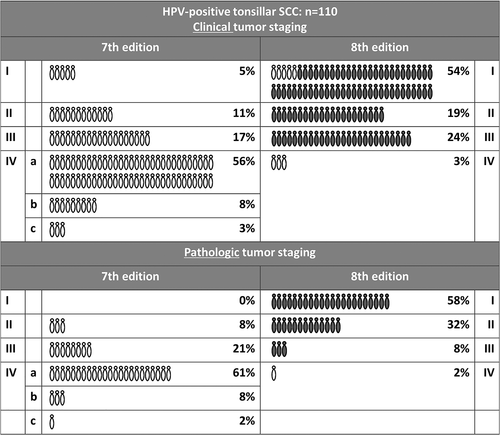
Using the 8th edition of the UICC system for clinical staging, restaging of the majority of HPV-positive tumors resulted in classifying only 3% of patients as having stage IV tumors, compared to 67% in the previous classification. Figure 2 depicts the shift between stages. Using the 8th edition, we found a decreasing survival with increasing clinical UICC tumor stage in HPV-positive tumors (p < 0.001) (Table 4). In the group of HPV-positive TSCCs which were treated surgically and thus staged pathologically (n = 38), we found no decrease in survival with increasing tumor stage.
3.4 Univariate analysis of survival of T, N, and M classification (8th edition UICC TNM classification) in patients with HPV-positive TSCC
Only nodes larger than 6 cm (cN3 status) were associated with a worse survival (p < 0.001), and no differences in survival were found for necks that were clinically staged cN0, cN1, and cN2 (Table 5). Remarkably, cN1 (unilateral nodes with a diameter of no more than 6 cm) seemed to be associated with a better survival than when no lymph nodes were affected. The number of contralateral or bilateral involved necks in TSCCs was very low in this study.
| T classification | p-value | HR | cN classification | p-value | HR | pN classification | p-value | HR |
|---|---|---|---|---|---|---|---|---|
| T1 | 1 | cN0 | 1 | pM0 | 1 | |||
| T2 | 0.259 | 1.980 | cN1 | 0.534 | 0.744 | pN1 | 0.966 | 95.604.162 |
| T3 | 0.152 | 2.686 | cN2 | 0.656 | 0.699 | pN2 | 0.969 | 40.630.224 |
| T4 | 0.459 | 1.761 | cN3 | 0.004 | 6.541 | |||
| T classification | 0.553a | cN classification | 0.003a | pN classification | 0.731a |
- a Log rank, Kaplan–Meier.
- Abbreviations: HR, hazard ratio; SCC, squamous cell carcinoma.
Also, no correlation with survival was seen when N classification was defined pathologically, and no difference in survival was found between HPV-positive pathologically staged necks pN1 and pN2.
Altogether, only lymph nodes that were both larger than 6 cm (cN3) or TSCCs that were seen with distant metastases (M1) correlated with a poor prognosis in HPV-positive TSCCs.
Table 6 presents the odds ratios (and 95% confidence intervals) for each considered explanatory variable obtained from Cox regression modeling of OS.
| Hazard ratio | 95% confidence interval | ||
|---|---|---|---|
| Age | >65 years | 2.30 | 1.05–5.06 |
| Sex | Male | 1.27 | 0.51–3.19 |
| Smoking status | Smokers versus nonsmokers | 4.64 | 1.10–9.19 |
| Smokers versus non- and former smokers | 5.32 | 1.58–17.91 | |
| Alcohol intake | <1 unit per day | 1 | |
| 1–2 units per day | 2.39 | 0.73–7.8 | |
| >2 units per day | 1.87 | 0.65–5.40 | |
| Tumor differentiation | G1 | 1 | |
| G2 | 1.40 | 0.37–5.26 | |
| G3 | 1.21 | 0.47–3.14 | |
| M classification | 1 | 8.77 | 2.60–29.64 |
| Treatment | Surgery as monotherapy | 1 | |
| Surgery with radiotherapy | 0.64 | 0.085–4.89 | |
| Surgery with combined chemoradiotherapy | 0.22 | 0.014–3.63 | |
| Palliative treatment | 11.5 | 1.15–98.25 |
3.5 Univariate analysis of survival of nonanatomical characteristics of patients with HPV-positive TSCC
Smoking, age >65 years, cN3 status and M classification were correlated with OS. Treatment category had no influence on OS, with the exception of palliative care for which a significantly lower OS was found
The rationale for choosing age 65 years as the cut-off point is as follows. The Youden Index was calculated to determine the optimal cut-off point in the ROC curve that displays the relation between sensitivity and specificity for the range of age value.46 The optimal value was 2.22 for both the total data set and for the HPV+ cases. The Youden Index corresponded to the age of 63.5 for the total data set and 66.5 for the patients with HPV+. Age >65 years showed a significant correlation with a worse prognosis (p < 0.001).
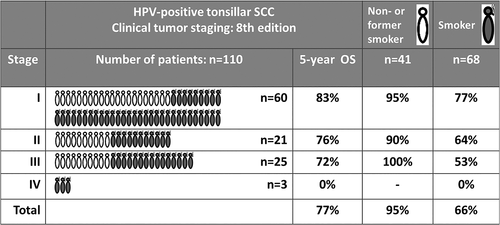
In the HPV-positive group, the 5-year OS in the non- or former smokers group (n = 41/110) was 95%, compared to 66% in the smoking group (n = 62/110) (p < 0.001). The number of pack-years had no further influence on prognosis. There was no difference in survival between never-smokers and former smokers (those having quit more than 10 years before diagnosis). Moreover, non- or former smokers were equally distributed over the different stages in the UICC 8th edition (Figure 3). Interestingly, being a non- or former smoker had a favorable prognosis independent of tumor stage. In stage I the overall survival of nonsmokers was 95%, in stage II 100%, and in stage III 90%.
An inverse relationship was found between age and smoking behavior in patients with HPV-positive TSCCs (χ2, p = 0.036): nonsmokers were more often older than 65 years, whereas smokers developed a HPV-positive TSCC more often at or under age 65. Smokers older than 65 had a worse overall survival (log rank, p = 0.049). Smoking and age were not correlated to T classification, N classification, and/or UICC tumor staging (8th edition).
3.6 Multiple regression analysis of survival including T, N, and M classification and nonanatomical characteristics of patients with HPV-positive TSCC
The multiple regression analysis resulted in a model with M classification, smoking category, and age 65 years or older (Table 7).
| p-value | Hazard ratio | |
|---|---|---|
| Smoking | 0.006 | 5.723 |
| Age >65 | 0.692 | 1.225 |
| M classification | 0.009 | 7.118 |
| N3 vs. N012 | 0.000 | 14.862 |
After analysis of the above-mentioned prognostic factors (smoking behavior, age, and N3 status), we developed a predictive model of four groups of patients with HPV-positive TSCCs for patients without hematogenous metastases (Figure 4). M classification was not included as the 5-year OS was 0%. Group I (n = 41) included non- or former smokers with a 5-year overall survival rate of 95.1%, regardless of age, number of pack-years or T and/or N classification, with the exception of cN3 classification. Group II (n = 45) included smokers of age 65 or younger with a 5-year OS of 75.6.1% (HR 5.649, 95%CI 1251–25498). The third group (n = 13) consisted of smokers older than 65 years of age with a 5-year OS of 46.2% (HR 14.165, 95%CI 2935–68368). Survival in group II and III was determined regardless of TNM staging. The fourth group contains patients staged with a N3 staged neck This group showed a 5-year OS of 33% (n = 6). Interestingly, the two patients who survived were nonsmokers who were seen with a cN3-staged neck.
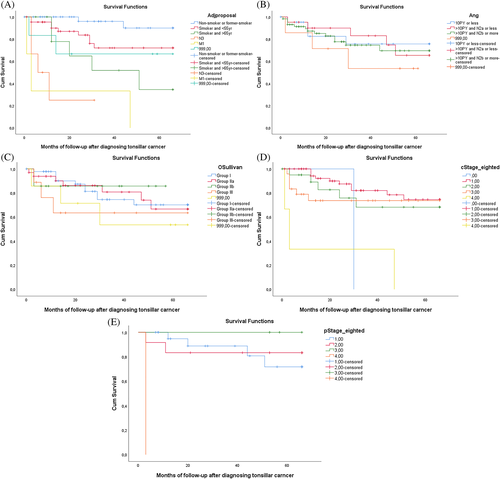
We then considered other prognostic classification models which utilize nonanatomical parameters, as introduced in the literature (Ang et al., O'Sullivan et al. [ICON-S], and Huang et al.). When applying these to our HPV-positive tonsillar carcinoma patients, no relation between the proposed staging and outcome was found (Figure 4).38, 40, 42 The model proposed by Ang et al. had been tested on the stage III/IV tumors treated with concomitant chemotherapy, and also on the total population.
4 DISCUSSION
The implementation of the 8th edition of the UICC classification system is a step forward for staging HPV-associated OPSCCs
In the literature, the predictive value of the 7th edition of the TNM classification for oropharyngeal carcinomas, and for N classification in particular, has shifted over time as a consequence of the epidemic of HPV-associated HNSCCs.13-34 This shift prompted the publication of a new edition of the UICC staging system. The 8th edition introduced a separate classifying system for HPV-positive tumors. In this study we investigated an unselected group of 368 patients with carcinomas of the tonsil, which is the site associated with the highest prevalence of HPV. In total, 110 tumors tested HPV-positive with p16, PCR, and/or FISH. The influence of HPV presence on the prognostic value of the 7th and 8th UICC tumor staging system as well as on individual T, N, and M classification was examined, taking into account the influence of patient-associated clinical variables including tumor differentiation grade, age, smoking behavior, alcohol consumption, and treatment.
Altogether, when we investigated the prognostic value of T, N, and M classification in both classification systems (7th and 8th edition), only two criteria—lymph nodes larger than 6 cm (cN3), and the presence of distant metastases (M1)—were correlated with a poor prognosis in HPV-positive TSCCs.
Regarding the 7th classification system, our study did not reveal a difference in survival between tumor stages I–IVa, which are the staging groups that represent 90% of the HPV-positive tumors. This prognosis, which is favorable even within the more advanced stages, might be the result of a better response to treatment by HPV-positive tumors. Another explanation for the favorable prognosis might lie in the tumor biology which is associated with HPV. HPV-positive tumors are associated with smaller, less advanced T stages despite the presence of more advanced N stages.
For N classification, no prognostic value in HPV-positive TSCCs could be demonstrated when using the 7th classification system. Only N3 classification was correlated with a worse survival. Our review of the literature on outcome of treatment of TSCCs demonstrated the loss of prognostic value for N classification over time (Table 1). The causal relationship between this shift in prognostic value of N classification and the rising incidence of HPV-associated carcinomas has been observed previously.35, 36 Again, the HPV-associated tumor biology, with smaller primary tumors despite advanced-stage lymph nodes, may have a beneficial effect on the response to therapy.
Restaging for HPV-associated carcinomas has been proposed in the literature since 2009. Spector et al. reported that the AJCC classification system for N classification might be unreliable in predicting outcome for HPV-positive oropharyngeal tumors.39 A new classification system was proposed: “HPV+ N1” was defined as a single node <6 cm, ipsilaterally or contralaterally; “HPV+ N2” was defined as a single node ≥6 cm or ≥2 nodes ipsilaterally/contralaterally or ≥3 nodes bilaterally; “HPV+ N3” was defined as matted nodes. Applying that classification to our study population, no differences were found between the proposed “HPV+ N1,” “HPV+ N2,” and “HPV+ N3” because the number of lymph nodes >6 cm was relatively small and survival for HPV-positive N2b necks was good.
Ang et al. proposed a new classification system for oropharyngeal tumors.38 In the HPV-positive groups, smoking status discriminated between mild and moderate risk. The same authors also reported a difference in outcome between N0-2a versus N2b-3. However, in the study of Ang et al. only stage III and IV OPSCCs were included, which were treated with radiotherapy and chemotherapy. In our patient population, in contrast, we did not find a survival difference between N0-2a and N2b-3, nor did we find it when correcting for smoking behavior.
Huang et al. divided 537 oropharyngeal carcinomas without distant metastases into four groups, based on N classification (N0–N2c vs. N3), T classification (T1–T3 vs. T4), smoking behavior (fewer vs. more than 20 pack-years history) and age (younger vs. older than 70 years): “T1–T3/N0–N2c and ≤20 pack-years of tobacco smoking,” “T1–T3/N0–N2c and >20 pack-years of tobacco smoking,” “T4 or N3 and age ≤70 years,” and “T4 or N3 and age >70 years.”40 The 5-year overall survival rates for the four groups were, respectively, 89%, 64%, 57% and 40%. Again, in our study no significantly different survival could be demonstrated between those groups.
Altogether, different cut-off points have been chosen in studies proposing an HPV-dependent tumor classification system. The results of the ICON-S study were finally adopted for the 8th edition of the UICC tumor staging system.42 Cramer et al. recently validated this classification system in a population of more than 15 000 patients (USA) and demonstrated a better stratification of the tumor staging for HPV-positive patients.44 Also for T, cN, and pN classification, the prognostic value could be validated in the HPV-positive population for that study.
Unlike Cramer et al., we focused on tonsillar carcinomas, studied a smaller patient population, and used HPV16-DNA PCR and/or FISH in addition to p16-IHC, whereas P16-IHC was the only detection method used by Cramer et al. These methodological choices might lead to differences in the results. Even so, in our study population, the 8th edition of the UICC tumor staging system was associated with a better prognostic value for tumor stage. The 8th edition was also associated with a better stratification for evaluating the impact of anatomical tumor characteristics on survival.
However, our study showed that there were no prognostic differences between N stages cN0 to cN2 and pN0 to pN2 (UICC 8th edition). Only cN3 and M1 classification were significantly associated with prognosis, and no differences were found between clinical N stages cN0 to cN2. Remarkably, there even seemed to be a better prognosis for cN1 than for cN0. As this study population included TSCCS only, the amount of contralateral or bilateral involved lymph nodes (cN2 classification) was very limited.
In literature, MacKenzie et al. also reported that only lymph nodes larger than 6 cm (cN3) were associated with worse survival.47 As mentioned, Cramer et al. had successfully validated the prognostic value of the clinical N classification (8th edition); however, their tables did not show a significant difference in survival between cN stages cN0 versus cN1 and cN2 M, and even so reported a better prognosis for cN1 versus cN0.44
All in all, the staging system for HPV-associated clinical N classification as now included in the 8th edition UICC TNM classification still does not appear to be fully representative of HPV-associated tumor biology. Additional variables need to be addressed to allow even more adequate stratification of HPV-positive tumors. Possible opportunities in our study population to improve the prognostic value of the 8th edition of the HPV-associated UICC TNM classification are discussed below.
4.1 Role of nonanatomical characteristics
The 8th edition of the clinical classification system is the result of a study published by O'Sullivan et al. (ICON-S).42 The predictive staging model adopted in that study included only anatomical features of the HPV-associated tumors. The earlier recursive partitioning analysis RTOG (2010) by Ang et al. included nonanatomical parameters such as age and tobacco smoking for the first time, and reported that tobacco smoking had important prognostic value.38 Results of the study by Ang et al. were validated by others.48, 49 Regarding smoking, Marur et al. noticed that treatment failures in a de-escalating regime of combining cetuximab with radiotherapy were seen in smokers (>10 pack-years).50 Although, in the study of Hawkin et al., number of pack-years were not correlated with survival, authors found a significant correlation between overall survival and a 1+ pack-year smoking history. Interestingly, 1+ pack-year smoking was not correlated with disease-free survival.51 Further study by Rietbergen et al., on the other hand, showed no differences in outcome regarding smokers versus nonsmokers.52 In that study, smoking status was defined based on the number of pack-years and no separate classification was performed for former-smoker status, which might have influenced the results for the smoking group.52 Broughman et al. postulated to leave the 10 pack-year rule, proposed by Ang et al. as a stratification tool in HPV-positive OPSCCs, because of the favorable prognosis of former-smoking status in regard of the number of pack-years in their recent study.38, 53 Haigentz et al. emphasized that including smoking in a predictive model has great limitations because of the lack of validated, prospective data, and the subjectivity of data collection on tobacco use.54 In our predictive model, non- or former smokers had a very favorable prognosis of more than 95% 5-year OS, even in more advanced tumor stages. It need to be emphasized that this groups of non- or former smokers include also patients that quitted smoking for more than 10 years despite having smoked numerous pack-years. Moreover, the number of pack-years was probably therefore not a predictor in prognosis in our patient population. Our study is the first to present that a time period of 10 years since cessation to smoking has a similar favorable outcome as nonsmokers with HPV-positive OPSCCs regardless of number of pack-years. This stresses the importance of adequate history taking regarding smoking status and period since cessation in the work-up of patients with HPV-positive OPSCCs.
Concerning the role of age, both Ang et al. and Huang et al. found that age influenced prognosis in OPSCCs in their studies38, 40 In our data, we observed a prognostic role for age with a cut-off point of 65 years.
In the present study, outcome of HPV-positive TSCCs was not predominantly TNM classification dependent, even when using the 8th edition. The most significant prognostic factors in HPV-positive TSCCs were smoking, age, N3 classification, and the presence of distant metastases. Therefore, we developed a new prognostic model comprising four groups. The first group consisted of non- or former smokers (patients who had quit smoking more than 10 years prior to the diagnosis of TSCC), and was associated with a 5-year OS of 95.1%, even in more advanced tumor stages and in former smokers who quitted smoking longer than 10 years ago the number of pack-years had no influence on prognosis. Group 2 included smokers aged 65 or younger with an associated overall survival rate of 75.6%. In group 3 patients who smoked and were older than 65 had a 5-year OS of 46.2%. Patients with N3 and M1 classification comprised group 4. Interestingly, the two patients who survived in this latter group were the only nonsmokers with N3-staged necks. Within the different groups, survival was not differentiated by T and/or N classification. Therefore, this proposed model appears to provide a simple additional tool for predicting outcome in the clinical setting. The current authors are aware of the relative small sample sizes on which this model is based. Therefore, an attempt to validate our predictive model was undertaken. A previous published database of almost 1000 patients with OPSCCs of which HPV-status and the concerning variables were collected and obtained with data transfer agreement. Nevertheless, smoking history was noted by pack-years only and times of cessation were not as such obtained in the dataset nor could be obtained out of original clinical records. This finding hampered validation of the in this study present predictive model. Nonetheless by presenting the model, the authors would like to emphasize the crucial role of smoking behavior including former-smoking, and age in the prediction of survival in HPV-related TSCCs, as both nonanatomical variables act almost independently of the UICC staging system (8th edition) with the exception of the N3-neck and the presence of distant metastases. The model highlights the role of adequate smoking history taking in the ongoing discussion whether or not smoking behavior is of influence on survival in HPV-positive TSCCs.
The role of nonanatomical parameters is not taken into account in the 8th version of the UICC classification system. Results of our research group on a prognostic model for OPSCCs was previously presented by Rios et al.55 These results were validated by Rietbergen et al. in a larger cohort, showing the large impact of performance status on outcome in the whole patient group.52 However, within the HPV-positive subgroup no further differentiation in risk profiles was provided. The present study is the first in which both age and smoking behavior play a role in classifying HPV-associated tumors in a way that is almost without reference to TNM classification. Our findings suggest that outcome in HPV-positive TSCCs is influenced by patient characteristics including senescence and intoxication (factors such as tobacco use) regardless of tumor stage. Furthermore, as a low number of locoregional and distant failures were noted, it appears that mechanisms through which senescence and intoxication influence overall survival are not necessarily tumor-related, as also Hawkins suggested.51 Nevertheless, these factors have proven to be unavoidable when considering response to therapy and rate of survival and when taking into account which patients might benefit from de-escalating therapeutic strategies.
4.2 How to address the N0 neck
In our patient population, a clinically negative neck classification was not associated with a better prognosis than for N1. The cut-off point for prognostic value in our study of tonsillar carcinomas was N3, which meant that very few bilaterally involved (and thus N2) neck stages were diagnosed. The prognosis for the N0 neck was even worse than for the N1 neck. As already mentioned regarding the study by Cramer et al., in which the 8th edition was validated in a population of more than 15000 HPV-positive oropharyngeal tumors, cN1 classification was associated with a significantly better survival than N0. Moreover, the bilaterally involved neck (cN2 classification) was not associated with a significantly worse survival than the clinically negative neck after adjustment for age, sex and race. Only cN3 classification was significantly associated with a worse survival.44 In previous research, it was noticed that patients with HPV-positive carcinomas more often had a lymph node as presenting symptom when compared to their HPV-negative counterparts. Presenting with that symptom may have led to an earlier discovery of the primary tumor.35 It is therefore possible that these presenting “alarming” nodes are associated with a better prognosis, as we found in our study. Also Fritsch et al. and Ang et al. found that patients with an HPV-positive single neck node between 3 and 6 cm in size (N2a, 7th edition) had a better outcome than patients without lymph node metastases.37, 38 Fritsch et al. compared outcome based on N classification between HPV-dominant (tonsillar fossa and base of tongue) and non-HPV-dominant oropharyngeal subsites in more than 15 000 oropharyngeal tumors.37 In their HPV-dominant population, cN2a (7th edition) had a better survival rate than the N0/1 patients. In the total population, no differences in outcome were noted as long as lymph node metastases were unilateral (<cN2c). Our study only included patients with TSCC; in this strongly HPV-dominant subgroup, similar results were found and a clinically negative neck (cN0) in HPV-positive TSCCs was not associated with a better survival than necks with lymph nodes smaller than 6 cm in diameter (i.e., cN1 and cN2 classification).
4.3 Role of oropharyngeal subsite
The influence of HPV on prognosis is often analyzed for all oropharyngeal subsites, without even discriminating between HPV-dominant and nondominant subsites. For the present study, we selected a group of 110 patients with squamous cell carcinomas of the tonsil, the most HPV-dominant oropharyngeal subsite. According to Sood et al., a bilaterally involved neck classification is predominantly seen in tumors at the base of the tongue, indicating that lymph node dissemination patterns differ even within the HPV-dominant oropharyngeal sites.44 This may explain why only a few bilaterally involved necks are seen in our population of tonsillar carcinomas. It may also explain the lack of significance of the cN2 neck in our study where we classify bilateral neck involvement in accordance with the 8th edition. The location of the tumor in the different subsites of the oropharynx therefore likely plays a prominent role in the development of advanced (N-) tumor stages in HPV-positive OPSCCs.
4.4 HPV-detection
P16 immunohistochemistry is a widely available, low-cost test, unlike the more complex HPV-in situ hybridization. In our study, p16 was combined with HPV-DNA PCR and/or FISH. We found that 15 out of 124 p16-positive patients were not HPV-positive (12%).
Nauta et al. raised the issue of consensus on the exact definition of HPV-associated OPSCC.56 HPV infection alone is not sufficient to classify an instance of OPSCC as HPV-related since the presence of HPV-DNA could merely reflect a transient infection. Detection of p16 alone is not specific for HPV-activity. Smeets et al. described an algorithm in which p16-IHC was combined with HPV 16 DNA PCR.57 Taberna et al. recently investigated the outcome of HPV-positive OPSCC in relation to the definition for HPV-positivity.58 They concluded that definitions of HPV positivity have an impact on TNM classification and patients' survival. Bussu et al. recently confirmed that p16-IHC alone may not be specific enough to become the diagnostic standard from the perspective of treatment de-intensification. Standardization of clinical use and of detection methods for HPV as a marker for molecular characterization in head and neck oncology is warranted.59
5 CONCLUSION
Altogether, our results are in line with those from other studies confirming that the introduction of the 8th HPV-associated tumor staging system is a step forward in staging HPV-associated OPSCC. However, our study indicates that the prognostic value is improved by including smoking history (nonsmokers and former smokers: cessation >10 years) and age as additional prognostic factors. These nonanatomical variables seem inevitable when the UICC staging system (8th edition) is used to predict outcome and stratify therapy groups, for example, for the purpose of de-escalation. To further optimize stratification, issues such as the relevance of N classification smaller than 6 cm, influence of subsite, and HPV-detection method remain to be addressed.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.